Vasogenic Edema Pattern in Brain Metastasis
By Ezel Yaltirik Bilgin, Ozkan Unal, Nazan CiledagAffiliations
doi: 10.29271/jcpsp.2022.08.1020ABSTRACT
Objective: To determine the relationship of the presence and amount of vasogenic edema with origin, type, and grade of primary cancer.
Study Design: Cross-sectional study.
Place and Duration of Study: Dr. Abdurrahman Yurtaslan Ankara Oncology Training and Research Hospital, Radiology Clinic, Ankara, Turkey, from July 2017 to October 2021.
Methodology: Brain MRI scans of 292 patients were retrospectively evaluated. Age, gender, origin, type, and grade of primary cancer were determined. Metastasis type, and presence of vasogenic edema accompanying metastatic lesion were questioned. In cases of vasogenic edema accompanying metastatic lesions, the largest diameter of the vasogenic edema mass complex was measured in T2 sequences. In the contrast-enhanced series, the largest diameter of the metastatic lesion was measured, and the edema-mass ratio (EMR) was calculated by proportioning the diameter of the edema mass complex to the diameter of the mass.
Results: The frequency of vasogenic edema was found higher in patients with lung cancer compared to other primaries. The EMR was found statistically significantly higher in patients with primary lung cancer (p=0.001). This was particularly evident in the adenocarcinoma group. In the patient group with primary breast cancer, EMR was found significantly lower in patients with invasive ductal carcinoma. (IDC→1.95±0.66 vs. Other→2.48±0.52, Z=-2.301, p=0.021).
Conclusion: The amount and presence of vasogenic edema in patients with brain metastases may differ according to the origin and type of primary tumour.
Key Words: Brain edema, Metastatic disease, Magnetic resonance imaging.
INTRODUCTION
Despite advanced diagnosis in imaging and treatment methods, brain metastases are still the most important cause of mortality and morbidity in approximately 20% of adult cancer patients. They are the most common malignant tumour of the central nervous system with a rate of 20-40%. The frequency of brain metastases has been increasing over the years due to the improved survival in cancer patients and advances in diagnostic tools such as magnetic resonance imaging (MRI).1
First applied clinically in the 1980s, MRI has replaced computerized tomography (CT) as the gold standard imaging method in the diagnosis of brain metastasis and the follow-up of response to the treatment.2
Although the most common involvement is in the parenchymal area, non-parenchymal structures such as the calvarial area, diploe distance, meninges, choroid plexus or pituitary gland can also be involved, and MRI is the gold standard for imaging these areas.3
Most brain metastases are associated with peritumoural vasogenic edema which increases intracranial pressure and causes neurological deficits. Although the pathogenesis of vasogenic edema remains unclear in general, it is considered to be due to the microvascular proliferation around the tumour and fluid transfer from the intravascular space to the interstitium.4
Although there are publications in the literature investigating the relationship between vasogenic edema and survival, there are not enough studies evaluating the relationship between vasogenic edema and primary cancer origin and type. The Edema-mass ratio (EMR) is calculated by proportioning the largest diameter of the vasogenic edema-mass complex to the largest diameter of the metastatic mass in the MRI examinations at the time of the first diagnosis in patients followed up in the clinic with the diagnosis of brain metastasis. The study aimed to determine the relationship between vasogenic edema and EMR with origin, type, and grade of primary cancer and demographic characteristics of the patient.
METHODOLOGY
Local Ethics Committee approval was obtained (2021-11/5). Consent was obtained from all the patients before contrast-enhanced MRI imaging. The clinical information files and contrast-enhanced brain MRI examinations of 480 patients who were followed up with brain metastases at Radiology Clinic, Dr. Abdurrahman Yurtaslan Ankara Oncology Training and Research Hospital, between July 2017 - Oct 2021, were retrospectively evaluated. Of these patients, those who had no brain MRI before surgery or radiation therapy, whose clinical information could not be obtained, who had no primary focus of cancer, who had been diagnosed with neurodegenerative disease, and who had received corticosteroid therapy for any reason, were excluded from the study.
