Survival and Prognostic Factors in Limited-stage Small-cell Lung Cancer
By Muhammed Mustafa Atci1, Abdullah Sakin1, Emre Uysal2, Ferdi Aksaray2, Oguzhan Selvi1, Orcun Can1Affiliations
doi: 10.29271/jcpsp.2021.12.1433ABSTRACT
Objective: To evaluate the factors affecting overall survival (OS) and progression-free survival (PFS) in patients with limited stage-small cell lung cancer (LS-SCLC).
Study Design: Descriptive study.
Place and Duration of Study: Prof. Dr. Cemil Tascioglu City Hospital, Istanbul, Turkey from January 2002 to October 2019.
Methodology: Data of 89 patients was analysed, who were treated with chemoradiotherapy (CRT) for LS-SCLC, of whom some had also received prophylactic cranial irradiation (PCI). The clinical course and survival rates of LS-SCLS patients treated with different treatment modalities, were evaluated and the prognostic factors were analysed by Cox-regression analysis.
Results: The median age of the patients was 59.6 (39 – 83) years-old; 82% were men. The median follow-up duration was 20 (1 – 189) months. The median PFS and OS were 16 (95% CI, 13-18) months and 33 (95% CI, 25-41) months. Patients, who underwent PCI had better OS compared to patients who did not [54 (95% CI, 27-87) months vs. 19 (95% Cl,, 13-25) months, log-rank, p = 0.004]. Grade 3-4 hematologic toxicities were observed in 12 (13.5%) patients and grade 3-4 esophagitis was observed in 25 (28.1%) patients. Younger age, ECOG 0-1, stage I-II disease, complete response to CRT were good prognostic factors on OS and PFS. A complete response to CRT was also a good independent factor in terms of PFS and OS.
Conclusion: In this study, younger age, better ECOG status, stage I-II disease, and complete response to CRT had a favourable impact on OS and PFS in LS-SCLC. In addition, PCI has been shown to increase survival in these patients.
Key Words: Limited-stage, Small-cell lung cancer, Thoracic radiotherapy, Chemoradiotherapy.
INTRODUCTION
Small cell lung cancer (SCLC), which accounts for 15 percent of lung cancers, is a poorly differentiated neuroendocrine tumor. SCLC is distinguished from other lung cancers by its more aggressive nature with a higher rate of metastasis.1 Since SCLC patients are usually diagnosed with disseminated disease, systemic treatment modalities play an essential role in the management of this disease. Although SCLC is a chemotherapy and radiotherapy sensitive disease, relapses are expected in a few months after treatment.2 Limited stage (LS) SCLC is defined as the disease involving ipsilateral hemithorax and ipsilateral regional lymph nodes; and 30-40% of the patients with SCLC have LS-SCLC at the time of diagnosis.3,4
In the LS-SCLC, early concurrent chemoradiotherapy (CRT) and prophylactic cranial irradiation (PCI) of the brain remain the gold standard treatment.5 Etoposide and cisplatin or carboplatin combined with thoracic radiotherapy (TRT) is the mainstay of treatment, as it improves survival and local control in LS-SCLC patients.6 In a meta-analysis chemoradiation was associated with better overall survival (OS) than chemotherapy alone.7 In another study, a concurrent CRTregimen with cisplatin plus etoposide was shown to be better than sequential radiotherapy after cisplatin plus etoposide in LS-SCLC.8 One of the most important factors determining the prognosis in SCLC is the the clinical stage. Survival was found to be worse in those patients with mediastinal lymph node involvement.9 The prognostic significance of PCI, chest radiation, chemotherapy, surgery, platelet/lymphocyte ratio, smoking cessation and age in LS-SCLC patients has been demonstrated previously.10
The aim of this study was to evaluate the demographic characteristics, clinical course, and survival rates of LS-SCLS patients treated with different treatment modalities.
