Treatment for Resistant Hypertension Under the Guidance of Pharmacogenomics: A Randomised Controlled Open-Label Trial
By Ai Wang, Muisha Bienvenue Mbikyo, Junzhe Zhang, Nan Cui, Zhao LiAffiliations
doi: 10.29271/jcpsp.2024.04.383ABSTRACT
Objective: To evaluate the efficacy and safety of pharmacogenomics (PGx)-guided treatment in individuals with resistant hypertension (RH).
Study Design: Randomised controlled open-label study.
Place and Duration of the Study: Department of Cardiology, The First Hospital of China Medical University, Shenyang, Liaoning Province, China, from June 2019 to November 2021.
Methodology: The study assigned RH patients to two groups. The intervention group (IG) received 12 weeks of PGx-guided treatment, while the control group (CG) followed a consensus-based approach. Examining 10 genes and their alleles with 31 antihypertensive drugs in the IG, the study provided specific medication advice. The primary outcome measured the difference in office systolic blood pressure (SBP) change from baseline at 12 weeks. Secondary outcomes included changes in diastolic blood pressure (DBP), hepatic and renal function, and major adverse cardiovascular events.
Results: Fifty-nine patients from the First Hospital of China Medical University participated, with 29 in the IG and 30 in the CG. Significant differences were noted in SBP reduction (IG: 31.26 ± 18.64 mmHg; CG: 14.61 ± 17.74 mmHg; p=0.001) and DBP reduction (IG: 19.61 ± 17.32 mmHg; CG: 7.81 ± 11.23 mmHg; p = 0.003) after 12 weeks. One IG patient had a heart attack, and one CG subject developed heart failure. At week 12, hepatic insufficiency was observed in one IG patient and six CG patients, while renal insufficiency occurred in five patients of both groups.
Conclusion: Treatment guided by PGx demonstrated significant reductions in both SBP and DBP compared to consensus-based treatment.
Key Words: Resistant hypertension, Treatment, Pharmacogenomics, Clinical study.
INTRODUCTION
Hypertension is the leading cause of chronic noncommunicable diseases.1 The global prevalence of hypertension stands at 31.1%, impacting a staggering 1.39 billion individuals across the globe.2 Resistant hypertension (RH) poses a significant public health concern, as it is linked to heightened risks of negative outcomes3 and cardiovascular events.4,5 Therapeutic efficacy and adverse reactions for treating RH vary considerably between individuals.
Genetic variations can lead to modifications in the quantity or functionality of metabolic enzymes, drug receptors, as well as transporters.6,7
Genetic polymorphism leads to significant differences in therapeutic efficacy, adverse effects, and drug tolerance of patients treated with the same medication.8 Pharmacogenomics (PGx) is a term in genome science relating to DNA variation across large populations and the regulation of gene expression. It detects and analyses genes that regulate drug metabolism and activity, influencing an individual's response to the drug. PGx involves leveraging individualised genetic variations of patients to inform the process of medication selection. PGx for clinical guidance of individualised medication treatment has gradually become a research topic of interest. For some specific drugs, such as neuropsychiatric, antineoplastic, and antiplatelet agents,9,10 it is essential to conduct genetic testing before prescribing the drugs. PGx in hypertension has seen some progress in recent years. The majority of studies conducted in this field have focused on either assessing the correlation between genotype and blood pressure or investigating treatment-related outcomes that exhibit variations based on genotype.11 Based on available data on hypertension genetics, there is convincing evidence that there are no genetic polymorphisms with huge effect sizes. Therefore, analysis of only one or two genes does not help guide treatment decisions. Hence, simultaneously detecting and analysing multiple relevant gene loci covering the patient's medication regimen may be a good choice.
The aim of this study was to investigate the efficacy and safety of RH treatment under the guidance of PGx, by testing various previously identified hypertension – associated genes and adjusting the medication use accordingly.
METHODOLOGY
This single-centric, open-label, parallel-group, non-inferiority randomised clinical trial (RCT) involved Chinese adults from Northeast China. It was registered in the Chinese Clinical Trial Registry (Registration number: ChiCTR2000035063, https:// www.chictr.org.cn/edit.aspx?pid=56786&htm=4).
Patients who signed informed consent form before any study mandated procedure were included. The inclusion criteria were 18 years or older; not assigned to other hypertension-related clinical studies; had RH, defined as uncontrolled blood pressure despite treatment with a stable regimen including maximum tolerated dosages of at least three antihypertensive medications from different classes, including a diuretic, or required ≥4 medications to achieve their targets,12 and had 24-h ambulatory systolic blood pressure (SBP) ≥140 mmHg during a screening period of 4–8 weeks before the procedure.