Apart from the excluded patients, 292 patients were evaluated. Age, gender, origin, type, and grade of primary cancer were evaluated. Contrast-enhanced brain MRI examinations of 292 patients before surgery, chemotheraphy, or radiotherapy were evaluated retrospectively. Type of metastasis (heterogeneous enhancing mass, focally enhancing focus, peripheral enhancement, hemorrhagic metastasis, and leptomeningeal involvement) (Figure 1) and presence of vasogenic edema accompanying metastatic lesion were questioned.
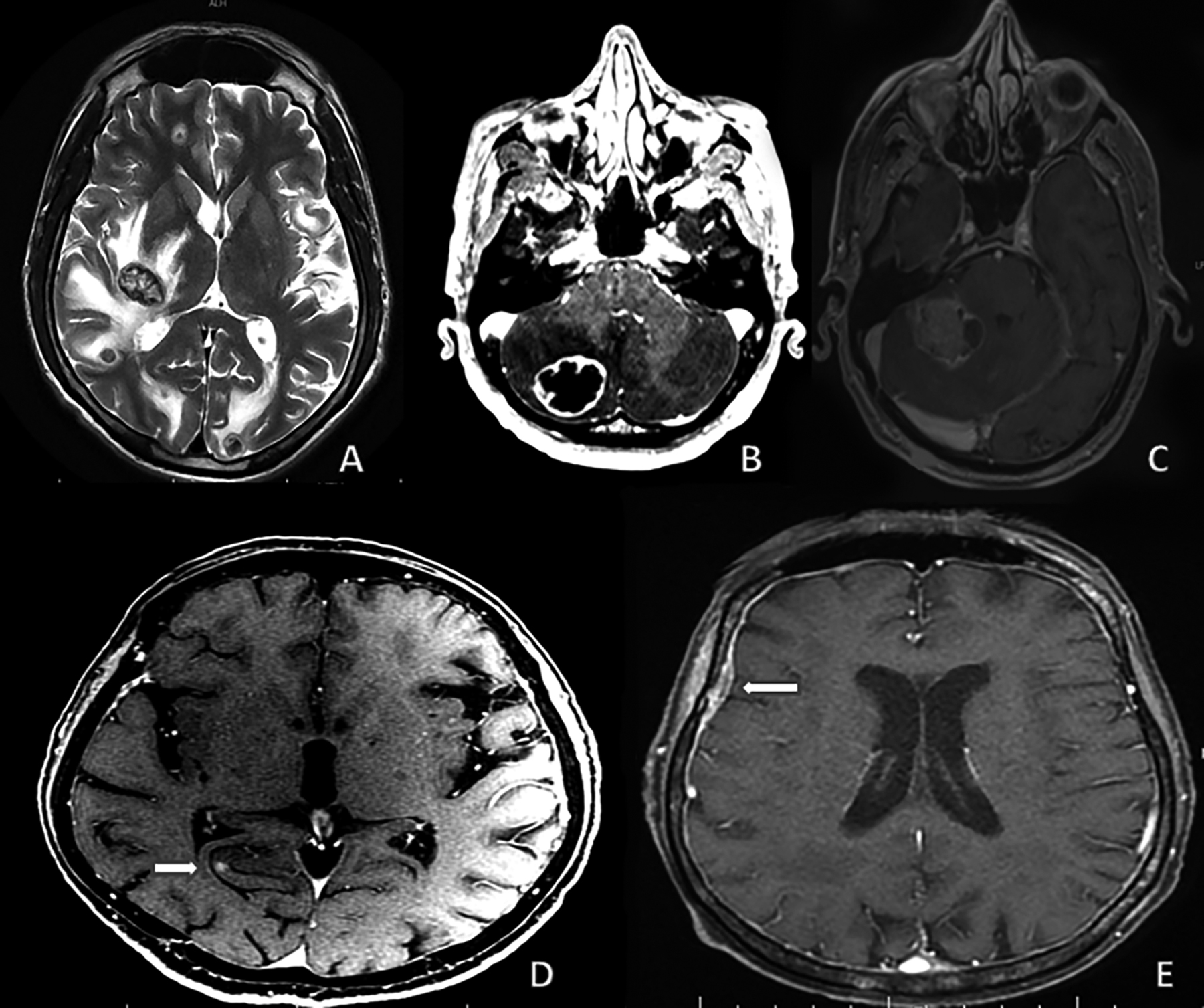 Figure 1: Types of metastases in MRI examination. (A): Hemorrhagic (B): Peripherally enhanced mass, (C): Heterogeneously enhanced mass, (D): Focally enhancing focus, (E): Leptomeningeal involvement.