METHODOLOGY
In this study, conducted at Prof. Dr. Cemil Tascioglu City Hospital, Istanbul, Turkey, among 422 SCLC patients followed-up between 2002 and 2019, 89 were included, who were evaluated as LS-SCLC. The diagnosis of all patients was based on histopathological examination, and staged based on American Joint Committee on Cancer (AJCC) 8th edition.11 Demographic information of LS-SCLC patients as well as their data regarding the Eastern Cooperative Oncology Group (ECOG) performance status, chemotherapy, radiotherapy, local recurrence, distant metastasis, and survival were recorded. Exclusion criteria were age <18 years, metastatic disease at the time of diagnosis, history of ischemic heart disease, and other additional malignancies. In addition, the patients who could not complete thoracic radiotherapy (TRT) or with missing data, were also excluded.
Chemotherapy was administered every 28 days concurrently with TRT. Chemotherapy was given as cisplatin (80 mg/m2 intravenous) or carboplatin area under the curve (AUC) on the first day and etoposide (100 mg/m2 intravenous) on the 1st, 2nd, and 3rd days. TRT was applied on day-2 of the first cycle of chemotherapy twice daily (1.5 Gy per fraction, with 4 hours or more between fractions) and a total dose of 45 Gy in 3 weeks. Primary tumor, ipsilateral hilum, subcarinal region, and, if present, involved lymph nodes were included in the treatment area of TRT. Additionally, the data regarding PCI performed on some patients, were also recorded.
Treatment response was evaluated by positron emission tomography (PET-CT) and contrast-enhanced thoracic computed tomography (CT) every three months, based on Response Evaluation and Criteria in Solid Tumors 1.1 (RECIST).12 Treatment-related side effects were evaluated with a version of Common Terminology Criteria for Adverse Events of the National Cancer Institute.13 Progression-free survival (PFS) was defined as the time from diagnosis to local recurrence or distant metastasis. OS was defined as the time from diagnosis to death.
