UGT1A1 Genetic Testing for Irinotecan Should be done before Starting the Treatment, Rather than after the Development of Serious Adverse Drug Reactions
By Lingti Kong, Li Rong, Muhua WangAffiliations
doi: 10.29271/jcpsp.2022.12.1651Sir,
It is well known that serious adverse reactions such as diarrhoea and myelosuppression of irinotecan are related to the UGT1A1 gene polymorphism, and the mutation frequencies of alleles such as *6, *28 and *93 vary among different races.1-3 Currently, genetic testing before treatment is not mandatory on the label for irinotecan. It is only recommended to reduce the dose if carrying genetic variation.
A 55-year male, with a body surface area of 1.63 m2, was diagnosed with advanced-stage small cell lung cancer. He had previously received 11 cycles of the EP regimen and developed grade II myelosuppression. On June 30th, 2021, he was admitted to the hospital due to dizziness and nausea. MRI of the head showed multiple abnormal signals in the upper and lower brain parenchyma. He received cranial radiotherapy at a dose of 40Gy/20F. On July 14th, he received irinotecan 300 mg single-agent regimen. On July 17th, he developed diarrhoea. No obvious haematological toxicity was noted. He was given loperamide capsules (2 mg/Q2h). Diarrhoea gradually increased to 6-7 episodes per day, and clostridium butyric dual viable bacteria capsules (1.26 g/Q12h) were added. Diarrhoea continued to worsen to 8-9 watery stools per day, and the patient developed grade IV myelosuppression. The clinical pharmacist suggested UGT1A1 genetic testing, plus octreotide infusion (50 μg/Qh), G-CSF (200 μg/Q12h), and mecapegfilgrastim infusion (6 mg/st), and suspended radiotherapy at the same time. The test results showed the patient to be heterozygous carrier of UGT1A1*28 and UGT1A1*6. On July 26th, the patient developed faecal incontinence and continuous leak of brick-red stool, and the amount of blood in the stool continued to increase. He was given hemocoagulase (1 U/st). On the same day in the evening, the patient’s family decided to stop further treatment and the patient was discharged from the hospital.
The changes of common haematologic parameters during hospitalisation are shown in Figure 1.