Figure 1: Types of metastases in MRI examination. (A): Hemorrhagic (B): Peripherally enhanced mass, (C): Heterogeneously enhanced mass, (D): Focally enhancing focus, (E): Leptomeningeal involvement.
In the cases with vasogenic edema accompanying metastatic lesions, the largest diameter of the vasogenic edema-mass complex was measured in T2 sequences. In the contrast-enhanced series, the largest diameter of the metastatic lesion was measured, and the edema-mass ratio (EMR) was calculated by proportioning the largest diameter of the edema mass complex to the largest diameter of the mass (Figure 2). In patients with multiple metastatic lesions, the largest lesion was evaluated, and measurement and characterisation were made from the largest lesion. Measurements were evaluated and agreed upon by the 2 radiologists with more than 15 years of MRI experience.
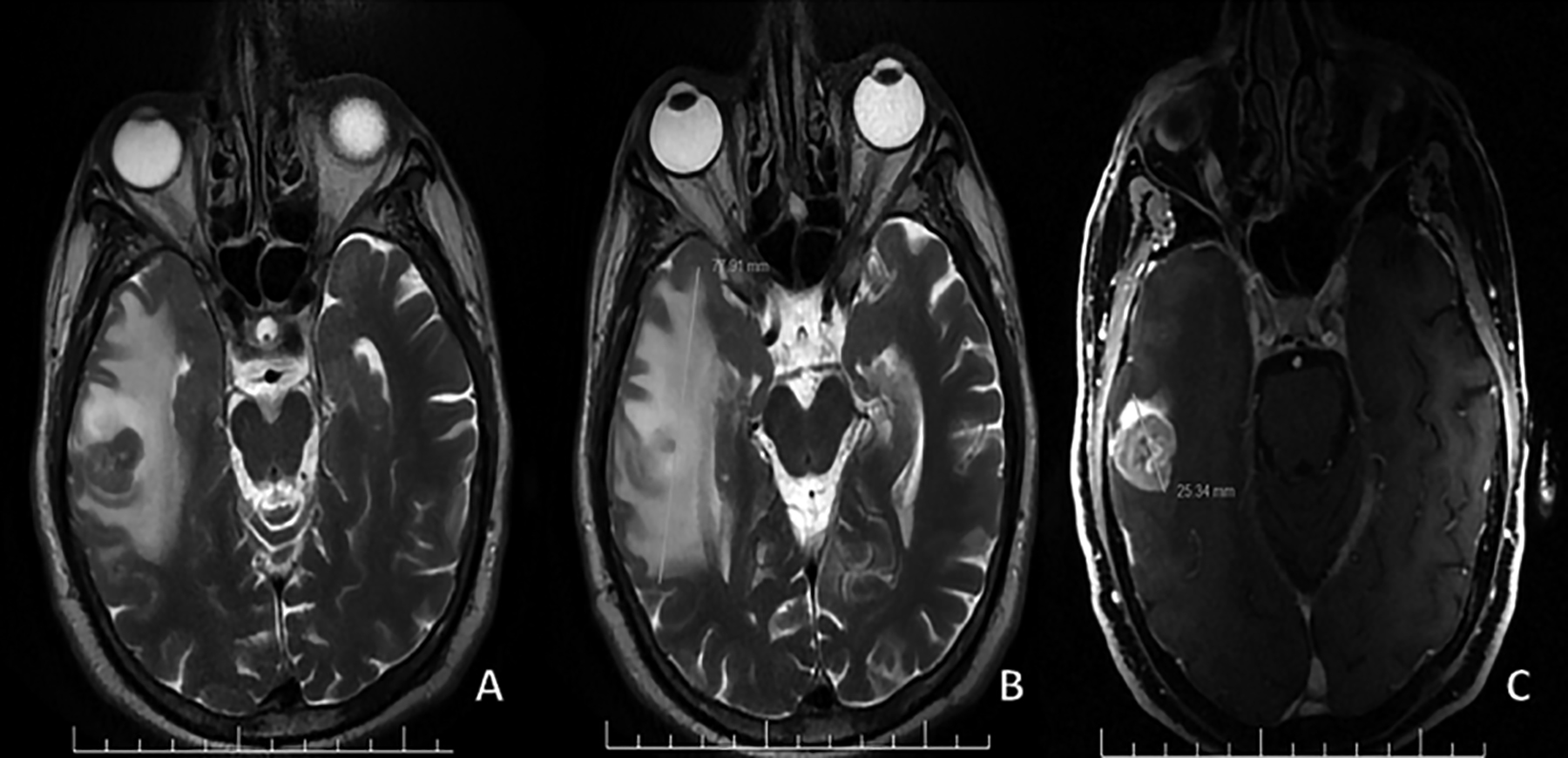 Figure 2: Edema-mass ratio of the largest size of vasogenic edema-mass complex to the largest size of metastasis.
Figure 2: Edema-mass ratio of the largest size of vasogenic edema-mass complex to the largest size of metastasis.
While evaluating the findings of the study, SPSS (Statistical Package for the Social Sciences) version 25.0 (IBM Corp., Armonk, NY, USA) program was used for statistical analyses. Descriptive statistics methods (number, percentage, median, IQR, etc.) were used to evaluate the study data. Whether the data showed normal distribution or not was evaluated with the Kolmogorov Smirnov test. In the analysis, age was