What does the Procalcitonin Level Tell us in Patients with Acute Pancreatitis?
By Berat Ebik1, Huseyin Kacmaz2, Elif Tugba Tuncel3, Medeni Arpa4, Feyzullah Ucmak5, Muhsin Kaya5Affiliations
doi: 10.29271/jcpsp.2022.10.1272ABSTRACT
Objective: To determine the factors affecting the procalcitonin level, and its association with the severity of pancreatitis in patients with acute pancreatitis (AP).
Study Design: Cross-sectional analytical study.
Place and Duration of Study: Division of Gastroenterology, University of Health Sciences, Diyarbakır Gazi Yasargil Education and Research Hospital and Department of Gastroenterology, Dicle University School of Medicine, Diyarbakır, Turkey, between April 2017 and June 2021.
Methodology: The study included 214 patients diagnosed with AP according to Atlanta criteria. By checking the PCT and CRP values of the patients in the first 12 hours, the relationship with these scales that predict the severity of pancreatitis was statistically examined.
Results: Hundred and fifty-two patients (71.0%) had mild, while 62 patients (29.0%) had severe pancreatitis. According to the Atlanta criteria, the mean PCT level of patients with mild pancreatitis was 1.4±0.7 ng/mL, while the mean PCT level of patients with severe pancreatitis was 9.0±12.3 ng/mL (p<0.001). The diagnostic performance of PCT was better for predicting severe AP. For the 0.94 ng/mL cut-off, PCT had 86.9% sensitivity and 50.7% specificity. (AUC=0.731[95% CI: 0.669-0.811]; p<0.001; LR: 1.7). In patients with severe pancreatitis, the PCT level was 4.7±18.5 ng/mL in patients without concomitant infection and 15.8±8.1 ng/mL in patients with concomitant infection (p<0.001).
Conclusion: High PCT value measured at the time of the first admission to the hospital may predict severe pancreatitis. In addition, a high PCT value at the time of admission to the hospital in patients with pancreatitis may indicate another concomitant infection.
Key Words: Acute pancreatitis, Coinfection, Procalcitonin, Severity of pancreatitis.
INTRODUCTION
Acute pancreatitis (AP) is a disease with a variable clinical course characterised by necroinflammatory changes in the pancreas. AP usually has a mild course and the inflammation is limited to the pancreas, in which case it results in clinical and laboratory improvement in a few days.
The course of AP may not always be this way. Severe AP may cause local and systemic complications that may result in mortality.1 Excessive inflammatory response, which starts with the development of systemic inflammatory response syndrome (SIRS) and progresses to multiorgan dysfunction syndrome (MODS), is responsible for severe pancreatitis.2
The most important thing to be determined from the time of hospitalisation in patients with AP is the severity of pancreatitis. In this way, patients can be effectively treated early and the possible need for intensive care can be determined in advance. Therefore, many scoring systems have been developed from the past to the present in order to predict the severity and prognosis of AP.3 Since some of these scores do not provide accurate information at first and some require 48-72 hours, researches on rapid and practical blood tests have been carried out. The most well-known example of this is CRP. In particular, there are studies stating that values above 150 mg/dl at the time of application are an indicator of severe pancreatitis.4
Procalcitonin (PCT) is an inactive propeptide that is secreted by monocytes as well as hepatocytes and C cells of the thyroid gland.5 Increased PCT levels in the blood are an indicator of sepsis, especially with bacterial and fungal infections.6
There have been studies showing the importance of PCT, especially in patients with severe pancreatitis who develop pancreatic abscess and necrosis.7,8 In addition, the PCT level was compared with scoring systems measuring the severity of pancreatitis and other laboratory parameters.9,10
In literature, not only local complications of pancreatitis but also the presence of additional infections/inflammations such as acute cholecystitis, acute cholangitis, and pneumonia have not been adequately compared in patients with pancreatitis. By comparing the PCT level with many scoring systems that measure the severity of AP, an attempt was made to understand what this means clinically. In addition, how another concomitant infection or inflammations may affect the PCT level in patients with AP. The objective of this study was to determine the factors affecting the procalcitonin level, and its association with the severity of pancreatitis in patients with acute pancreatitis (AP).