Exclusion criteria were pseudo-resistant hypertension result-ing from the white coat effect, medical inertness, sub-optical adherence to treatment or secondary causes of hypertension (except sleep apnea), confirmed grade 3 hypertension (SBP ≥180 mmHg or diastolic blood pressure [DBP] ≥110 mmHg measured at two different time points), pregnant and lactating women, clinically significant unstable heart disease, severe renal insufficiency (defined as estimated glomerular filtration rate [eGFR] <30 ml/min/1.73m² calculated using the Chronic Kidney Disease Epidemiology Collaboration formula13), or any known factor, illness, or clinically relevant medical or surgical condition that may pose a risk to the subjects and influence treatment compliance, progress of the research, or interpretation of results. Pseudo-resistant hypertension caused by irregular medication or inadequate dosage and poor adherence was identified by reviewing medical documentation and conducting patient questionnaires.12 White-coat hypertension was defined as elevated office blood pressure (BP) despite normal ambulatory BP.
During initial eligibility assessments, participants underwent thorough screening based on predefined criteria. Those meeting the criteria and providing informed consent post-randomisation proceeded with the questionnaire. The first intervention session, scheduled within 2 weeks of screening, followed by questionnaire completion. To control for expectation bias, participants decided on study participation before random assignment to an intervention group. They were informed about the study's goal to compare two antihypertensive therapies, emphasising the current uncertainty regarding intervention superiority.
The sample size calculation for this study was conducted using the PASS 15 software.14 The primary outcome used for the calculation was the change in SBP from baseline to the 12-week follow-up. Previous clinical studies provided insights indicating that the overall standard deviation for patients with RH was 18.1 mmHg.15 To establish a non-inferiority threshold, a value of 15 mmHg was considered based on the aforementioned findings. With a desired power of 80% and a one-sided type I error rate of 5%, a sample size of 24 participants per trial arm was initially calculated to detect the intended effect. However, accounting for an estimated drop-out rate of 20%, the sample size was increased to 30 participants per arm, resulting in a total of 60 participants for the study.
Following screening, patients were randomly assigned to the intervention group (IG) with PGx-guided treatment or the control group (CG) with consensus-based treatment. Randomisation, performed using SAS V9.4 software before the study, created a secure computer-generated sequence for the designated statistician. Sealed and numbered envelopes were used to minimise selection bias, keeping investigators and participants unaware of allocations until interventions.
Before randomisation, subjects underwent evaluations for secondary causes of hypertension, excluding conditions, such as primary aldosteronism, pheochromocytoma, Cushing synd-rome, renal parenchymal disease, renovascular hypertension, drug-induced hypertension, and others. Eligible subjects were then randomly assigned to IG or CG using a computer-generated coding system that produced random numbers. To rule out white coat hypertension, all patients underwent 24-hour dynamic blood pressure monitoring before drug intervention.
In the IG, oral mucosa specimens were collected using cotton swabs, and genotyping of fourteen polymorphic sites associated with 22 antihypertensive drugs was performed using the Mass ARRAY iPLEX platform (Sequenom, San Diego, CA). The relationship between genotypes and medicines was obtained from https://www.pharmgkb.org/, and medicine suggestions were compiled based on this information. Doctors have the flexibility to adjust drug dosage or change medications based on these lists, considering individual metabolic characteristics, drug efficacy, and potential side effects. Medication adjustments prioritised antihypertensive medicines with the highest efficacy and minimal adverse reactions. For instance, if a subject had an AA allele in AGTR1, it was known from PharmGKB that patients with this genotype exhibited lower sensitivity to nitrendipine. As a result, the dose of nitrendipine was increased accordingly. All medication adjustments were made within the recommended dosage guidelines for each specific drug. In CG, patients were treated according to clinical guidelines and expert consensus.16 Patients in both groups received a combination of complementary antihypertensive agents. Antihypertensive drugs were administered at the highest tolerable doses. All subjects followed a Mediterranean diet and engaged in regular exercise (such as jogging, bicycling, and swimming) for at least 30 minutes, two or more times per week, achieving 50-74% of their maximal heart rate.17
The primary outcome measured the difference between the two groups in terms of the change in office SBP from baseline at the 12-week mark. The secondary outcomes included the between-group difference in the change in office DBP from baseline at 12 weeks, as well as assessments of liver dysfunction, renal dysfunction, and occurrences of major adverse cardiovascular events (MACE), such as cardiovascular death, myocardial infarction, and stroke. Safety measurements encompassed evaluations of liver dysfunction, renal dysfunction, and MACE events. Baseline assessments included anthropometric measurements (weight, height, BMI), and BP was recorded at screening, baseline, and weeks 4, 8, and 12. Office BP was measured with OMRON HEM-907, and ambulatory BP used SunTech Oscar2 24-HR ABP initially. Baseline blood analysis included XN-9000 for cells, liver, renal function, and electrolytes. Hitachi 7600-110 analysed serum, plasma, and urine. Safety evaluations covered reported MACE, lab measurements, and clinical assessments throughout. Transaminases and creatinine were assessed at the last follow-up. The intervention group rated PGx treatment on a 1 to 10 scale.