found to be normally distributed, but the edema-mass ratio was not normally distributed. Quantitative comparisons between the groups were made by using the Mann-Whitney U-test and the Kruskal-Wallis H-test. Bonferroni correction was used to determine from which group the difference originated in more than two-group comparisons. In qualitative comparisons between groups, chi-square tests (Pearson Chi-square Test, Continuity Correction Test, and Fisher's Exact Test) were used. The results were evaluated within a 95% confidence interval, and significance was evaluated at p<0.05 level.
RESULTS
The mean age of 292 patients with brain metastasis during follow-up after the primary diagnosis included in the study was 59.80±11.47 years, 45.9% (n=134) of the patients were females and 54.1% (n=158) of the patients were males.
Of the patients with brain metastasis, 29.5% (n=86) had a primary tumour of the breast, 50% (n=146) of the lung, 3.1% (n=9) of the head and neck, and 4.8% (n=14) of the digestive system. It was determined that 7.2% (n=21) were urogenital, 3.8% (n=11) were skin (malignant melanoma), and 1.7% (n=5) were from other localisations.
One-hundred and ninety-one (65.4%) patients had vasogenic edema with metastases. In patients with edema, the mean diameter of metastasis was 22.30±12.81 mm, the mean diameter of edema mass complex was 45.74±23.11 mm, and the mean edema- mass ratio was 1.80±0.85.
Of the 79 patients with known grade classification of primary cancer, 40.5% were low/intermediate grade cancer and 59.5% were high.
