The Effect of HALP Score on the Prognosis of Gastric Adenocarcinoma
By Zeynep Gok Sargin, Ibrahimhalil DusunceliAffiliations
doi: 10.29271/jcpsp.2022.09.1154ABSTRACT
Objective: To investigate the predictive value of haemoglobin and albumin levels and lymphocyte and platelet (HALP) counts in gastric cancer patients.
Study Design: Descriptive study.
Place and Duration of Study: Department of Gastroenterology, Zonguldak Bulent Ecevit University, Faculty of Medicine, Zonguldak, Turkey, from January 2017 to January 2022.
Methodology: Clinical data of 204 patients with gastric cancer were reviewed. The median value of the HALP score of 23.87, was considered to be cut-off. According to this cut-off value, patients are separated into two categories. Kaplan-Meier method was used to identify factors associated with the overall survival.
Results: There was no statistical difference in the mean HALP score according to gender, tumour localisation, histological type, TNM stage, and adjuvant or palliative chemotherapy groups. The mean HALP score was significantly lower in those older than 64 years (p=0.04). When all the patients were divided into the low and high HALP scores, gender, age, histological subtypes, tumour location, adjuvant or palliative treatment status, TNM stage, CEA, and CA19-9 levels were statistically similar between the two groups. A significant difference was found in overall survival of the patients with low and high HALP groups (p=0.05). There was an insignificant difference in the overall survival of those with low and high HALP scores in the adjuvant and palliative chemotherapy groups.
Conclusion: Gastric adenocarcinoma patients with a high HALP score had a better overall survival rate.
Key Words: Gastric adenocarcinoma, Haemoglobin, Albumin, Lymphocyte, Platelet, HALP score.
INTRODUCTION
Gastric adenocarcinoma is the fifth most common malignancy and the third major cause of cancer-related deaths.1 The prognosis is poor because most patients with gastric adenocarcinoma are diagnosed at an advanced stage of the disease.2 Surgery, systemic chemotherapy, radiotherapy, immuno-therapy, and targeted therapies are effective treatments, therefore, multidisciplinary management is essential.3 However, gastric cancer mortality is still high due to the recurrence and metastasis. Thus, palliative management, including systemic chemotherapy, chemoradiation, and/or best supportive care are recommended for all patients with unresectable or metastatic cancer.4
The TNM stage, which comprises the tumour's degree of invasion, lymph node metastasis, and distant metastasis is currently the most critical determinant in determining the prognosis.5 The fact that TNM staging is insufficient in prognosis, with newly developed treatments, such as immunotherapy and targeted therapies, have necessitated continuous updating. It is critical to establish separate risk categories based on different biomarkers for the patients at various stages of the disease, and clinical performance that requires personalised treatment regimens. As a result, possible biomarkers are critical for forecasting disease prognosis, planning therapy regimens, and follow-up protocols.
Haemoglobin is a molecule that carries oxygen to the tissues in the body. In the presence of anaemia, relative hypoxia occurs due to the decrease in oxygen transported to the tumoural tissues.6 Hypoxia causes a change in gene expression and subsequent proteomic changes (e.g. vascular endothelial growth factor, epidermal growth factor, erythropoietin, glucose transporters, and glycolytic enzymes), which may catalyse tumour survival, proliferation, invasion, and metastasis, resulting in a worse prognosis for the patient.7
Table I: Mean and median HALP score levels by clinicopathological subgroups.