Continuous variables' normality was assessed using the Kolmogorov-Smirnov test (p>0.05 indicated normal distribution). Mean and standard deviation were reported for normally distributed variables, and median with quartiles for non-normally distributed ones. Categorical variables were presented as counts and percentages. Group comparisons employed t-tests or Mann–Whitney tests based on variable distribution. Chi-square or Fisher's exact test assessed qualitative variables. Adjusted odds ratios and 95% confidence intervals were calculated via binary logistic regression, adjusting for potential confounders. IBM SPSS Statistics 25 and GraphPad Prism 8.0.1 were used for analysis and visualisation. Significance was set at p<0.05.
RESULTS
Figure 1 illustrates participant recruitment. Medical records of 150 adults with high BP were reviewed; 100 eligible individuals were identified. Seven were excluded (haemodialysis: n=5, pregnant: n = 1, malignant tumour: n = 1). Of 93 invited, 19 declined mainly due to limited time. The remaining 74 were randomly allocated to IG (36) or CG (38). Five patients in IG and four in CG were lost to follow-up. Two in IG and four in CG withdrew consent. After 12 weeks, 29 in IG and 30 in CG (78.95%) completed the trial. No documented deaths occurred. Data integrity exceeded 70% for all subjects (Figure 1). Due to COVID-19, some subjects could not go to the hospital, so home-based BP self-measurement was acceptable. Table I shows the baseline characteristics of all participants. Medicine in the trial included ACEI/ARB, β-blockers, CCB, diuretics, and α-receptor blockers. Drug ratios in both groups were similar (Table I) with CCB most prescribed at baseline (IG 89.66%, CG 93.67%). As shown in Table I, there was no significant difference in SBP between the IG (162.43 ± 14.37 mmHg) and CG (159.16 ± 12.54 mmHg) at baseline, p = 0.36.
Table I: Demographic and baseline clinical characteristics of study patients.
|
Characteristics |
Intervention group (n = 29) |
Control group (n = 30) |
p-values |
|
Age, mean (SD), y |
42.03 ± 14.59 |
47.90 ± 11.57 |
0.09 |
|
Gender, male, % |
62.07% (18) |
66.67% (20) |
0.59 |
|
Nation, Han% |
75.86% (22) |
96.67% (29) |
0.03 |
|
Height, m |
170.00 (167.00-176.50) |
169 (163.25-173.25) |
0.20 |
|
Weight, kg |
77.67 ± 13.66 |
80.27 ± 14.73 |
0.49 |
|
BMI, kg/m2 |
26.32 ± 3.62 |
27.95 ± 3.91 |
0.10 |
|
Haemoglobin, g/L |