Table I: Patient characteristics (n=292).|
Characteristics |
Category |
n (%) |
Mean (SD) |
Median (IQR) |
|
Age |
All |
292 (100) |
59.80 (11.47) |
|
|
Age group |
≤60 |
149 (51) |
|
|
|
|
>60 |
143 (49) |
|
|
|
Gender |
Female |
134 (45.9) |
|
|
|
|
Male |
158 (54.1) |
|
|
|
Origin of primary tumour |
Breast |
86 (29.5) |
|
|
|
|
Lung |
146 (50) |
|
|
|
|
Head and neck |
9 (3.1) |
|
|
|
|
Digestive system |
14 (4.8) |
|
|
|
|
Urogenital system |
21 (7.2) |
|
|
|
|
Skin(malignant melanoma) |
11 (3.8) |
|
|
|
|
Other |
5 (1.7) |
|
|
|
Presence of vasogenic edema |
Yes |
191 (65.4) |
|
|
|
|
No |
101 (34.6) |
|
|
|
Edema-mass Ratio (EMR) |
All |
292 (100) |
1.80 (0.85) |
1.59 (1-2.3) |
|
Type of metastasis |
Leptomeningeal involvement |
30 (10.3) |
|
|
|
|
Hemorrhagic |
22 (7.5) |
|
|
|
|
Focally enhancing focus |
34 (11.6) |
|
|
|
|
Heterogeneous enhancing mass |
107 (36.6) |
|
|
|
|
Peripheral enhancement |
99 (33.9) |
|
|
|
Grade (n=79) |
Low/medium |
32 (40.5) |
|
|
|
|
High |
47 (59.5) |
|
|
|
Histological type-breast |
Invasive ductal carcinoma |
73 (84.9) |
|
|
|
|
Other |
13 (15.1) |
|
|
|
Histological type-lung |
Adenocarcinoma |
88 (60.3) |
|
|
|
|
Squamous cell carcinoma |
25 (17.1) |
|
|
|
|
Small cell carcinoma |
26 (17.8) |
|
|
|
|
Other |
7 (4.8) |
|
|
|
SD: Standard deviation, IQR: Interquartile range. |
||||
Table II: The frequency of vasogenic edema and edema-mass ratio by primary cancer origin and type.
|
|
|
Frequency of vasogenic edema |
|
|
Edema-mass ratio |
|
|
|
|
n |
n(%) |
χ2 |
p-value |
Mean(SD) |
Median(IQR) |
p-value |
|
Origin of primary tumour |
|
|
|
|
|
|
0.002b** |
|
Breast1 |
86 |
42(48.8) |
18.120a |
<0.001* |
1.50(0.69) |
1(1-1.8) |
dif=1<2 |
|
Lung2 |
146 |
102(69.9) |
|
|
1.91(0.89) |
1.7(1-2.4) |
|
|
Head and neck3 |
9 |
8(88.9) |
|
|
2.36(1.11) |
2.1(1.5-3.4) |
|
|
Digestive system4 |
14 |
10(71.4) |
|
|
1.79(0.69) |
1.6(1-2.6) |
|
|
Urogenital system5 |
21 |
16(76.2) |
|
|
2.12(1.01) |
2(1.2-2.5) |
|
|
Skin (malign melanoma)6 |
11 |
8(72.7) |
|
|
1.56(0.49) |
1.5(1-1.9) |
|
|
Other7 |
5 |
5(100) |
|
|
2.04(0.57) |
1.9(1.5-2.6) |
|
|
Type of breast cancer |
|
|
|
|
|
|
0.526b |
|
Invasive ductal carcinoma |
73 |
36(49.3) |
0.000b |
1.000 |
1.47(0.66) |
1(1-1.8) |
|
|
Other |
13 |
6(46.2) |
|
|
1.68(0.84) |
1(1-2.5) |
|
|
Type of lung cancer |
|
|
|
|
|
|
0.001b** |
|
Adenocarcinoma1 |
88 |
69(78.4) |
18.874a |
<0.001* |
2.09(0.94) |
2(1.3-2.7) |
dif=3<1,2 |
|
Squamous cell carcinoma2 |
25 |
20(80) |
|
|
1.95(0.76) |
1.8(1.4-2.5) |
|
|
Small cell carcinoma3 |
26 |
10(38.5) |
|
|
1.40(0.62) |
1(1-1.6) |
|
|
Other4 |
7 |
3(42.9) |
|
|
1.35(0.64) |
1(1-1.6) |
|
|
*=p<0.05, χ2=Chi-square tests (a=Pearson Chi-square, b=Continuity correction); **=p<0.05, a=Mann-whitney U-test, b=Kruskal wallis-H Test; IQR: Interquartile range. |
|||||||
The histologic tumour type in the majority of patients diagnosed with primary breast cancer was invasive ductal carcinoma. The tumour type in 60.3% (n=88) of the patients with primary lung cancer was determined to be adenocarcinoma, 17.1% (n=25) of them were squamous cell carcinoma, 17.8% (n=25) were small cell carcinoma and 4.8% (n=7) of them were other tumour types. The characteristics of the patients are summarised in Table I.