|
Category |
|
n (%) |
Mean HALP Levels (±SD) |
Median HALP Levels (25%-75%) |
p-value |
|
Gender |
Female |
60 (29.4%) |
27.13 (±14.27) |
24.96 (16.02-36.94) |
0.83* |
|
Male |
144 (70.6%) |
28.51 (±18.92) |
23.55 (14.80-35.69) |
||
|
Age |
<64 |
104 (51%) |
31.06 (±19.56) |
27.02 (16.58-41.19) |
0.04* |
|
>64 |
100 (49%) |
25.04 (±14.91) |
22.89 (13.79-31.60) |
||
|
Body-mass index |
<22.7 |
102 (50%) |
29.50 (±18.34) |
26.37 (15.49-38.7) |
0.226* |
|
≥22.7 |
102 (50%) |
26.71 (±16.91) |
22.89 (14.88-34.82) |
||
|
Tumour localisation |
Cardia |
49 (24%) |
27.53 (±17.94) |
23.68 (15.86-32.57) |
0.31** |
|
Corpus |
56 (27.5%) |
26.15 (±17.23) |
23.19 (12.58-33.06) |
||
|
Antrum |
74 (36.3%) |
28.92 (±18.84) |
23.63 (14.16-38.70) |
||
|
Unknown |
25 (12.3%) |
31.20 (±14.51) |
29.58 (20.00-39.19) |
||
|
Histopathological subtypes |
Tubular |
77 (37.7%) |
24.98 (±15.51) |
21.96 (12.51-35.46) |
0.14** |
|
Signet ring cell |
28 (13.7%) |
26.40 (±14.82) |
23.97 (16.04-32.59) |
||
|
Mixed type |
25 (12.3%) |
38.53 (±23.05) |
32.81 (21.28-53.74) |
||
|
Mucinous |
18 (8.8%) |
29.90 (±21.88) |
24.87 (14.73-44.25) |
||
|
Poorly cohesive |
12 (5.9%) |
31.03 (±22.83) |
26.07 (14.20-37.0) |
||
|
Unknown |
44 (21.6%) |
27.21 (±14.23) |
26.91 (16.63-35.36) |
||
|
Chemotherapy regimen |
Adjuvant |
136 (66.7%) |
29.13 (±17.94) |
24.20 (15.60-38.66) |
0.18* |
|
Palliative |
68 (33.3%) |
26.07 (±17.01) |
22.52 (13.49-32.34) |
||
|
T stage |
T1-3 |
87 (42.6%) |
29.74 (±20.01) |
24.30 (14.88-38.52) |
0.5* |
|
T4 |
117 (57.4%) |
26.89 (±15.66) |
23.54 (15.12-35.46) |
||
|
Lymph node metastasis |
Yes |
182 (89.2%) |
28.20 (±18.06) |
23.76 (14.73-37.00) |
0.76* |
|
No |
22 (10.8%) |
27.31 (±14.12) |
26.64 (15.06-36.53) |
||
|
Distant metastasis |
Yes |
58 (28.4%) |
26.37 (±16.31) |
22.99 (14.73-32.57) |
0.41* |
|
No |
146 (71.6%) |
28.79 (±18.17) |
24.02 (15.06-38.52) |
||
|
* Mann-Whitney U Test; ** Kruskal-Wallis Test. |
|||||
Furthermore, gastric carcinoma often results in malnutrition and weight loss, affecting patients' prognosis and quality of life. The progression and prognosis of gastric cancer are highly associated with the systemic inflammatory response and nutrition status.8 Decreased absorption of nutrients by the gastrointestinal tract in gastric cancer patients may lead to decreased albumin levels. Albumin represents nutritional status and also acts as an antioxidant or carrier. Finally, because albumin is a negative acute-phase protein, hypoalbuminemia may indicate an elevated inflammatory state in the patient, potentially leading to poor results.9 Lymphocytes play an essential role in recognising tumour cells and indirectly inhibit tumour cell growth through immune regulation and tumour cells killing.10 Platelets induce angiogenesis by releasing pro-angiogenic factors and angiogenesis inhibitors and also release proteolytic enzymes by platelet-derived growth factors, which ultimately contribute to the angiogenesis during tumour development and metastasis formation.11 Based on the above information, high-haemoglobin, albumin, and lymphocyte levels positively affect cancer patients' prognosis, while high-platelet levels should be considered negative factors. As a result, a variety of nutrition and inflammation-related markers, such as the prognostic nutritional index (PNI), platelet to lymphocyte ratio (PLR), and neutrophil to lymphocyte ratio (NLR), based on albumin levels, neutrophil, platelet, and lymphocyte counts, have been used to predict the prognosis of patients with gastric cancer in recent years.12,13 The haemoglobin, albumin levels, and lymphocytes count appear to be positively correlated with the prognosis, but platelet count is negatively associated with the prognosis. Haemoglobin, albumin, lymphocyte, and platelet (HALP) score, a novel composite marker related to the nutritional and inflammatory status, are easily tested in clinical practice. It has been reported that the HALP score correlates positively with the prognosis of many cancers.14–17 However, there were only two studies in the literature investigating the importance of the HALP score in gastric adenocarcinoma prognosis.16,17 The goal of this study was to determine the predictive value of HALP in patients with gastric cancer and to identify subgroups with a significant risk of poor survival.