Statistical analyses were performed in SPSS version 15.0 (IBM). Descriptive statistics are number, and percentage for categorical variables, mean, standard deviation, median, minimum, and maximum for numerical variables. The rates in the groups were analysed by Chi-square test, survival rates by Kaplan-Meier and risk factors by Cox-Regression. The statistical significance level of alpha was accepted as p <0.05.
RESULTS
In total, 89 patients diagnosed with LS-SCLC, were included in this study. The median follow-up duration was 20 months (ranging from 1 - 189 months). The demographic information and clinical characteristics are shown in Table I. The median age of the patients was 59.6 (39 – 83) years, of whom 16 (18.0%) were women, and 73 (82.0%) were men. Most patients’ (79.8%) performance status was good (ECOG 0-1), and 73 (82%) patients were determined to be stage III.
Table I: Demographic information and clinical features of the LS-SCLC patients.
|
Age, years |
59.6 ±9.4 (39-83) |
|
Gender |
|
|
Women |
16 (18.0%) |
|
Men |
73 (82.0%) |
|
BMI (kg/m2) |
25.7 ±5.0 (16.2-41) |
|
Smoking (pack-year) |
58.7 ±30.1 (0-160) |
|
Performance status |
|
|
ECOG 0 |
50 (56.2%) |
|
ECOG 1 |
21 (23.6%) |
|
ECOG 2 |
10 (11.2%) |
|
ECOG 3 |
2 (2.2%) |
|
Unknown |
6 (6.7%) |
|
Localization |
|
|
Right lung |
54 (60.7%) |
|
Left lung |
35 (39.3%) |
|
Tumor size (cm) |
5.8 ±2.5 (2-15) |
|
Stage |
|
|
I |
2 (2.2%) |
|
II |
14 (15.7%) |
|
III |
73 (82.0%) |
|
Concurrent CT |
|
|
Cisplatin plus etoposide |
82 (92.1%) |
|
Carboplatin plus etoposide |
7 (7.9%) |
|
The number of CT cures |
5.0 ±1.4 (1-8) |
|
Prophylactic cranial irradiation |
|
|
None |
44 (49.4%) |
|
Received |
45 (50.6%) |
|
Response after CRT |
|
|
Complete response |
52 (58.4%) |
|
Partial response |
27 (30.3%) |
|
Stable |
4 (4.5%) |
|
Progression |
6 (6.7%) |
|
Final situation |
|
|
Alive |
37 (41.6%) |
|
Exitus |
52 (58.4%) |
|
BMI: Body mass index; CT: Chemotherapy; CRT: Chemoradiotherapy; PCI: Prophylactic cranial irradiation. |
|
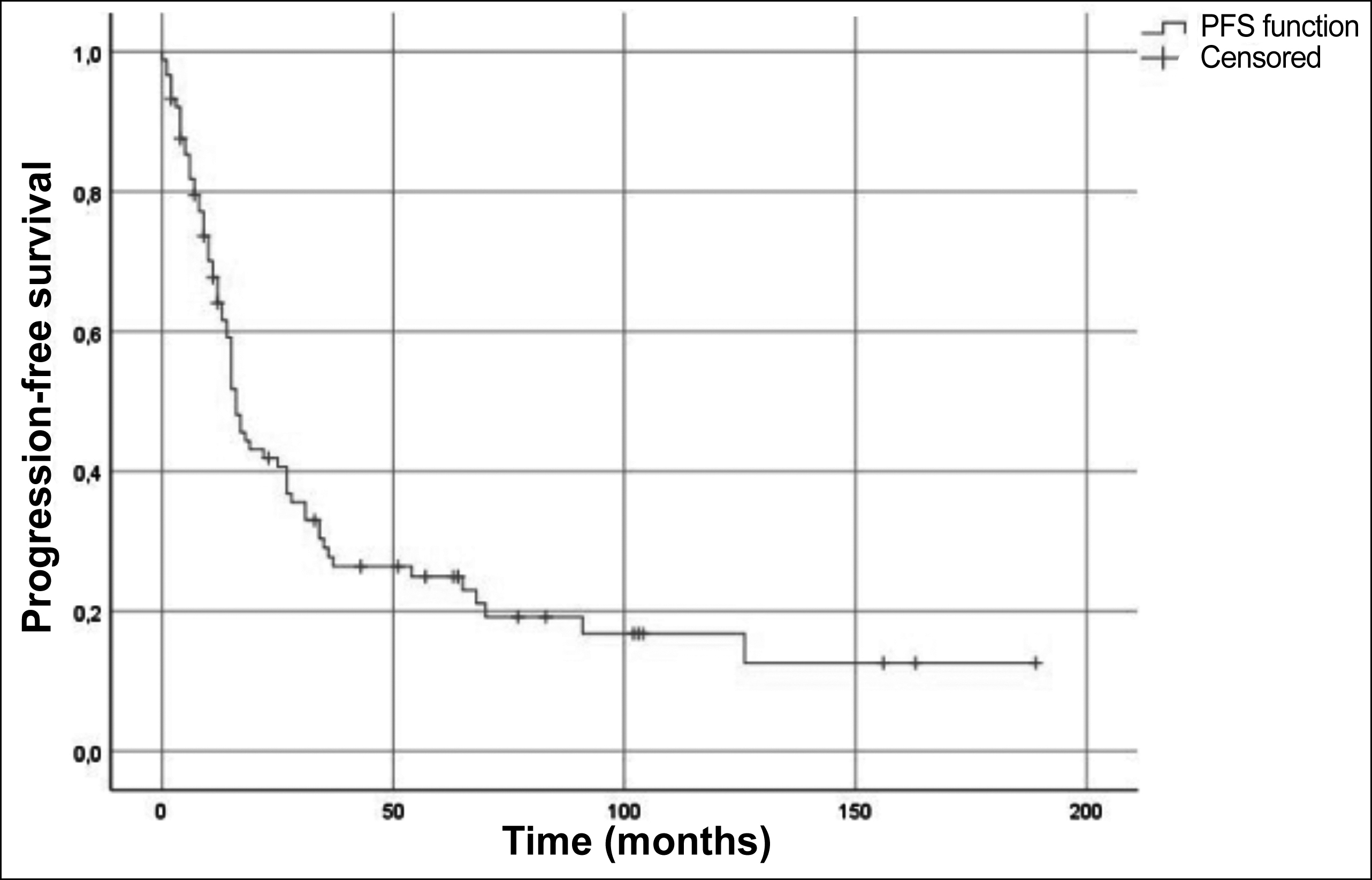 Figure 1: Progression-free survival curve.
Figure 1: Progression-free survival curve.