METHODOLOGY
A total of 214 patients, with acute pancreatitis, were included to the study, between April 2017 and June 2021. Approval was obtained for this study from the Ethics Committee of the University of Health Sciences, Gazi Yasargil Education and Research Hospital (Date: 20.06.2019; Issue No:236). The diagnosis of acute pancreatitis was made according to the Atlanta criteria.11
Abdominal pain compatible with AP; (spreading towards the back in the upper right quadrant and epigastric region), increase in amylase or lipase level more than 3 times the upper limit of normal interval, and presence of imaging findings specific to AP (with USG, CT or MR) patients who had 2 of the criteria were accepted as AP.11 Patients with previously known chronic pancreatitis, patients with a history of intra-abdominal surgery and resection, patients with acute abdomen, patients with pancreatic and other organ malignancies, and patients with shock and multiple organ dysfunction when admitted to hospital (elevated amylase and lipase levels could not be explained by pancreatitis alone) were excluded.
The selected patients were then divided into groups with mild and severe according to the Atlanta criteria. Cases without local or systemic complications were evaluated as mild pancreatitis, and cases with organ failure lasting more than 48 hours were evaluated as severe pancreatitis.11
In order not to complicate statistically (to avoid including the same patients twice), patients whose organ failure findings improved in the first 48 hours were included in the analysis in the mild pancreatitis group. However, patients with signs of organ failure lasting longer than 48 hours and local or systemic complications of pancreatitis were included in the analysis among patients with severe pancreatitis.
In addition to other laboratory parameter; CRP and procalcitonin levels were measured in the first 12 hours after hospitalisation. Procalcitonin level was measured by the immunomineometric method. The reference value determined for this test is <0.05 ng/mL.12 CRP was measured by the immunonephelometric method. The 0-5 mg/L range is designated as the reference range for this test.
Other scores measuring pancreatitis severity were calculated together with clinical and laboratory parameters as recommended by the guidelines. APACHE-2 scores of patients admitted to the intensive care unit were calculated. Cases below 8 points were considered mild, and cases above 8 points were considered severe pancreatitis.13 The age, state of consciousness, urea, pleural effusion, presence of SIRS parameters, and BISAP scores of the patients were calculated. Patients with a BISAP score <1 were evaluated as mild pancreatitis, and patients with ≥2 were evaluated as severe pancreatitis.14 Contrast-enhanced CT was performed in patients with fever, whose abdominal pain was persistent, and amylase and lipase values did not regress, despite 72-96 hours have passed since hospitalisation. Balthazar scores of CT patients were calculated. Patients with 0-6 points were evaluated as mild pancreatitis, and patients above ≥7 points were considered as severe pancreatitis.15 Ranson scores were calculated by checking complete blood count (CBC), AST, LDH, glucose, calcium, urea, and bicarbonate level in blood gas analysis at the time of hospitalisation and in the 48th hour. Oral and intravenous fluid intake and urine output of the patients were calculated. In addition, the fluid deficit was tried to be calculated by measuring central venous pressure in patients with central venous catheters. Those with a Ranson score ≥3 were considered as severe pancreatitis.16
With these calculated scores, the procalcitonin and CRP levels of the patients were statistically compared.
In addition, patients were evaluated for other concomitant infections at the time of admission to the hospital. Patients with upper right quadrant pain, positive Murphy's findings on physical examination, and more than 4 mm of gallbladder wall thickness or hydropic gallbladder were considered acute cholecystitis.17 In addition to the diagnosis of acute pancreatitis, patients with right upper quadrant pain, common bile duct dilatation on imaging, fever, and a total bilirubin level above 4 mg/dl were regarded as acute cholangitis.18
Apart from acute cholecystitis and cholangitis, other existing infections of the patients were also detected (such as pneumonia, and urinary tract infection). Thus, the authors have formed 4 patient groups. Inclusion and analysis of patients with mild and severe pancreatitis, with and without infection, allowed them to take a broader perspective on the effect of PCT on acute pancreatitis.