METHODOLOGY
This study was retrospectively evaluated. Two hundred and four patients older than 18 years of age diagnosed with gastric adenocarcinoma in the gastroenterology clinic, between January 2017 and January 2022, were included. Patients with gastric tumours (except gastric adenocarcinoma), extragastric malignancies, haematological diseases (other than iron deficiency anaemia), smoking, alcoholism, chronic kidney failure, chronic liver disease, chronic heart failure, and cerebrovascular diseases were excluded.
The files of the patients were retrospectively reviewed from the hospital database. Demographic information, chemotherapy regimen (adjuvant or palliative), localisation of the gastric tumour in the stomach, tumour histological type, tumour differentiation grade, T staging in TNM classification, lymph node involvement, and distant metastasis status were recorded for the analysis. Whether the patients were exitus or not in the final stage, was recorded. The overall survival (OS) was defined as the time from the diagnosis to death or the last follow-up visit. The primary endpoint was defined as OS.
Table II: Clinicopathologic characteristics of the patients according to HALP groups.
|
Features. |
|
HALP score |
|
|
|
|
|
<23.8 |
>23.8 |
p-value |
|
Gender |
Female |
27 (45%) |
33 (55%) |
0.49* |
|
Male |
74 (51.4%) |
70 (48.6%) |
||
|
Age |
≤64 |
46 (44.2%) |
58 (55.8%) |
0.16* |
|
>64 |
55 (55%) |
45 (45%) |
||
|
Body-mass index |
<22.7 |
47 (46.1%) |
55 (53.9) |
0.327* |
|
≥22.7 |
54 (52.9%) |
48 (47.1) |
||
|
Tumour localisation |
Cardia |
25 (51%) |
24 (49%) |
0.78* |
|
Corpus |
29 (51.8%) |
27 (48.2%) |
||
|
Antrum |
37 (50%) |
37 (50%) |
||
|
Unknown |
10 (40%) |
15 (60%) |
||
|
Histopathological subtypes |
Tubular |
45 (58.4%) |
32 (41.6%) |
0.31* |
|
Signet Ring Cell |
13 (46.4%) |
15 (53.6%) |
||
|
Mixed Type |
8 (32%) |
17 (68%) |
||
|
Mucinous |
9 (50%) |
9 (50%) |
||
|
Poorly Cohesive |
6 (50%) |
6 (50%) |
||
|
Unknown |
20 (45.5%) |
24 (54.5%) |
||
|
Chemotherapy regimen |
Adjuvant |
64 (47.1%) |
72 (52.9%) |
0.4* |
|
Palliative |
37 (54.4%) |
31 (45.6%) |
||
|
T stage |
T1-3 |
41 (47.1%) |
46 (52.9%) |
0.65* |
|
T4 |
60 (51.3%) |
57 (48.7%) |
||
|
Lymph node metastasis |
Yes |
91 (50%) |
91 (50%) |
0.85* |
|
No |
10 (45.5%) |
12 (54.5%) |
||
|
Distant metastasis |
Yes |
31 (53.4%) |
27 (46.6%) |
0.58* |
|
No |
70 (47.9%) |
76 (52.1%) |
||
|
CEA |
≤2.35 |
48 (47.1%) |
54 (52.9%) |
0.57* |
|
>2.35 |
53 (52%) |
49 (48%) |
||
|
CA19-9 |
≤14.05 |
44 (43.1%) |
58 (56.9%) |
0.09* |
|
>14.05 |
57 (55.9%) |
45 (44.1%) |
||
|
* Chi-square test. |
||||
The laboratory parameters were obtained from the registration system when the first application was made to the outpatient clinic. HALP score was calculated by haemoglobin level (g/L) × albumin level (g/L) × lymphocytes count (/L) /platelet count (/L). The concentrations of serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were also recorded.