144.50 ± 19.03 |
141.17 ± 17.44 |
0.49 |
|
Urea, mmol/L |
4.12 (3.73-5.82) |
4.90 (3.99-7.13) |
0.19 |
|
Cr, μmol/L |
70 (56-83) |
67.00 (53.00-81.50) |
0.47 |
|
eGFR, mL/min/1.73m2 |
109.50 (96.05-119.00) |
108.45 (85.7-111.98) |
0.43 |
|
ALT, U/L |
17.50 (10.00-30.00) |
20.00 (14.00-25.00) |
0.04 |
|
AST, U/L |
16.00 (13.00-20.00) |
20.00 (14.00-25.00) |
0.04 |
|
TG, mg/dL |
2.03 (1.28-8.85) |
1.88 (1.63-2.97) |
0.65 |
|
TC, mmol/L |
4.69 (3.20-5.18) |
4.86 (4.30-5.21) |
0.41 |
|
HDL-C, mg/dL |
0.98 (0.91-1.16) |
1.01 (0.88-1.26) |
0.89 |
|
LDL-C, mg/dL |
3.1 (2.26-3.60) |
3.40 (2.65-3.73) |
0.19 |
|
Office blood pressure |
- |
- |
- |
|
SBP, mmHg |
162.43 ± 14.37 |
159.16 ± 12.54 |
0.36 |
|
DBP, mmHg |
99.53 ± 14.34 |
93.53 ± 14.01 |
0.11 |
|
Renal dysfunction |
17.24% (5) |
15% (3) |
0.43 |
|
Hepatic dysfunction |
13.79% (4) |
25% (5) |
0.37 |
|
CHD |
24.14% (7) |
33.33% (10) |
0.39 |
|
DM |
24.14% (7) |
23.33% (7) |
>0.99 |
|
Medicine |
- |
- |
- |
|
ACEI /ARB |
68.97% (20) |
66.67% (20) |
>0.99 |
|
βRB |
65.51% (19) |
60% (18) |
0.79 |
|
CCB |
89.66% (26) |
93.67% (28) |
0.67 |
|
Diuretics |
55.17% (16) |
63.33% (19) |
0.60 |
|
αRB |
55.17% (16) |
46.67% (14) |
0.19 |
|
ABPM, mmHg |
- |
- |
- |
|
24-h SBP |
146.88 ± 20.72 |
152.56 ± 23.33 |
0.47 |
|
24-h DBP |
94.00 ± 15.31 |
90.88 ± 19.19 |
0.61 |
|
Daytime SBP |
148.94 ± 22.40 |
155.38 ± 23.97 |
0.44 |
|
Daytime DBP |
95.88 ± 16.44 |
86.50 ± 27.14 |
0.25 |
|
Nighttime SBP |
138.69 ± 17.17 |
141.94 ± 23.79 |
0.66 |
|
Nighttime DBP |
87.75 ± 12.98 |
83.69 ± 18.22 |
0.47 |
|
BMI, Body mass index; eGFR, Estimated glomerular filtration rate; ALT, Alanine transaminase; AST, Aspartate aminotransferase; TG, Triglyceride; TC, Total cholesterol; HDL-C, High-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; SBP, Systolic blood pressure, DBP, Diastolic blood pressure, CHD, Coronary heart disease; DM, Diabetes Mellitus; ACEI, Angiotensin-converting enzyme inhibitors; ARB, Angiotensin receptor blocker; CCB, Calcium channel blocker; βRB, β Receptor blockers; αRB, α Receptor blockers; ABPM, Ambulatory blood pressure monitoring.**p-values for the difference between the intervention and the control groups at baseline were analysed by t-test for parametric variables, Mann–Whitney U test for non-parametric, and χ2 test for categorical variables. |
|||
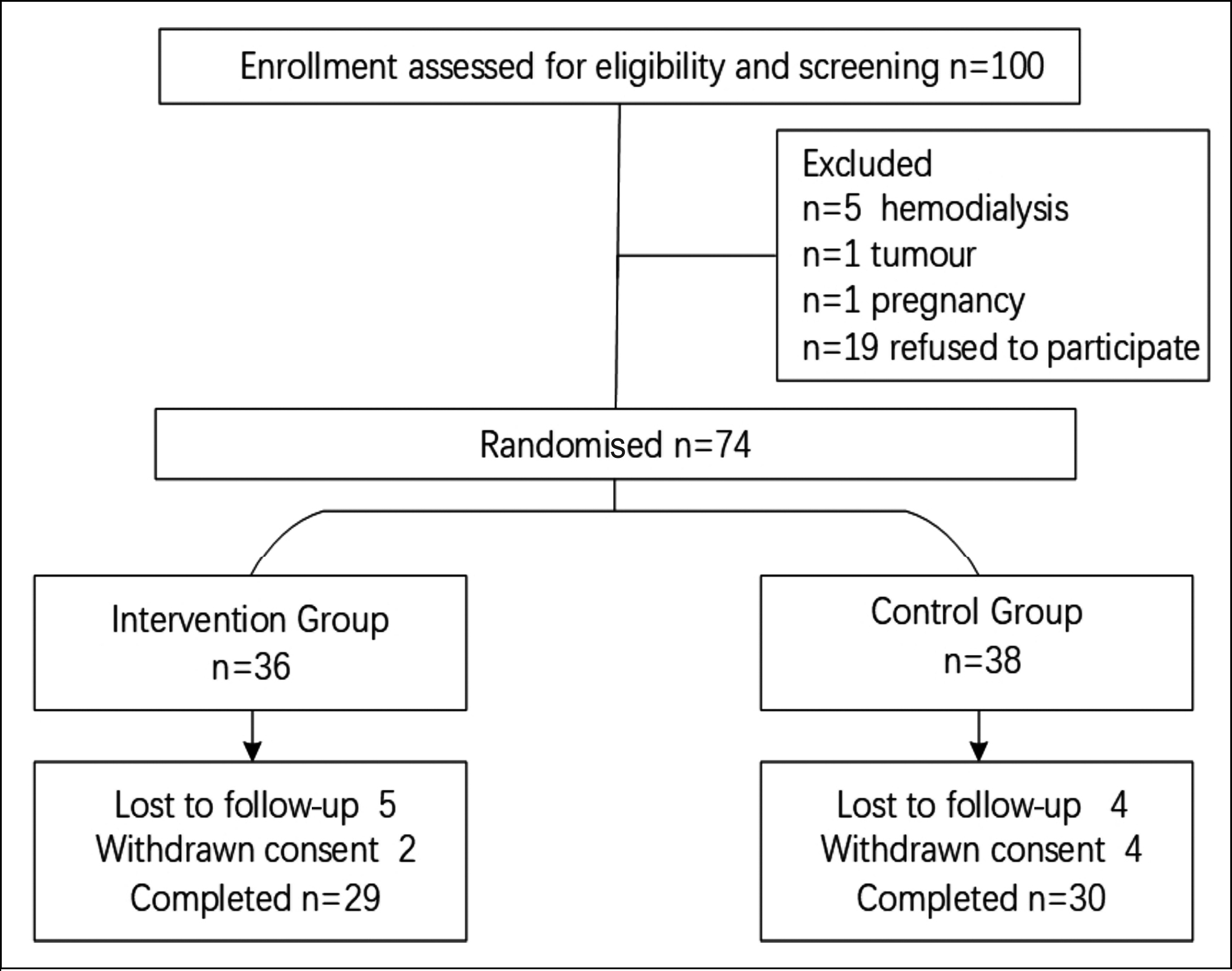 Figure 1: Flow chart of the randomisation.