Table I: Demographic data of patients with acute pancreatitis.|
|
Mild Pancreatitis (n=152) |
Severe Pancreatitis (n=62) |
p |
|
Age |
54.7 (17-90) |
59.7 (18-95) |
0.105 |
|
Gender F/M (n/%) |
101/51 (66.4%-33.6%) |
38/24 (61.3%-38.7%) |
0.473 |
|
Aetiology Biliary Idiopathic Hyperlipidemia Alcohol Medicines Infections |
110 (72.4%) 31 (20.4%) 5 (3.3%) 2 (1.3%) 3 (2.0%) 1 (0.6%) |
43 (69.4%) 10 (16.1%) 5 (8.1%) 1 (1.6%) 2 (3.2%) 1 (1.6%) |
0.244 |
|
Comorbid diseases None DM HT CAD CHF CKD Other |
83 (54.6%) 19 (12.5%) 35 (23.0%) 10 (6.5%) 4 (2.6%) 3 (1.9%) 5 (3.2%) |
25 (40.3%) 13 (20.9%) 17 (27.4%) 8 (12.9%) 3 (4.8%) 3 (4.8%) 2 (3.2%) |
0.431 |
|
Concomitant infection None A. Cholecystitis A. Cholangitis UTI Pneumonia Other |
102 (67.1%) 26 (17.1%) 14 (9.2%) 4 (2.6%) 3 (2.0%) 3 (2.0%) |
38 (61.3%) 11 (17.8%) 8 (12.9%) 2 (3.2%) 2 (3.2%) 1 (1.6%) |
0.432 |
|
Death |
0 |
6 |
<0.001 |
|
DM: Diabetes mellitus; HT: Hypertension; CAD: Coronary artery disease; CHF: Congestive heart failure, CKD: Chronic kidney disease, UTI: Urinary tract infection. Fisher’s exact test and the independent t-test were also used. |
|||
Table II: PCT and CRP levels of patients according to scores.
|
|
Procalcitonin (ng/mL) |
p |
CRP (mg/dL) |
p |
||
|
|
Mild AP |
Severe AP |
Mild AP |
Severe AP |
||
|
Atlanta |
1.4±0.7 |
9.0±12.3 |
<0.001 |
41.1±49.8 |
84.8±85.9 |
0.001 |
|
BISAP |
1.6±4.0 |
16.5±19.3 |
0.011 |
47.8±69.8 |
91.7±89.7 |
0.022 |
|
Balthazar |
4.1±10.3 |
13.2±23.0 |
0.259 |
52.7±53.8 |
74.1±80.5 |
0.401 |
|
APACHE-2 |
0.6±1.6 |
10.7±19.4 |
<0.001 |
47.5±59.7 |
73.6±81.6 |
0.008 |
|