IBM SPSS 22.0 (Armonk, NY, USA) was used for data analysis. The qualitative data were given as numbers and proportions, while quantitative as mean ± SD and median (IQR: Interquartile range 25th-75th percentiles). Shapiro-Wilk and Kolmogorov-Smirnov tests evaluated whether the data were normally distributed. Mann-Whitney U and Kruskal-Wallis tests were used for the non-normally distributed variables. Pearson’s chi-square test was used to compare the differences among the categorical variables. The median level was used to find cut-off values for the HALP level. Survival times were given with Kaplan-Meier statistics, and it was decided whether there was a difference in the survival times between the groups with the Log-Rank Test. A p-value ≤0.05 was considered statistically significant.
Approval from the Zonguldak Bulent Ecevit University Faculty of Medicine Non-Interventional Clinical Research Ethics Committee was obtained for the study (Protocol No: 2022/02 Approval Date: 26 January 2022). The study protocol conforms to the ethical guidelines of the 1964 Declaration of Helsinki.
RESULTS
Of the 204 patients included in this study, there was a predominance of males compared to females. The mean age of all the patients was 63.7±11.8 years. When patients were classified according to the T stage, most were T4 (n=117, 57.4%). One hundred and eighty-two (89.2%) patients had lymph node metastases, with 22 (10.8%) patients having no metastases. Fifty-eight (28.4%) patients had distant metastases and 146 (71.6%) had not. Of the tumours, 74 (36.3%) were in the antrum, 56 (27.5%) in the corpus, 49 (24%) in the cardia, and 25 (12.3%) in the unknown regions. The histopathological subtypes of the adenocarcinoma pathology preparations included in the study were such that: 77 (37.7%) had tubular, 28 (13.7%) had signet ring cell, 25 (12.3%) had mixed type, 18 (8.8%) had mucinous, 12 (5.9%) had poorly cohesive adenocarcinoma, and 44 (21.6%) had unknown subtype. One hundred and thirty-six (66.7%) individuals underwent adjuvant chemotherapy, and 68 (33.3%) patients received palliative chemotherapy, according to the findings (Table I).
When all the patients were divided into two groups with the median age of 64 (57-71) years as a reference, the mean HALP score under 64 years of age was 31.06±19.56, and the mean HALP score above that was 25.04±14.91. The HALP score was statistically different between these two groups (p=0.04). There was no significant difference in HALP score between the genders (p=0.83). When all the patients were divided into two groups with the median body mass index of 22.7 (19.54-25.85) Kg/m2 as a reference, the mean HALP score under 22.7 Kg/m2 was 29.50±18.34, and the mean HALP score above it was 26.71 ±16.91 (p=0.259).
When evaluated, according to the tumour localisation, histological type, T staging, lymph node metastasis, distant metastasis, and adjuvant or palliative chemotherapy, there was no statistical difference in the HALP score (p=0.031, p=0.14, p=0.5, p=0.76, p=0.41, and p=0.18, Table I), respectively.
Table III: Clinical characteristics and survival of the study group.