The cisplatin-etoposide (EP) combination with TRT was the most commonly (92.1%) used concurrent CRT regimen. Additionally, PCI was performed on about half (50.6%) of the patients. The complete response rate to CRT was 58.4%. The median PFS and OS were 16 (95% CI, 13-18) months and 33 (95% CI, 25-41) months, respectively. While 1, 3 and 5-year PFS were 64.1%, 27.7% and 24.9% (Figure 1); 1, 3 and 5-year OS were 81.5%, 43.3% and 34.3% (Figure 2); respectively.
Table II: Univariate and multivariate analysis for OS.
|
Variables |
Univariate |
Multivariate |
||||||
|
p |
HR |
%95 CI |
p |
HR |
%95 CI |
|||
|
Age |
<0.001 |
1.061 |
1.029 |
1.094 |
0.710 |
0.987 |
0.923 |
1.056 |
|
Gender (Ref: Women) |
||||||||
|
Men |
0.094 |
2.198 |
0.873 |
5.530 |
0.626 |
0.544 |
0.047 |
6.275 |
|
BMI (kg/m2) |
0.011 |
0.897 |
0.825 |
0.976 |
0.227 |
0.895 |
0.748 |
1.071 |
|
Smoking |
0.433 |
1.003 |
0.995 |
1.011 |
0.028 |
1.019 |
1.002 |
1.037 |
|
Performance status (Ref: ECOG 0) |
||||||||
|
ECOG 1 |
<0.001 |
8.611 |
3.443 |
21.538 |
0.007 |
6.198 |
1.639 |
23.440 |
|
ECOG 2 |
<0.001 |
16.049 |
5.768 |
44.655 |
0.044 |
6.565 |
1.051 |
41.004 |
|
ECOG 3 |
0.002 |
13.357 |
2.660 |
67.071 |
0.197 |
5.776 |
0.403 |
82.822 |
|
Localization (Ref: Left lung) |
||||||||
|
Right lung |
0.389 |
1.280 |
0.730 |
2.245 |
0.527 |
0.736 |
0.284 |
1.907 |
|
Tumor size |
0.758 |
0.981 |
0.871 |
1.106 |
0.610 |
0.941 |
0.744 |
1.190 |
|
Stage (Ref: I-II) |
||||||||
|
III |
0.030 |
2.428 |
1.090 |
5.409 |
0.393 |
1.920 |
0.429 |
8.588 |
|
CRT (Ref: cisplatin + etoposide) |
||||||||
|
Carboplatin + etoposide |
0.085 |
2.318 |
0.891 |
6.032 |
0.391 |
0.327 |
0.025 |
4.217 |
|
The number of CT cures |
<0.001 |
0.644 |
0.522 |
0.795 |
0.001 |
0.352 |
0.194 |
0.637 |
|
PCI |
0.005 |
0.448 |
0.256 |
0.785 |
0.864 |
0.892 |
0.243 |
3.274 |
|
Response after CRT (Ref: Complete response) |
||||||||
|
Partial response |
<0.001 |
4.590 |
2.413 |
8.730 |
0.014 |
5.527 |
1.413 |
21.628 |
|
Stable |
<0.001 |
16.538 |
5.145 |
53.152 |
0.717 |
1.414 |
0.217 |
9.211 |
|
Progression |
<0.001 |
13.727 |
4.650 |
40.524 |
0.579 |
2.317 |
0.119 |
45.197 |
|
OS: Overall survival; BMI: Body mass index; CRT: Chemoradiotherapy; CT: Chemotherapy; PCI: Prophylactic cranial irradiation. |
||||||||
Table III: Univariate and multivariate analysis for PFS.