Ranson |
1.8±0.6 |
10.1±4.1 |
0.004 |
46.6±5.2 |
89.2±14.8 |
0.010 |
|
BISAP: Bedside index of severity in acute pancreatitis; APACHE-2: Acute physiology and chronic health evaluation. Independent samples t-test was used. |
||||||
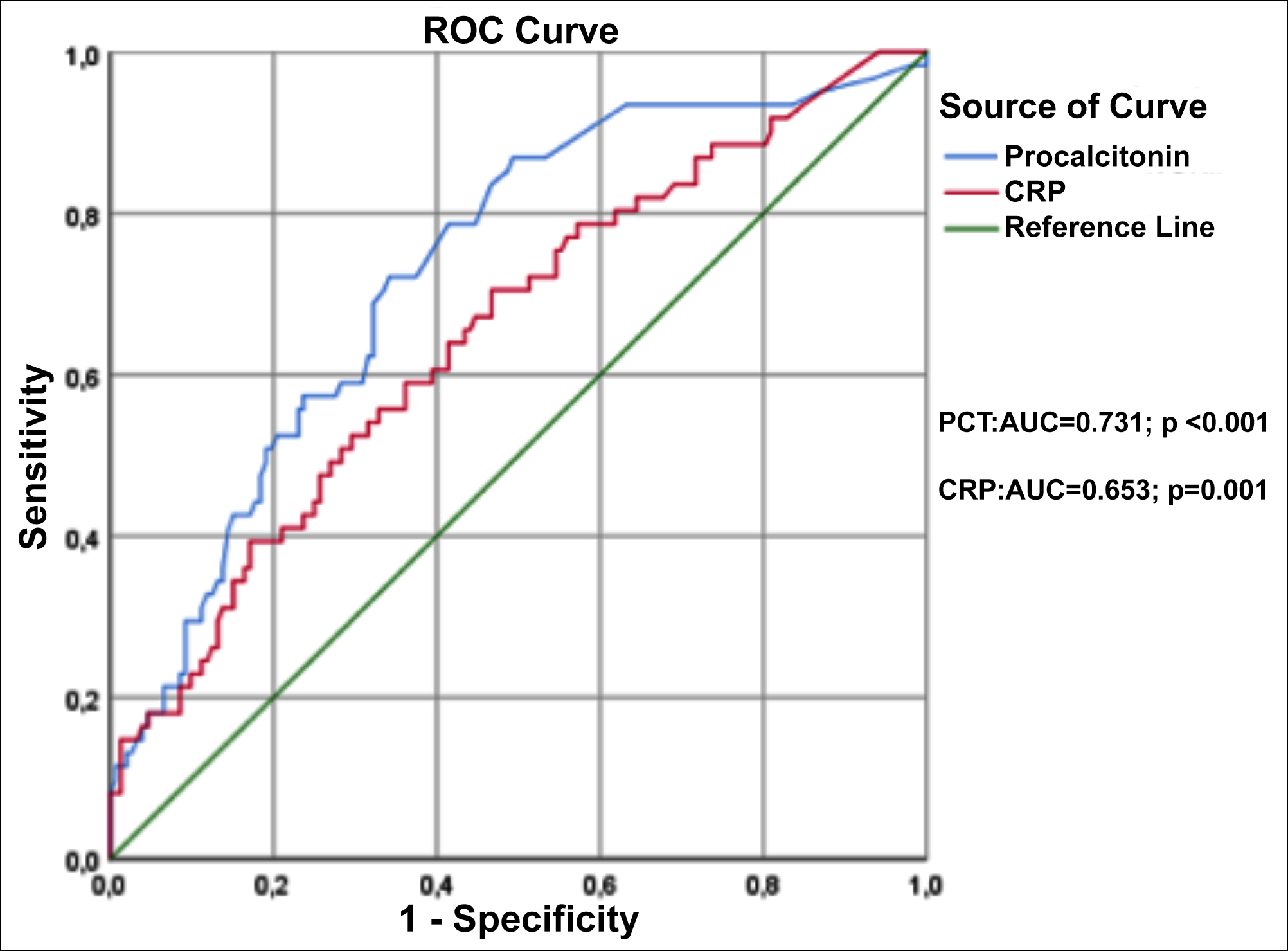 Figure 1: The effect of procalcitonin and C-reactive protein levels in predicting severe AP according to the Atlanta criteria.
Figure 1: The effect of procalcitonin and C-reactive protein levels in predicting severe AP according to the Atlanta criteria.
The effect of the presence or absence of a local (pancreatic abscess or infected necrosis) or systemic infection on the procalcitonin level in acute pancreatitis was analysed by statistical methods.
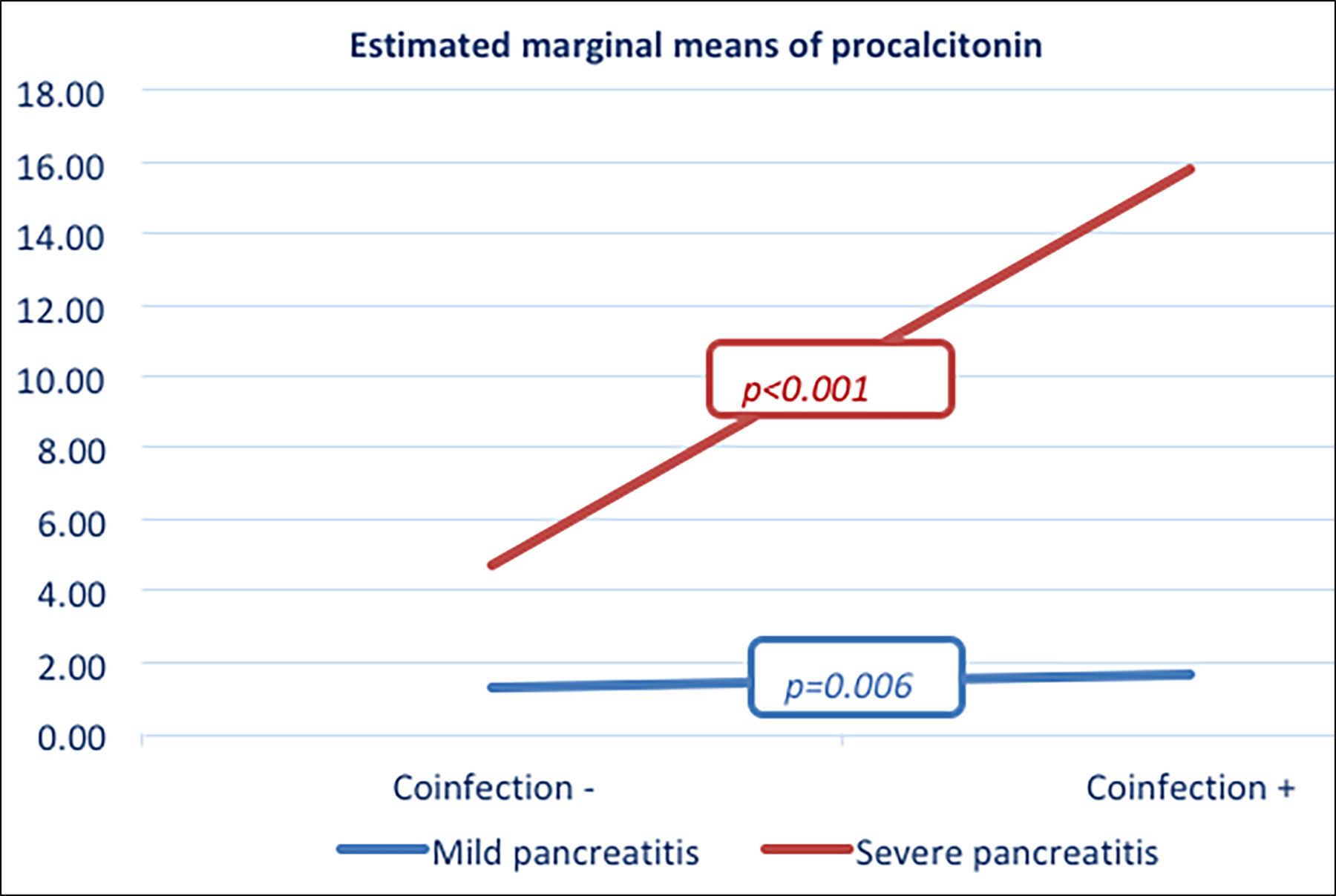 Figure 2: Effect of concomitant infection on PCT.
Figure 2: Effect of concomitant infection on PCT.
Kolmogorov-Smirnov, Shapiro-Wilk test, coefficient of variation, skewness, and kurtosis methods were used to control the normal distribution of patient data. While mean and standard deviation values were specified in continuous variables, categorical variables were expressed as counts and percentages. Fisher’s exact test was used to examine the difference between age, gender, etiological causes, comorbid conditions, and concomitant infection rates among groups in patients with mild to severe pancreatitis. Independent t-test was used to determine the mortality difference between the groups. Independent Samples t-test was used to determine the difference between groups in terms of procalcitonin and CRP in patients divided into