It is obvious that the clinician did not test the UGT1A1 gene polymorphism before starting treatment, which resulted in serious adverse reactions. If tested in advance, according to the test results, the dose can be reduced, and close monitoring can be carried out at the same time, which might be beneficial to avoid the occurrence of serious adverse reactions. Genetic testing was carried out after the occurrence of adverse reactions according to the clinical pharmacist's recommendation.
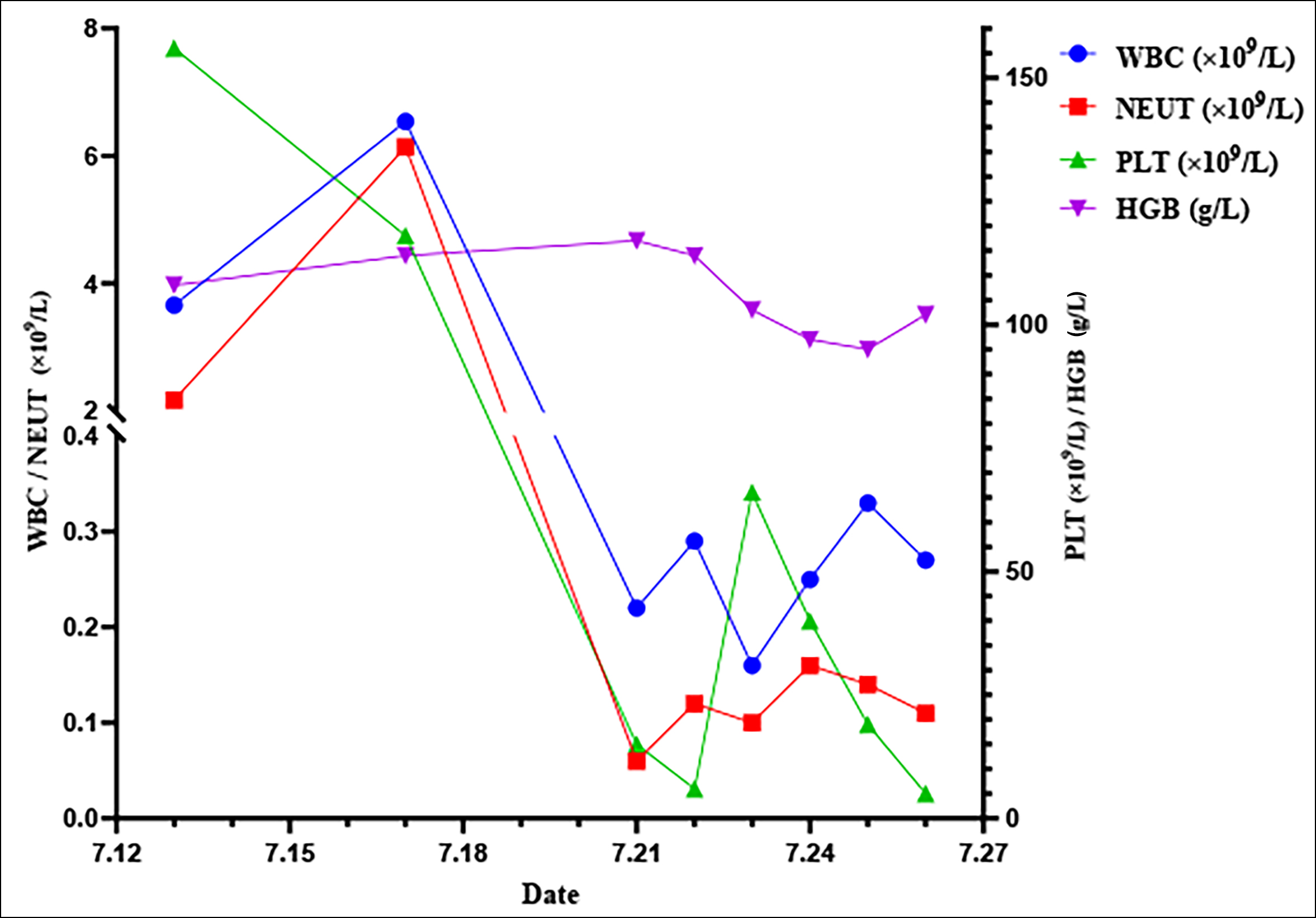 Figure 1: The changes of common blood tests.
Figure 1: The changes of common blood tests.
WBC: White blood cell; PLT: Platelet; HGB: Haemoglobin; NEUT: Neutrophilshttps://cn.bing.com/dict/search?q=cell&FORM=BDVSP6&cc=cn.
Studies have shown the UGT1A1 gene polymorphism is associated with toxicity, clinical efficacy, and cost-effectiveness of irinotecan.3-5 Therefore, we strongly recommend that all patients be tested for genetic polymorphisms prior to starting irinotecan therapy, so as to promote its rational use.
FUNDING:
This work was financially supported by the key research and development program project of Anhui Province (No. 202004j07020008) and talent training plan of Bengbu Medical College (No. by51201316).
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
LTK: Substantial contribution, conception, and design of work.
LR: Drafting the manuscript.
MHW: Data collection and writing critical review.
All the authors have approved the final of the manuscript to be published.
REFERENCES
- Karas S, Innocenti F. All you need to know about UGT1A1 genetic testing for patients treated with irinotecan: A practitioner-friendly guide. JCO Oncol Pract 2021; OP2100624. doi.org/10.1200/op.21.00624.
- Mhandire DZ, Goey AKL. The value of pharmacogenetics to reduce drug-related toxicity in cancer patients. Mol Diagn Ther 2022; 26(2):137-51. doi.org/10.1007/s40291-021- 00575-x.
- Hulshof EC, de With M, de Man FM, Creemers GJ, Deiman B, Swen JJ, et al. UGT1A1 genotype-guided dosing of irinotecan: A prospective safety and cost analysis in poor metaboliser patients. Eur J Cancer 2022; 162:148-57. doi.org/10.1016/j.ejca.2021.12.009.
- Hulshof E, Deenen M, Guchelaar H, Gelderblom H. Pre-therapeutic UGT1A1 genotyping to reduce the risk of irinotecan-induced severe toxicity: Ready for prime time. Eur J Cancer 2020; 141:9-20. doi.org/10.1016/j.ejca. 2020.09. 007.
- Iwasa S, Muro K, Morita S, Park Y, Nakamura M, Kotaka M, et al. Impact of UGT1A1 genotype on the efficacy and safety of irinotecan-based chemotherapy in metastatic colorectal cancer. Cancer Sci 2021; 112(11):4669-78. doi.org/10.1111/cas.15092.