|
Variables |
Univariate |
Multivariate |
||||||
|
p |
HR |
%95 CI |
p |
HR |
%95 CI |
|||
|
Age |
<0.001 |
1.048 |
1.021 |
1.075 |
0.238 |
1.033 |
0.979 |
1.090 |
|
Gender (Ref: Women) |
||||||||
|
Men |
0.169 |
1.638 |
0.811 |
3.308 |
0.616 |
0.706 |
0.181 |
2.752 |
|
BMI |
0.050 |
0.937 |
0.879 |
1.000 |
0.202 |
0.927 |
0.824 |
1.042 |
|
Smoking |
0.252 |
1.004 |
0.997 |
1.011 |
0.856 |
1.001 |
0.987 |
1.016 |
|
Performance status (Ref: ECOG 0) |
||||||||
|
ECOG 1 |
<0.001 |
3.556 |
1.862 |
6.793 |
0.223 |
1.732 |
0.716 |
4.187 |
|
ECOG 2 |
<0.001 |
6.513 |
2.910 |
14.577 |
0.465 |
1.957 |
0.323 |
11.858 |
|
ECOG 3 |
0.125 |
3.143 |
0.728 |
13.576 |
0.781 |
1.382 |
0.141 |
13.548 |
|
Localization (Ref: left lung) |
||||||||
|
Right lung |
0.214 |
1.375 |
0.832 |
2.272 |
0.627 |
1.206 |
0.567 |
2.562 |
|
Tumor size |
0.422 |
1.042 |
0.942 |
1.154 |
0.721 |
0.972 |
0.832 |
1.135 |
|
Stage (Ref: I-II) |
||||||||
|
III |
0.002 |
3.542 |
1.612 |
7.781 |
0.040 |
4.111 |
1.064 |
15.888 |
|
CRT (Ref: cisplatin + etoposide) |
||||||||
|
Carboplatin + etoposide |
0.021 |
2.581 |
1.155 |
5.766 |
0.611 |
0.671 |
0.144 |
3.123 |
|
The number of CT cures |
0.010 |
0.782 |
0.650 |
0.942 |
0.271 |
0.789 |
0.517 |
1.203 |
|
PCI |
0.035 |
0.593 |
0.365 |
0.964 |
0.714 |
1.183 |
0.482 |
2.906 |
|
Response after CRT (Ref: Complete response) |
||||||||
|
Partial response |
<0.001 |
4.782 |
2.663 |
8.585 |
0.011 |
3.048 |
1.284 |
7.232 |
|
Stable |
<0.001 |
17.917 |
5.575 |
57.582 |
0.078 |
5.539 |
0.823 |
37.291 |
|
Progression |
<0.001 |
11.512 |
4.478 |
29.591 |
0.068 |
10.608 |
0.837 |
134.47 |
|
PFS: Progression-free survival; BMI: Body mass index; CRT: Chemoradiotherapy; CT: Chemotherapy; PCI: Prophylactic cranial irradiation. |
||||||||
Patients who underwent PCI had better OS compared with patients who did not undergo PCI median 54 (95% CI, 21-87) months vs. 19 (95% CI, 13-25) months, (log-rank, p = 0.004, Figure 3).
In the univariate analysis of prognostic factors affecting OS, older age, higher body mass index (BMI), poor performance status and stage III disease were found as bad prognostic factors. In addition, PCI, complete response to CRT, an increased number of CT cures were good prognostic factors. In the multivariate analysis, while smoking history, poor performance status were bad prognostic factors, an increased number of CT cures were found to be a good prognostic factor. Besides, partial response compared to complete response to CRT was statistically significantly associated with poor OS (Table II).
In the univariate analysis in which prognostic factors affecting PFS were investigated, older age, poor performance status, advanced stage disease were determined as poor prognostic factors; and receiving PCI, showing complete response to CRT, receiving increased number of CT cures, and concurrent cisplatin plus etoposide (compared to carboplatin plus etoposide) were good prognostic factors. In the multivariate analysis, stage III disease was determined as an independent prognostic factor for PFS. In addition, although the partial response was statistically significant as a poor prognostic factor, compared to the complete response, there was a trend towards significance in stable response and progression (Table III).
Hematologic toxicities, such as grade 3-4 leukopenia, thrombocytopenia, and anemia were observed in 12 (13.5%) patients. Grade 3-4 acute esophageal toxicity and grade 1-2 pulmonary toxicity were observed in 25 (28.1%) and 7 (7.8%) patients, respectively. Due to these side effects, chemotherapy was postponed and dose reduction was made in these patients.