|
|
n (%) |
Median OS (95% confidence interval) |
Chi-square |
p-value |
|
Gender |
||||
|
Female |
60 (29.4%) |
55 (26.5-83.4) |
0.797 |
0.37 |
|
Male |
144 (70.6%) |
33 (21.6-44.3) |
||
|
Age (years) |
||||
|
<64 years |
104 (51%) |
33 (21.3-44.6) |
1.234 |
0.26 |
|
>64 years |
100 (49%) |
46 (23.9-68.0) |
||
|
Tumour Localisation |
||||
|
Unknown |
25 (12.3%) |
73 ( - ) |
3.972 |
0.26 |
|
Corpus |
56 (27.5%) |
33 (17.6-48.3) |
||
|
Cardiac |
49 (24%) |
34 (14.5-53.4) |
||
|
Antrum |
74 (36.3%) |
30 (16.6-43.3) |
||
|
Histopathological Subtypes |
||||
|
Unknown |
44 (21.6%) |
13 (9.7-16.2) |
28.341 |
<0.001 |
|
Tubular |
77 (37.7%) |
55 (31.5-78.4) |
||
|
Mucinous |
18 (8.8%) |
33 (0.0-71.2) |
||
|
Signet ring cell |
28 (12.7%) |
25 (3.7-46.2) |
||
|
Poorly cohesive |
12 (5.9%) |
46 (35.6-56.3) |
||
|
Mixed type |
25 (12.3%) |
62 (20.3-103.6) |
||
|
Chemotherapy Regimen |
||||
|
Adjuvant |
136 (66.7%) |
65 (35.2-94.7) |
93.559 |
<0.001 |
|
Palliative |
68 (33.3%) |
10 (6.9-13.0) |
||
|
HALP Levels |
||||
|
<23.8 |
101 (49.5%) |
25 (15.1-34.8) |
3.726 |
0.05 |
|
>23.8 |
103 (50.5%) |
55 (32.0-77.9) |
|
|
|
CEA |
||||
|
≤2.35 |
102 (50%) |
55 (34.7-75.2) |
5,730 |
0.01 |
|
>2.35 |
102 (50%) |
24 (15.0-32.9) |
|
|
|
CA19-9 |
||||
|
≤14.05 |
102 (50%) |
40 (21.1-58.8) |
0.666 |
0.4 |
|
>14.05 |
102 (50%) |
30 (21.1-38.8) |
|
|
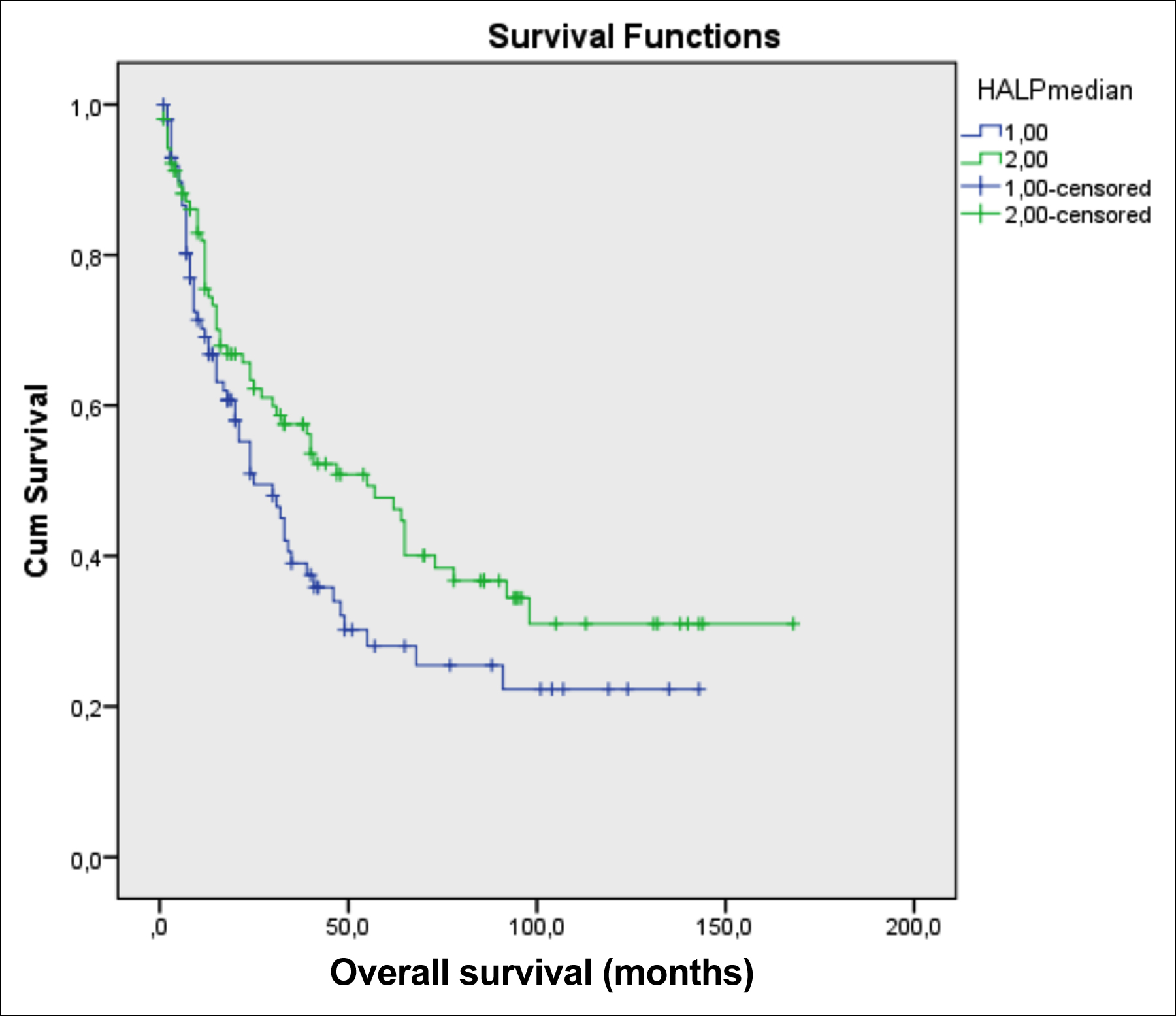 Figure 1: The effect of HALP on overall survival in gastric adenocarcinoma.
Figure 1: The effect of HALP on overall survival in gastric adenocarcinoma.