Figure 1: Flow chart of the randomisation.
There was a cross point just before week 4, after which the SBP of the IG was always lower than that of CG. The SBP level did not significantly differ between CG and IG until 8 weeks (Figure 2a), whereas there was a significant difference in DBP between the two groups at week 12 (Figure 2c). Reduction in SBP levels significantly differed at week 8 and week 12 (Figure 2b). Moreover, reductions in DBP levels significantly differed at week 4, week 8 and week 12 (Figure 2f).
Table II: Binary logistic regression analysis for blood pressure control (140/90mmHg) and pharmacogenomics.|
Variables |
Model 1 |
Model 2 |
Model 3 |
|||||||||
|
OR |
95% CI |
p |
OR |
95% CI |
p |
OR |
95% CI |
p |
||||
|
PGx |
7.41 |
1.84 |
29.83 |
0.01 |
6.28 |
1.37 |
28.75 |
0.02 |
6.41 |
1.2 |
34.16 |
0.03 |
|
Age |
1.05 |
0.99 |
1.11 |
0.11 |
1.05 |
0.98 |
1.13 |
0.18 |
1.08 |
0.99 |
1.18 |
0.09 |
|
Gender |
1.3 |
0.37 |
4.64 |
0.69 |
1.63 |
0.39 |
6.72 |
0.5 |
4.51 |
0.66 |
31.00 |
0.13 |
|
BMI |
0.86 |
0.72 |
1.04 |
0.11 |
0.85 |
0.69 |
1.04 |
0.12 |
0.83 |
0.65 |
1.05 |
0.12 |
|
CHD |
- |
- |
- |
- |
0.44 |
0.08 |
2.53 |
0.36 |
0.34 |
0.048 |
2.36 |
0.12 |
|
DM |
- |
- |
- |
- |
6.65 |
0.98 |
45.18 |
0.05 |
11.59 |
1.33 |
101.34 |
0.27 |
|
Dtroke |
- |
- |
- |
- |
0.52 |
0.10 |
2.59 |
0.42 |
0.39 |
0.06 |
2.65 |
0.33 |
|
Dyslipidemia |
- |
- |
- |
- |
0.56 |
0.13 |
2.38 |
0.43 |
0.62 |
0.13 |
2.86 |
0.54 |
|
Smoke |
- |
- |
- |
- |
- |
- |
- |
- |
0.09 |
0.01 |
0.98 |
0.05 |
|
Drink |
- |
- |
- |
- |
- |
- |
- |
- |
5.60 |
0.30 |
103.59 |
0.25 |
|
Model 1: Adjusted for age, gender, and BMI. Model 2: Adjusted for factors in model 1 and CHD, DM, stroke, and dyslipidemia. Model 3: Adjusted for factors in model 2 and smoke, drink history |
||||||||||||
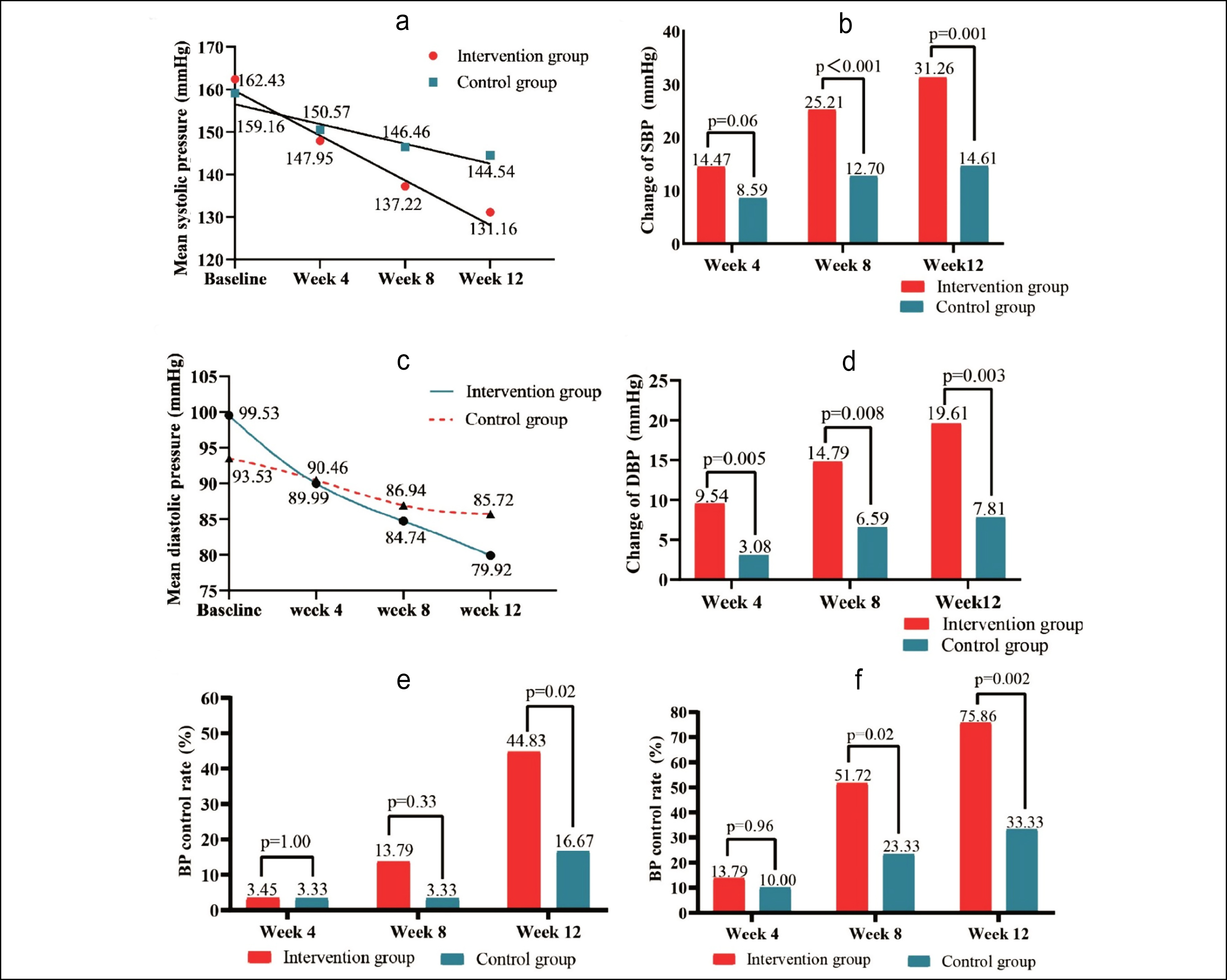 Figure 2: (a) Variation tendency of SBP in the follow-up period. (b) Decline SBP level from baseline of IG and CG. (c) Variation tendency of DBP in the follow-up period. (d) Decline DBP level from baseline of IG and CG. (e) BP control rate when the target BP was less than 130/80 mmHg. (f) BP control rate when the target BP was less than 140/90 mmHg.