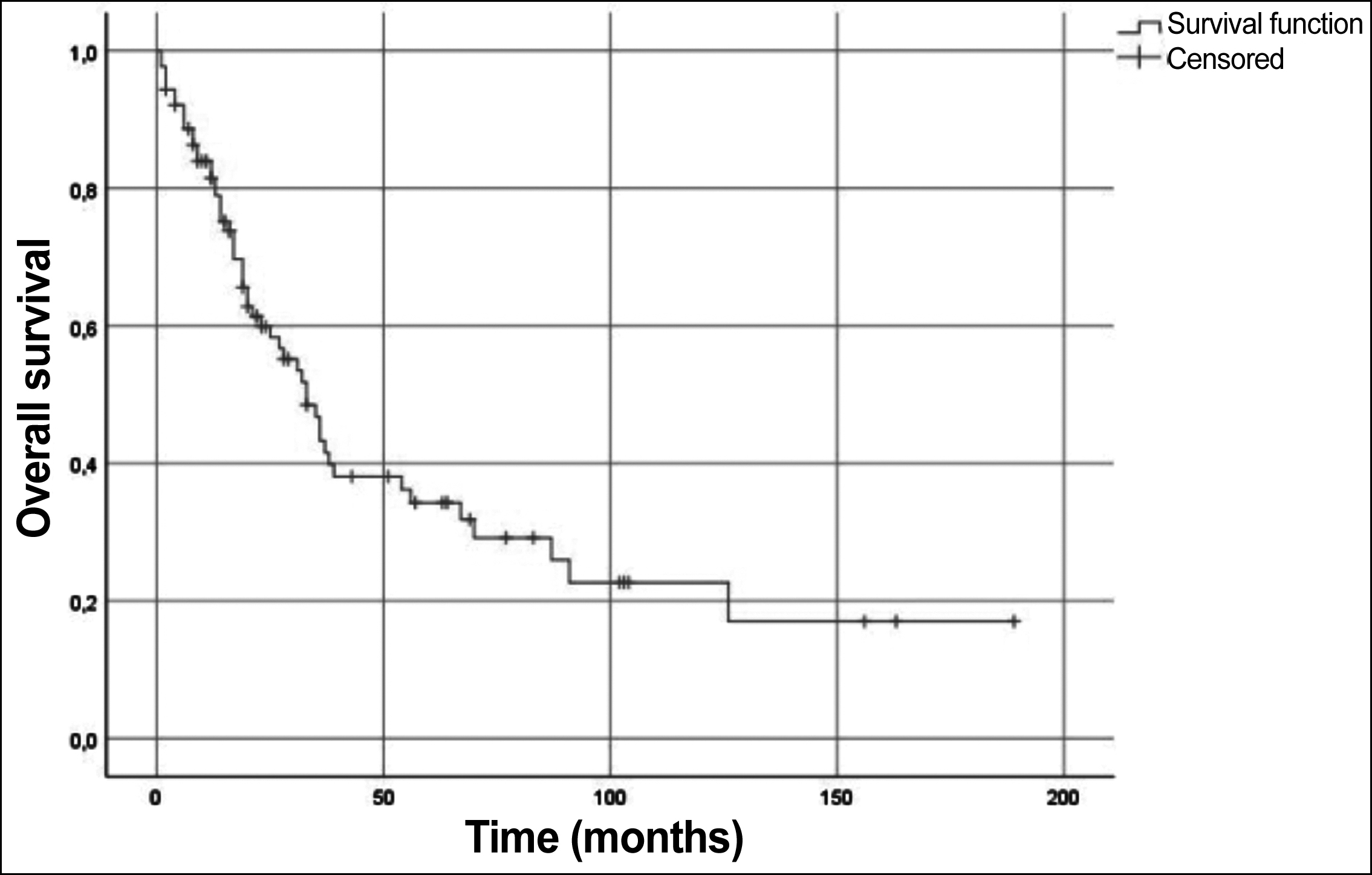 Figure 2: Overall survival curve.
Figure 2: Overall survival curve.