The frequency of vasogenic edema in patients according to the origin of primary cancer was statistically and significantly different. The lowest edema rate was 48.8% (p=0.006) in the patient group diagnosed with primary breast cancer. The frequency of vasogenic edema by primary cancer origin and type is presented in Table II.
The frequency of vasogenic edema in the patients over 60 years of age with brain metastases was statistically and significantly higher than in patients aged 60 years and younger (72% vs 59.1%, p=0.020). In the univariate modeling, the factors associated with the incidence of vasogenic edema were determined to be primary tumour region and age (p<0.05).
In 79 patients whose primary cancer grade information was available, the incidence of vasogenic edema was 32% in low / medium grade cancers and 47% in high-grade cancers. No significant difference was found in the incidence of vasogenic edema between low-intermediate grade and high-grade cancers (p=0.179).
EMR had a statistically significant difference according to the primary tumour site (p=0.002). In the subgroup analyses, this difference was most significant between the primary breast cancer patient group and the primary lung cancer patient group (Table II). The mean EMR according to the tumour histological type in the patient group with primary lung cancer differed statistically and significantly (p=0.001). In subgroup analyses, this difference was found significantly higher in adenocarcinoma type. In the patient group with primary breast cancer, EMR was found to be statistically and significantly lower in patients with invasive ductal carcinoma. (IDC→1.95±0.66 vs. Other→2.48±0.52, p=0.021). No statistically significant difference was found in EMR measurement between low-intermediate grade cancers and high-grade cancers in 79 patients whose primary cancer grade information was accessed (p=0.45).
DISCUSSION
Metastases are the most common brain tumours in adulthood.5,6 30-40% of the cancer patients develop brain metastases at some point during the course of their disease.7,8 Metastases to the brain are a well-established but incompletely understood process.9
Patients can be diagnosed with brain metastases on screening after the diagnosis of primary cancer. However, in some cases, brain metastases may be the first finding.
Despite improvements in advanced imaging techniques over the past decades, conventional structural magnetic resonance imaging (MRI) remains the standard of care imaging method for neurooncologic practice.10,11 The primary roles of structural MRI in the initial brain tumour evaluation include determining the lesion location, involvement, and mass effect upon the brain.12,13
The primary tumours that most frequently metastasise to the brain are lung (≥50%), breast (15-25%), melanoma (5-20%), and less frequently testicular, kidney, colon-rectum, and thyroid cancers in order of frequency.14 However, generally any cancer subtype can metastasise to the brain. Similar to the literature, in this study, the most frequently defined primary tumour was lung cancer.