In the ROC analysis, no significant cut-off value for the HALP level was found (AUC=0.496, p=0.919). Therefore, the patients were divided into two groups according to their HALP levels, and this value was used as a cut-off. The median value of the HALP score of the study population was 23.8, which was considered to be the cut-off. According to this cut-off value, patients were separated into two categories. The gender, age, tumour localisation, histopathological subtype, adjuvant or palliative chemotherapy status, T staging, lymph node metastasis status, presence of distant metastasis, CEA, and CA19-9 levels were not significantly different between the high and low HALP score groups (Table II).
The association between HALP score and overall survival was examined, and it was observed that there was a substantial difference in the overall survival of the study population. The median survival time, for the patients with low and high HALP scores, was 25 and 55 months, respectively (p=0.05, Figure 1, Table III).
Even though the study groups’ median overall survival was 34 (24.3±43.6) months, there was no significant relationship among age (p=0.26), gender (p=0.37), tumour localization (p=0.26), CA19-9 levels (p=0.41), and survival. However, survival was significantly different according to histopathological subtype (p<0.001), adjuvant or palliative chemotherapy status (p<0.001), and CEA (p=0.01) levels (Table III). In the multi-variant analysis of these parameters that had an effect on OS, only the difference was observed between the adjuvant and palliative chemotherapy groups (p<0.001).
When the study participants were divided into subgroups based on adjuvant and palliative treatments, in the adjuvant treatment group, the median overall survival was 49 and 92 months in the low and high HALP score groups, respectively (p=0.15). In the palliative treatment setting, the median survival was eight months in the low HALP score group, while 12 months overall survival was observed in the patients with a high HALP score (p=0.38).