groups according to Atlanta, BISAP, Balthazar, Ranson, and APACHE-2. Thresholds of procalcitonin and CRP in patients with severe pancreatitis were determined using receiver operating characteristic (ROC) curves. Sensitivity, specificity, and likelihood ratios were calculated. To examine the effect of coinfection on procalcitonin level, two-way repeated measures ANOVA test was performed and a partial effect value was calculated. All tests were two-sided and p-value <0.05 was considered statistically significant. Statistical analyses were carried out using the SPSS 24.0 for Windows (SPSSInc. Chicago, IL, USA) package program.
RESULTS
A total of 214 patients were included in the study. Hundred and fifty-two patients (71.0%) were mild according to the Atlanta criteria, while 62 patients (29.0%) had severe pancreatitis. Biliary causes were the most common etiologic cause in patients with both mild and severe pancreatitis [(mild AP, n=110 (72.4%); severe AP, n=43 (69.4%)]. Idiopathic pancreatitis was the most common aetiologic cause after biliary causes. No concomitant infection was detected in 67.1% (n=102) of patients with mild AP at the time of admission. There was no other concomitant infection in 61.3% (n=38) of patients with severe AP. Of the patients with mild AP, 17.1% (n=26) had acute cholecystitis and 9.2% (n=14) had acute cholangitis at the time of admission. Similarly, 17.8% (n=11) of patients with severe AP had acute cholecystitis and 12.9% (n=8) had acute cholangitis at the time of admission. Of the patients developing severe pancreatitis, 9.6% (n=6) died (Table I).