Figure 2: (a) Variation tendency of SBP in the follow-up period. (b) Decline SBP level from baseline of IG and CG. (c) Variation tendency of DBP in the follow-up period. (d) Decline DBP level from baseline of IG and CG. (e) BP control rate when the target BP was less than 130/80 mmHg. (f) BP control rate when the target BP was less than 140/90 mmHg.
Figure 2(e and f) displays BP control rates in CG and IG at each follow-up point. When target BP was <140/90 mmHg, a significant difference was observed at week 8 (IG: 51.72%; CG: 23.33%; p=0.02) and week 12 (IG: 75.86%; CG: 33.33%; p=0.002). A significant difference in control rate was noted at week 12 (p = 0.02) for target BP <130/80 mmHg. One subject in the IG developed heart failure, and one subject in the CG had a myocardial infarction. Cases of liver dysfunction in the CG increased from five at baseline to six after 12 weeks. In contrast, liver dysfunction in the IG decreased from four at baseline to one after 12 weeks. Renal insufficiency in the IG remained at five at baseline, while in the CG, it increased from three to five at week 12.
Binary logistic regression analysed blood pressure control and PGx. After adjustments for age, gender, and BMI, the IG had a 15% lower risk of inadequate blood pressure control than the CG (OR: 7.41, 95% CI: 1.84-29.83, p = 0.01). Further adjustments for comorbidities revealed that the CG had a 10-fold higher risk (OR: 6.28, 95% CI: 1.37-28.75, p = 0.02) compared to the IG targeted at 140/90 mmHg at week 12. Full adjustments, including PGx, showed a significant association between PGx and BP control rate (OR: 6.41, 95% CI: 1.20-34.16, p = 0.03 Table II).
At the end of the follow-up, the IG underwent an attitude survey on PGx-based drug efficacy testing, 89.67% participated, with over 80% rating treatment satisfaction above 6.5 on a scale of 10.