The location of the primary cancer is unknown in 15% of the cases. Brain metastases usually develop in the advanced stages of the disease, and less often they may be the first clinical manifestation of cancer or may be diagnosed simultaneously with the primary tumour.15
The most common sites of metastasis within the brain are the cerebral hemispheres (80%), the cerebellum (15%), and the basal nuclei (5-10%).16
There are some studies investigating the relationship between primary cancer origin and brain metastasis location. Mampre et al. found in their study of 388 patients and 669 lesions that malignant melanoma metastases were observed more frequently in the lateral and medial lenticulostriate artery irrigation areas and less frequently in the cerebellar region.17
Most metastases are associated with vasogenic edema which increases intracranial pressure and causes neurological deficits.18 Although the pathogenesis of vasogenic edema is not exactly known, it is thought to result from an increase in microvessels and fluid transport from the intravascular space.4
Vasogenic edema around metastasis is observed as hyperintense in the T2-weighted series and hypointense in the T1-weighted series. Edema is usually limited to white matter, and cortical areas are usually spared. The presence of involvement in cortical areas should suggest other pathologies such as vasogenic edema due to primary brain tumours.1
In addition, some studies aimed to differentiate primary brain tumour and metastasis according to the diffusion characteristics of edema. It was found that no difference was observed for any of the diffusion parameters in peritumoural edema.19
Some studies in the literature aim to differentiate primary brain tumours from the brain metastasis according to the vasogenic edema. In a study by Meyer et al. conducted with 23 patients (9 metastases, 8 low-grade gliomas, and 6 GBM), they found that mean T1 p-values were significantly elevated in the vasogenic edema surrounding intracranial metastases when compared to the infiltrative edema associated with either LGG or GBM, and no significant difference was found between low-grade gliomas and glioblastomas.20
There is no study in the English literature that aimed to predict the relation between origin, type, grade of primary cancer, and amount of vasogenic edema.
In studies on edema in the literature, the size of edema is measured independently of the size of the lesion. EMR, which was evaluated in this study, provides a new measurement in this area by proportioning the edema size to the lesion size. In addition, EMR is a measurement method that is repeatable and that can be done easily by any radiologist with any device, does not require equipment or technological support, and can be done in a short time.
The rate of accompanying vasogenic edema was higher in patients over 60 years of age and tumours originating from the lung. EMR values were higher in lung cancer, especially in adenocarcinoma. In patients with primary breast cancer, the rates of EMR were lower than in metastases originating from lung. In the group of patients diagnosed with breast cancer, the rate of EMR was significantly lower in patients with invasive ductal carcinoma. These significant results are likely related to tumour behavior and cellularity. However, these findings need to be supported by further studies. Retrospective data collection is the main limitation of this study.
CONCLUSION
The presence and amount of vasogenic edema in brain metastasis cases differ according to the origin and type of primary cancer in the present study.
ETHICAL APPROVAL:
Ethics Committee approval for this study was received from the Dr. Abdurrahman Yurtaslan Oncology Trainning and Research Hospital (2021-11/5).
PATIENTS’ CONSENT:
Written informed consents were obtained from all the patients included in the study.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
EY: Designed and coordinated the study, evaluated images, and written the article.
OU: Analysed the data and created study plan.
NC: Selected patients and evaluated images.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Bertolini F, Spallanzani A, Fontana A, Depenni R, Luppi G. Brain metastases: an overview. CNS Oncol 2015; 4(1): 37-46. doi: 10.2217/cns.14.51.
- Graif M, Bydder GM, Steiner RE, Niendorf P, Thomas DG, Young IR. Contrast-enhanced MR imaging of malignant brain tumours. AJNR Am J Neuroradiol 1985; 6(6):855-62.
- Maroldi R, Ambrosi C, Farina D . Metastatic disease of the brain: Extra-axial metastases (skull, dura, leptomeningeal) and tumour spread. Eur Radiol 2015; 15(3):617-26. doi: 10.1007/s00330-004-2617-5.