According to Atlanta criteria, procalcitonin levels of patients with mild pancreatitis were 1.4±0.7 ng/mL, while the procalcitonin level of severe pancreatitis was 9.0±12.3 ng/mL (p<0.001). The mean PCT level of patients with mild and severe pancreatitis was statistically different according to BISAP criteria (1.6±4.0 vs. 16.5±19.3; p=0.011). Although, PCT levels of patients with mild and severe pancreatitis were different according to Balthazar criteria, this difference was not statistically significant (4.1±10.3 vs. 13.2±23.0; p=0.259). According to the APACHE-2 criteria, the mean PCT value of patients with mild pancreatitis was 0.6±1.6 ng/mL, while the mean procalcitonin value of patients with severe pancreatitis was 10.7±19.4 ng/mL (p<0.001). According to Ranson criteria, the mean procalcitonin values of patients with mild and severe pancreatitis were significantly different (1.8±0.6 vs. 10.1±4.1; p=0.004, Table II).
In the ROC analysis, where the diagnostic performances of PCT and CRP in predicting severe AP were compared, it was seen that the diagnostic performance of PCT was better. For the 0.94 ng/mL cut-off, PCT had 86.9% sensitivity and 50.7% specificity. (AUC=0.731[95%CI: 0.669-0.811]; p<0.001; LR: 1.7). In CRP, it was found to have a sensitivity of 67.2% and a specificity of 55.3% for a cut-off of 80.1 mg/dL (AUC=0.653 [95%CI: 0.575-0.737]; p=0.001; LR: 1.5, Figure 1).
Concomitant infection was found to have an effect on the PCT level. According to the Atlanta classification, concomitant infection increased the PCT level in both mild and severe pancreatitis patients. The mean PCT level of patients with mild pancreatitis without concomitant infection was 1.27±3.10 ng/mL, while it was 1.66±4.33 ng/mL in patients with infection (p=0.006; partial Eta2=0.035). The mean PCT level of patients with severe pancreatitis without concomitant infection was 4.7±18.5 ng/mL, while it was 15.8±8.1 ng/mL in patients with infection (p<0.001; partial Eta2=0.092, Figure 2).
DISCUSSION
PCT levels were found to be significantly increased in patients with severe pancreatitis. PCT level, which increased significantly in severe pancreatitis according to the Atlanta criteria, was similarly found to be higher in severe pancreatitis in the APACHE-2, BISAP and Ranson scoring systems that predict the severity of pancreatitis. CRP levels were similarly higher in severe pancreatitis compared to mild pancreatitis.
There was no relationship between PCT level and Balthazar score. This is because the Balthazar score only predicts necrosis. This is not reflected in PCT level unless necrotic tissue is infected and an overly inflammatory response occurs, not giving enough information about necrosis in the first 72-96 hours. Studies indicate that Balthazar score is particularly weak in predicting severe pancreatitis compared to Atlanta and APACHE-2.19,20
PCT had a stronger model than CRP in predicting severe pancreatitis according to the Atlanta criteria. The sensitivity of PCT was found to be higher than CRP. In particular, the sensitivity of PCT levels is high even at low cut-off values in patients with severe pancreatitis, but this may not be a direct indicator of severe pancreatitis, and additional laboratory parameters and imaging tests may be needed.