DISCUSSION
In this study, genetic testing of multiple hypertension-related loci was carried out in patients with RH. The medication was guided by the correlation between specific genotypes and the metabolism and efficacy of drugs in the human body. The results of the study showed that the SBP of CG decreased significantly at weeks 8 and 12. From 0 to 12 weeks, the SBP of IG decreased by 31.26 mmHg, which was consistent with previous studies.18 Compared with drug lowering alone, the BP control rate of IG and CG in the study both increased in a relatively short time, which is the result of the combined effect of diet control, exercise, and therapeutic effect. These results showed that multi-site gene testing is valuable in drug adjustment for RH.
The current method for selecting antihypertensive therapy is mainly empirical and often involves a trial-and-error approach.19 Individualised treatment may serve as a novel approach to control BP in RH and improve the current unsatisfactory BP control rates,20 which would have broad clinical application prospects.
Preliminary studies have found that some specific gene sites are associated with the effects or adverse effects of antihypertensive medicines.21 However, most studies focused on a particular gene and the relationship between genotype and drug metabolism or between BP change and genotype directly under a specific antihypertensive drug.22,23 Therefore, those findings have to be further verified in actual clinical use. Hypertension is not a monogenetic controlled disease; comprehensive interventions are the right choice for treating hypertension.
The trial's strength is its randomised controlled design with sufficient power to detect efficacy differences. In contrast, pharmacogenetic data are from trials addressing unresolved clinical issues, and there is no comparable RCT for evaluating the efficacy and safety of pharmacogenomics-guided treatment for RH. Its open-label design and relatively short follow-up time introduce potential biases and limit the observation of events such as MACE. Long-term prospective studies are crucial to thoroughly assess the safety of PGx-guided treatment for individuals with RH.
The trial revealed improved BP control rates in subjects with RH randomised to the new therapy compared to the usual treatment. It demonstrates the feasibility and efficacy of utilising PGx for BP control in RH management. These findings suggest practical applications in clinical settings. This work serves as an initial exploration of PGx for therapeutic guidance, urging larger-scale trials for long-term efficacy and safety assessment in BP control. The approach of simultaneously measuring multiple gene loci and guiding medications could be extended beyond hypertension treatment to other diseases. For complex drug situations, such as varied drug responses based on genotypes, employing multi-site gene detection technology can facilitate medication guidance. However, the study has limitations, including an open-label design, introducing potential bias and placebo effects. Additionally, the relatively short follow-up duration limits insights into events such as MACE, warranting consideration for future research with longer follow-up periods.
CONCLUSION
Patients who received antihypertensive intervention guided by PGx achieved significantly greater improvements in BP levels and BP control rates compared to the control group, who received care guided by consensus guidelines. These findings provide valuable clinical evidence supporting the effectiveness of PGx in managing patients with RH and suggest potential public health benefits.
AVAILABILITY OF DATA AND MATERIAL:
Derived data supporting the findings of this study are available from the corresponding author upon reasonable request.
ETHICAL APPROVAL:
The Medical Scientific Research Ethics Committee of the First Hospital of China Medical University approved the study ([2020]2020-224-2).
PATIENTS’ CONSENT:
Informed consent and the last follow-up details were obtained by examining medical records and conducting telephone interviews.
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
AW: Wrote the final manuscript and collected the data.
MBM: Critically reviewed the manuscript.
JZ: Performed the data analysis and data visualisation.
NC: Provided editing assistance and formatted the data and tables.
ZL: Conceived the idea and design of the manuscript.
All authors approved the final version of the manuscript to be published.
REFERENCES
- GBD 2015 Risk Factors collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: A systematic analysis for the global burden of disease study 2015. Lancet 2016; 388(10053): 1659-724. doi: 10.1016/S0140-6736(16)31679-8.
- Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: A Systematic analysis of population-based studies from 90 countries. Circulation 2016; 134(6):441-50. doi: 10.1161/CIRCULATIONAHA.115.018912.
- Bundy JD, Li C, Stuchlik P, Bu X, Kelly TN, Mills KT, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: A systematic review and network meta-analysis. JAMA Cardiol 2017; 2(7):775-81. doi: 10. 1001/jamacardio.2017.1421.
- Tsioufis C, Kasiakogias A, Kordalis A, Dimitriadis K, Thomo-poulos C, Tsiachris D, et al. Dynamic resistant hypertension patterns as predictors of cardiovascular morbidity: A 4-year prospective study. J Hypertens 2014; 32(2):415-22. doi: 10.1097/HJH.0000000000000023.
- Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 2012; 125(13):1635-42. doi: 10.1161/CIRCULATIONAHA.111.06 8064.
- Baudin B. Angiotensin II receptor polymorphisms in hypertension. Pharmacogenomic considerations. Pharmacogenomics 2002; 3(1):65-73. doi: 10.1517/14622416.3.1.65.