- Stummer W. Mechanisms of tumour-related brain edema. Neurosurg Focus 2007; 22(5):E8. doi: 10.3171/foc. 2007.22.5.9.
- Lamba N, Wen PY, Aizer AA. Epidemiology of brain metastases and leptomeningeal disease. Neuro Oncol 2021; 23(9):1447-56. doi: 10.1093/neuonc/noab101.
- Cho SJ, Sunwoo L, Baik SH, Bae YJ, Choi BS, Kim JH. Brain metastasis detection using machine learning: A systematic review and meta-analysis. Neuro Oncol 2021; 23(2):214-25. doi: 10.1093/neuonc/noaa232.
- Kotecha R, Gondi V, Ahluwalia MS, Brastianos PK, Mehta MP. Recent advances in managing brain metastasis. F1000Res 2018; 7:F1000Faculty Rev-1772. doi: 10.12688/f1000 research.15903.1.
- Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, et al. Brain metastases. Nat Rev Dis Primers 2019; 5(1):5. doi: 10.1038/s41572-018-0055-y.
- Buller M, Chapple KM, Bird CR. Brain metastases: Insights from statistical modeling of size distribution. AJNR Am J Neuroradiol 2020; 41(4):579-82. doi: 10.3174/ajnr.A6496.
- Ellingson BM, Bendszus M, Boxerman J, Barboriak D, Erickson BJ, Smits M, et al. Consensus recommendations for a standardised brain tumour ımaging protocol in clinical trials. Neuro Oncol 2015; 17(9):1188-98. doi: 10.1093/ neuonc/nov095.
- Davis PC, Hudgins PA, Peterman SB, Hoffman JC. Diagnosis of cerebral metastasis: Double-dose delayed CT vs. contrast-enhanced MR imaging. Am J Neuroradiol 1991; 12(12): 293-300.
- Cha S. Update on brain tumour imaging. Curr Neurol Neurosci Rep 2005; 5(3):169-77. doi: 10.1007/s11910- 005-0044-x.
- Arnold SM, Patchell RA. Diagnosis and management of brain metastases. Hematol Oncol Clin N Am 2001; 15(6): 1085-107. doi: 10.1016/s0889-8588(05)70269-0.
- Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin 2003; 21(1):1-23. doi: 10.1016/s0733-8619(02)00035-x.
- Nieder C, Spanne O, Mehta MP, Grosu AL, Geinitz H. Presentation, patterns of care, and survival in patients with brain metastases: What has changed in the last 20 years? Cancer 2011; 117(11):2505-12. doi: 10.1002/cncr. 25707.
- Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist 2007; 12(7):884-98. doi: 10.1634/theoncologist.12-7-884.
- Mampre D, Ehresman J, Alvarado-Estrada K, Wijesekera O, Sarabia-Estrada R, Quinones-Hinojosa A, et al. Propensity for different vascular distributions and cerebral edema of intraparenchymal brain metastases from different primary cancers. J Neurooncol 2019; 143(1):115-22. doi: 10.1007/ s11060-019-03142-x.
- Drappatz J. Management of vasogenic edema in patients with primary and metastatic brain tumours, in: UpToDate. Waltham, MA: UpToDate (Accessed on May 29, 2021).
- Mao J, Zeng W, Zhang Q, Yang Z, Yan X, Zhang H, et al. Differentiation between high-grade gliomas and solitary brain metastases: A comparison of five diffusion-weighted MRI models. BMC Med Imag 2020; 20(1):124. doi: 10.1186/s12880-020-00524-w.
- Villanueva-Meyer JE, Barajas RF, Mabray MC, Chen W, Shankaranarayanan A, Koon P, et al. Differentiation of brain tumour-related edema based on 3D T1rho imaging. Eur J Radiol 2017; 91:88-92. doi: 10.1016/j.ejrad.2017.03.022.