In a study in which patients with pancreatitis were divided into mild and severe groups according to Atlanta criteria, serum PCT level was found to be similar to APACHE-2 and better than Balthazar scoring when cut-off was taken as 1.77 ng/mL.21 In another parallel study, the PCT level measured at admission and at the 48th hour was found to be correlated with the APACHE-2 score in patients with severe pancreatitis hospitalised in the intensive care unit.22 Again, in a meta-analysis in which 59 studies were evaluated, it was stated that PCT level could be a predictor of both severe and necrotizing pancreatitis when cut off >0.5 ng/mL.23 In another study, PCT values above 2 ng/mL were found to be 100% sensitive to predicting severe pancreatitis.24
The importance of PCT, which is a rapid biochemical test, becomes clearer when we consider the following: it takes 48 hours to calculate the Ranson score, the APACHE-2 score is based on 16 parameters and is not reliable for the first 24 hours and was not developed for pancreatitis, the Balthazar score is only sensitive to necrosis.25
PCT levels were found to be higher, especially in patients with pancreatitis with concomitant infection. This difference was especially more pronounced in patients with severe pancreatitis and any concomitant infection. Concomitant infection with severe pancreatitis is likely to have a cumulative effect on the PCT level. In addition, regardless of whether the patient has mild or severe pancreatitis, it is necessary not to attribute the PCT level to this condition completely, and not to ignore other focus of infection, especially the additional common cholecystitis and cholangitis.
The most important disadvantage of this study is that it is based on a single PCT measurement. By making sporadic measurements at certain hours, a more useful algorithm can be developed between increasing PCT level and severity. Contrary to other studies, using all scales and scoring systems that measure the severity of pancreatitis, and making an extensive analysis by adding existing comorbid infections/inflammations constitute the strength and originality of this study.
CONCLUSION
High PCT value measured at the initial admission to the hospital, may be predictive of severe pancreatitis. CRP level is also a useful helper in this regard. Evaluating these two together will strengthen the clinical diagnosis. Furthermore, the high PCT value at the time of admission to the hospital in patients with pancreatitis may indicate another concomitant infection.
ETHICAL APPROVAL:
Ethics committee approval was received for this study from the Ethics Committee of the University of Health Sciences, Gazi Yasargil Education and Research Hospital, who approved this study protocol (Ethics committee approval; Date: 20.06.2019 issue: 236).
PATIENTS’ CONSENT:
Informed consents were obtained from the patients who participated in this study.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
BE, HK: Concept.
BE: Design, data collection and/or processing, drafting.
ETT, HK: Materials.
MA, FU: Analysis and/or interpretation.
HK: Literature search.
FU, MK: Critical reviews.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Mitchell RM, Byrne MF, Baillie J. Pancreatitis. Lancet 2003; 361(9367):1447-55. doi: 10.1016/s0140-6736(03)13139-x.
- Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response syndrome, severity of multi-organ dysfunction and death in acute pancreatitis. Br J Surg 2006; 93(6):738-44. doi: 10.1002/bjs.5290.
- Park JY, Jeon TJ, Ha TH, Hwang JT, Sinn DH, Oh TH, et al. Choi WC. Bedside index for severity in acute pancreatitis: comparison with other scoring systems in predicting severity and organ failure. Hepatobiliary Pancreat Dis Int 2013; 12(6):645-50. doi: 10.1016/s1499-3872(13)60101-0.
- Cardoso FS, Ricardo LB, Oliveira AM, Canena JM, Horta DV, Papoila AL, et al. C-reactive protein prognostic accuracy in acute pancreatitis: timing of measurement and cutoff points. Eur J Gastroenterol Hepatol 2013; 25(7):784-9. doi: 10.1097/MEG.0b013e32835fd3f0.
- Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin Infect Dis 2004; 39(2):206-17. doi: 10.1086/421997.
- Azzini AM, Dorizzi RM, Sette P, Vecchi M, Coledan I, Righi E, Tacconelli E. A 2020 review on the role of procalcitonin in different clinical settings: An update conducted with the tools of the evidence based laboratory medicine. Ann Transl Med 2020; 8(9):610. doi: 10.21037/atm-20-1855.
- Adachi T, Kishihara Y, Okano H, Honzawa H, Hirayama M, Higashi H, et al. The utility of procalcitonin for the patients with infected pancreatic necrotic and pancreatic abscess. Intensive Care Med Exp 2015; 3(Suppl 1):A113. doi: 10. 1186/2197-425X-3-S1-A113.
- Chen HZ, Ji L, Li L, Wang G, Bai XW, Cheng CD, et al. Early prediction of infected pancreatic necrosis secondary to necrotizing pancreatitis. Medicine (Baltimore) 2017; 96(30):e7487. doi: 10.1097/MD.0000000000007487.