- Wu X, Yin C, Ma J, Chai S, Zhang C, Yao S, et al. Polyoxypregnanes as safe, potent, and specific ABCB1-inhibitory pro-drugs to overcome multidrug resistance in cancer chemotherapy in vitro and in vivo. Acta Pharm Sin B 2021; 11(7):1885-902. doi: 10.1016/j.apsb.2020.12.021.
- Cars T, Lindhagen L, Malmström RE, Neovius M, Schwieler J, Wettermark B, et al. Effectiveness of drugs in routine care: A model for sequential monitoring of new medicines using dronedarone as example. Clin Pharmacol Ther 2018; 103(3):493-501. doi: 10.1002/cpt.751.
- He W, Eriksson M, Eliasson E, Grassmann F, Bäcklund M, Gabrielson M, et al. CYP2D6 genotype predicts tamoxifen discontinuation and drug response: A secondary analysis of the KARISMA trial. Ann Oncol 2021; 32(10):1286-93. doi: 10.1016/j.annonc.2021.07.005.
- Giacoppo D, Matsuda Y, Fovino LN, D'Amico G, Gargiulo G, Byrne RA, et al. Short dual antiplatelet therapy followed by P2Y12 inhibitor monotherapy vs. prolonged dual antiplatelet therapy after percutaneous coronary intervention with second-generation drug-eluting stents: A systematic review and meta-analysis of randomized clinical trials. Eur Heart J 2021; 42(4):308-19. doi: 10.1093/eurheartj/ehaa739.
- Iniesta R, Campbell D, Venturini C, Faconti L, Singh S, Irvin MR, et al. Gene Variants at loci related to blood pressure account for variation in response to antihypertensive drugs between black and white individuals. Hypertension 2019; 74(3):614-22. doi: 10.1161/HYPERTENSIONAHA.118.12177.
- Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, et al. American heart association professional/public education and publications committee of the council on hypertension; Council on cardiovascular and stroke nursing; council on clinical cardiology; council on genomic and precision medicine; Council on peripheral vascular disease; council on quality of care and outcomes research; and stroke council. Resistant Hypertension: Detection, evaluation, and management: A scientific statement from the American heart association. Hypertension 2018; 72(5):e53-90. doi: 10.1161/HYP.0000000000000084.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. CKD-EPI (chronic kidney disease epidemiology collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150(9):604-12. doi: 10.7326/0003-4819-150-9-200905050-00006.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): A randomised controlled trial. Lancet 2010; 376 (9756):1903-9. doi: 10.1016/S0140-6736(10)62039-9.
- Spiering W, Williams B, Van der Heyden J, van Kleef M, Lo R, Versmissen J, et al. CALM-FIM_EUR investigators. Endovascular baroreflex amplification for resistant hypertension: A safety and proof-of-principle clinical study. Lancet 2017; 390(10113):2655-61. doi: 10.1016/S0140-6736(17)32337-1.
- Ahmad T, Voora D, Becker RC. The pharmacogenetics of antiplatelet agents: Towards personalized therapy? Nat Rev Cardiol 2011; 8(10):560-71. doi: 10.1038/nrcardio.2011.111.
- Pelliccia A, Solberg EE, Papadakis M, Adami PE, Biffi A, Caselli S, et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: Position statement of the sport cardiology section of the European Association of Preventive Cardiology (EAPC). Eur Heart J 2019; 40(1): 19-33. doi: 10.1093/eurheartj/ehy730.
- Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet 2014; 383(9932):1899-911. doi: 10.1016/ S0140-6736(14)60685-1.
- Savoia C, Volpe M, Grassi G, Borghi C, Agabiti Rosei E, Touyz RM. Personalized medicine-a modern approach for the diagnosis and management of hypertension. Clin Sci (Lond) 2017; 131(22):2671-85. doi: 10.1042/CS20160407.
- January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation 2019; 140(2):e125-51. doi: 10.1161/CIR.0000000000 000665.
- Bigazzi R, Zagato L, Lanzani C, Fontana S, Messaggio E, Delli Carpini S, et al. Hypertension in high school students: Genetic and environmental factors: The HYGEF Study. Hypertension 2020; 75(1):71-8. doi: 10.1161/HYPERTENSIONAHA.119.13818.
- Hemnes AR, Rathinasabapathy A, Austin EA, Brittain EL, Carrier EJ, Chen X, et al. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur Respir J 2018; 51(6):1702638. doi: 10.1183/13993003.02638-2017.
- Singh P, Song CY, Dutta SR, Pingili A, Shin JS, Gonzalez FJ, et al. 6β-Hydroxytestosterone promotes angiotensin II-Induced hypertension via enhanced cytosolic phospholipase A2α activity. Hypertension 2021; 78(4):1053-66. doi: 10.1161/ HYPERTENSIONAHA.121.17927.