DISCUSSION
The HALP score is a composite indicator that includes components of a patient's nutritional and immune status and has demonstrated to have a prognostic role in various cancer types. The median HALP value of 23.8 was used as a cut-off in this study, and those with a HALP value over this threshold had a considerably higher overall survival rate. Although it did not reach statistical significance for an inoperable early-stage gastric cancer patient who received adjuvant therapy, survival was 43 months longer in those with high HALP scores than low HALP scores. Similarly, survival was four months longer in those who received palliative treatment with high HALP scores in advanced gastric cancer, even though statistical significance could not be reached. A low HALP score was significantly determined as an adverse prognostic factor in gastric adenocarcinoma patients. These results support that a high HALP score is associated with better outcomes in gastric adenocarcinoma prognosis, in line with the literature.16,17
To reflect inflammation and nutritional status, patients' HALP scores were calculated based on the haemoglobin, albumin, lymphocyte, and platelet levels. As a result, the HALP score was found to be lower in elderly patients. This can be explained by the lower nutritional status of older patients.18
Chen et al. took a cut-off value of 56.8 for the HALP score, revealing that gender, T-stage, and tumour size were independently associated with HALP. They also showed that the high HALP group had a significantly better prognosis for gastric cancer than the low HALP group. In that study, which is the only one in the literature evaluating the relationship between overall survival and HALP score in gastric cancer, the cut-off value was calculated with the X-tile program.16 However, when the median value was accepted as 23.8 for the HALP score cut-off, a significant overall survival difference, between low and high HALP score groups, was found. The number of participants in their study was enormously higher than this, which may be the reason why the present results differed.
Wang et al. reported that a low HALP score (HALP≤ 35.3) was found to be an independent risk factor for lymph node metastases in gastric cancer.17 Since the present study was a retrospective study, it could not determine the lymph node metastasis status of these patients at follow-up. When the lymph node status at the time of admission was evaluated, no difference could be identified most likely due to the large number of patients in this study group who had lymph node metastasis (182 patients) against those who did not have lymph node metastasis (22 patients).
The studies investigating pathological prognostic data have emphasised that the Laurén subtype is associated with gastric cancer prognosis.19 Similarly, the survival of the study group was significantly different according to the histopathological subtype. The results showed that high CEA is associated with poor prognosis, in line with Wang et al. study results.20
The present study revealed that the overall survival of those who received adjuvant chemotherapy was statistically more prolonged than those who received palliative chemotherapy, consistent with the literature.21 It is due to the differences in the stage of disease; neoadjuvant is meant for those with the potential candidacy for the surgery, while palliative is for those in a more advanced stage.
This research was a single-centre retrospective designed study with a limited sample size, which may have influenced the accuracy of the results that were its major limitations. The researchers need to conduct prospective, randomised, and well-designed studies to find the best cut-off value. Another limitation of this study is that HALP is an independent predictor in many tumours, and its sensitivity and specificity are not high. The study has a cross-sectional design, which cannot evaluate the HALP score during disease progression.
CONCLUSION
The HALP score can be measured easily, effectively, and reproducibly during clinical practice in patients with early and advanced gastric adenocarcinoma. Furthermore, this score was associated with an improved overall survival rate in gastric carcinoma patients. However, to determine the predictive value of the HALP score in patients with gastric cancer, large-scale prospective studies are required.
ETHICAL APPROVAL:
Approval from Zonguldak Bulent Ecevit University Faculty of Medicine Non-Interventional Clinical Research Ethics Committee was obtained for the study (Protocol No. 2022/02 Approval Date: 26 January 2022).
PATIENTS’ CONSENT:
This is a retrospective study so patients’ consent is not required.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
ZGS: Contributed to the design, data analysis, writing, and supervision of the manuscript.
ID: Contributed to data collection and data analysis.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71(3): 209-49. doi: 10.3322/caac.21660.
- Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: Review and considerations for future directions. Ann Surg 2005; 241(1):27-39. doi: 10.1097/01.sla.0000149300.28588.23.
- Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin 2021; 71(3): 264-79. doi: 10.3322/caac.21657.
- Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 2022; 20(2): 167-92. doi: 10.6004/jnccn.2022.0008.
- Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International gastric cancer association staging project. Gastric Cancer 2017; 20(2):217-25. doi: 10.1007/s10120-016-0601-9.
- Huang XZ, Yang YC, Chen Y, Wu CC, Lin RF, Wang ZN, et al. Preoperative anaemia or low haemoglobin predicts poor prognosis in gastric cancer patients: A meta-analysis. Dis Markers 2019; 2019:7606128. doi: 10.1155/2019/760 6128.
- Roma-Rodrigues C, Mendes R, Baptista P V, Fernandes AR. Targeting tumour microenvironment for cancer therapy. Int J Mol Sci 2019; 20(4):840. doi: 10.3390/ijms20040840.