- Kumar S, Jalan A, Patowary BN, Bhandari U. To access the role of serum procalcitonin in predicting the severity of acute pancreatitis. Kathmandu Univ Med J (KUMJ) 2017; 15(57):19-24. PMID: 29446357.
- Venkatesh NR, Vijayakumar C, Balasubramaniyan G, Chinnakkulam Kandhasamy S, Sundaramurthi S, et al. Comparison of different scoring systems in predicting the severity of acute pancreatitis: A prospective observational study. Cureus 2020; 12(2):e6943. doi: 10.7759/cureus. 6943. PMID: 32190494.
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Acute pancreatitis classification working group. Classification of acute pancreatitis-2012: revision of the atlanta classification and definitions by international consensus. Gut 2013; 62(1):102-11. doi: 10.1136/gutjnl- 2012-302779.
- Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta-analysis. Lancet Infect Dis 2013; 13(5):426-35. doi: 10.1016/S1473-3099(12)70323-7.
- Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and chronic health evaluation (APACHE) IV: Hospital mortality assessment for today's critically ill patients. Crit Care Med 2006; 34(5):1297-310. doi: 10.1097/01.CCM. 0000215112.84523.F0.
- Singh VK, Wu BU, Bollen TL, Repas K, Maurer R, Johannes RS, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104(4):966-71. doi: 10.1038/ajg. 2009.28.
- Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, et al. Comparative evaluation of the modified CT severity index and CT severity index in assessing severity of acute pancreatitis. AJR Am J Roentgenol 2011; 197(2): 386-92. doi: 10.2214/AJR.09.4025.
- Kim YJ, Kim DB, Chung WC, Lee JM, Youn GJ, Jung YD, et al. Analysis of factors influencing survival in patients with severe acute pancreatitis. Scand J Gastroenterol 2017; 52(8):904-8. doi: 10.1080/00365521.2017.1310291.
- Strasberg SM. Clinical practice. Acute calculous cholecystitis. N Engl J Med 2008; 358(26):2804-11. doi: 10.1056/NEJMcp0800929.
- Okamoto K, Takada T, Strasberg SM, Solomkin JS, Pitt HA, Garden OJ, et al. Tokyo guideline revision committee. TG13 management bundles for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci 2013; 20(1):55-9. doi: 10.1007/ s00534-012-0562-2.
- Cho JH, Kim TN, Chung HH, Kim KH. Comparison of scoring systems in predicting the severity of acute pancreatitis. World J Gastroenterol 2015; 21(8):2387-94. doi: 10.3748/ wjg.v21.i8.2387.
- Papachristou GI, Muddana V, Yadav D, O'Connell M, Sanders MK, Slivka A, et al. Comparison of BISAP, Ranson's, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol 2010; 105(2):435-41; quiz 442. doi: 10. 1038/ajg.2009.622.
- Su Mi W, Myung Hwan N, Byung Geun K, Chien Ter H, Ji Sun H, Seung Hee R, et al. Comparison of serum procalcitonin with Ranson, APACHE-II, glasgow and balthazar CT severity index scores in predicting severity of acute pancreatitis. Korean J Gastroenterol 2011; 58(1):31-7. doi: 10.4166/kjg. 2011.58.1.31.
- Choudhuri AH, Duggal S, Biswas PS, Uppal R. A comparison of acute physiology and chronic health evaluation II score and serum procalcitonin change for predicting mortality in acute pancreatitis. Indian J Crit Care Med 2020; 24(3): 190-4. doi: 10.5005/jp-journals-10071-23377.
- Mofidi R, Suttie SA, Patil PV, Ogston S, Parks RW. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review. Surgery 2009; 146(1):72-81. doi: 10.1016/j.surg.2009.02.013.
- Dias BH, Rozario AP, Olakkengil SA, V A. Procalcitonin strip test as an independent predictor in acute pancreatitis. Indian J Surg 2015; 77(Suppl 3):1012-7. doi: 10.1007/ s12262-014-1112-8.
- Yeung YP, Lam BY, Yip AW. APACHE system is better than Ranson system in the prediction of severity of acute pancreatitis. Hepatobiliary Pancreat Dis Int 2006; 5(2): 294-9. PMID: 16698595.