- Sachlova M, Majek O, Tucek S. Prognostic value of scores based on malnutrition or systemic inflammatory response in patients with metastatic or recurrent gastric cancer. Nutr Cancer 2014; 66(8):1362-70. doi: 10.1080/01635581.2014. 956261.
- Kim S, McClave SA, Martindale RG, Miller KR, Hurt RT. Hypoalbuminemia and clinical outcomes: What is the mechanism behind the relationship? Am Surg 2017; 83(11): 1220-7. doi: 10.1177/000313481708301123.
- Zhang X, Zhao W, Yu Y, Qi X, Song L, Zhang C, et al. Clinicopathological and prognostic significance of platelet-lymphocyte ratio (PLR) in gastric cancer: an updated meta-analysis. World J Surg Oncol 2020; 18(1):191. doi: 10. 1186/s12957- 020-01952-2.
- Sierko E, Wojtukiewicz MZ. Platelets and angiogenesis in malignancy. Semin Thromb Hemost 2004; 30(1):95-108. doi: 10.1055/s-2004-822974.
- Hirahara N, Matsubara T, Kaji S, Uchida Y, Hyakudomi R, Yamamoto T, et al. Influence of nutrition on stage-stratified survival in gastric cancer patients with postoperative complications. Oncotarget 2022; 13:183-97. doi: 10.18632/ oncotarget.28179.
- Konopka K, Micek A, Ochenduszko S, Streb J, Potocki P, Kwinta Ł, et al. Combined neutrophil-to-lymphocyte and platelet-volume-to-platelet ratio (NLR and PVPR score) represents a novel prognostic factor in advanced gastric cancer patients. J Clin Med 2021; 10(17):3902. doi: 10.3390/ jcm10173902.
- Xu SS, Li S, Xu HX, Li H, Wu CT, Wang WQ, et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol 2020; 26(8):828-38. doi: 10.3748/ wjg. v26.i8.828.
- Feng JF, Wang L, Yang X. The preoperative haemoglobin, albumin, lymphocyte and platelet (HALP) score is a useful predictor in patients with resectable esophageal squamous cell carcinoma. Bosn J basic Med Sci 2021; 21(6):773-81. doi: 10.17305/bjbms.2021.5666.
- Chen XL, Xue L, Wang W, Chen HN, Zhang WH, Liu K, et al. Prognostic significance of the combination of preoperative haemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: A retrospective cohort study. Oncotarget 2015; 6(38): 41370-382. doi: 10.18632/ oncotarget.5629.
- Wang X, He Q, Liang H, Liu J, Xu X, Jiang K, et al. A novel robust nomogram based on preoperative haemoglobin and albumin levels and lymphocyte and platelet counts (HALP) for predicting lymph node metastasis of gastric cancer. J Gastrointest Oncol 2021; 12(6): 2706-18. doi: 10.21037/ jgo-21-507.
- Kim J, Hurria A. Determining chemotherapy tolerance in older patients with cancer. J Natl Compr Canc Netw 2013; 11(12):1494-1502. doi: 10.6004/jnccn.2013.0176.
- DIaz Del Arco C, Estrada Muñoz L, Molina Roldán E, Ortega Medina L, García Gómez de Las Heras S, Chávez Á, et al. Proposal for a clinicopathological prognostic score for resected gastric cancer patients. Saudi J Gastroenterol 2021; 27(1):44-53. doi: 10.4103/sjg.SJG_208_20.
- Wang K, Jiang X, Ren Y, Ma Z, Cheng X, Li F, et al. The significance of preoperative serum carcinoembryonic antigen levels in the prediction of lymph node metastasis and prognosis in locally advanced gastric cancer: A retrospective analysis. BMC Gastroenterol 2020; 20(1): 100. doi: 10. 1186/s12876-020-01255-6.
- Aznab M, Beiki O, pia KE, Setayeshi K, Hesami MA, Vrae H. Evaluation the survival of patients with gastric cancer treated with adjuvant or palliative chemotherapy. J Gastrointest Cancer 2017; 48(1):31-7. doi: 10.1007/s12029-016- 9868-7