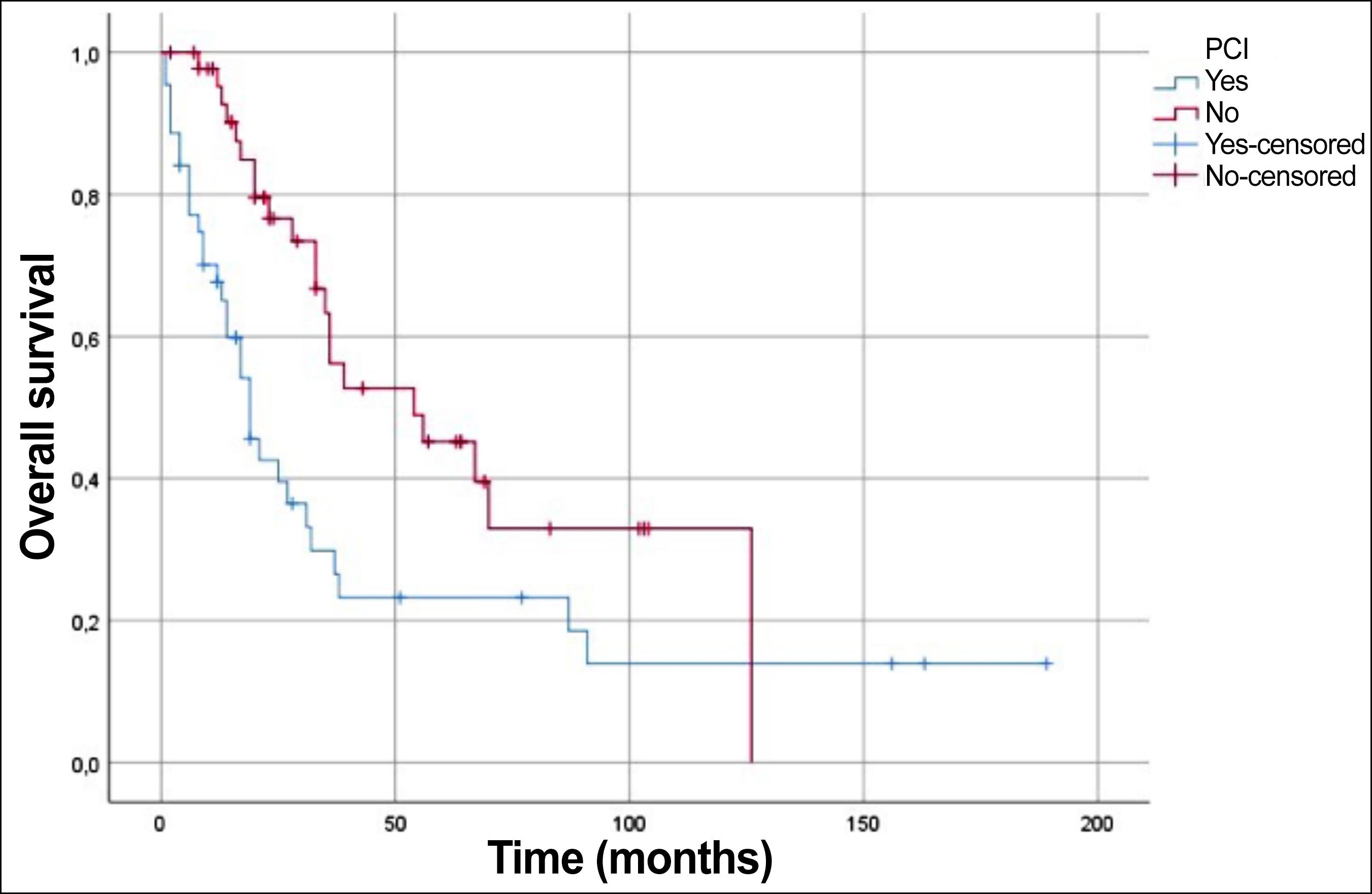 Figure 3: Comparison of overall survival between PCI and non-PCI patients.
Figure 3: Comparison of overall survival between PCI and non-PCI patients.
The solid line shows that the overall survival curve for the patients receiving prophylactic cranial irradiation (PCI). The dotted line shows the overall survival curve for patients not receiving PCI.
DISCUSSION
SCLC is a poorly differentiated tumor that usually presents with disseminated disease. Due to its aggressive nature, defining the prognostic factors and precise management of LS-SCLC patients is essential to get better PFS and OS in these patients.1-4 In this analysis of 89 LS-SCLC patients, multiple factors were found to be associated with the survival of these patients. Younger age, better ECOG status, stage I-II disease, complete response to CRT had a favorable impact on OS and PFS.
SCLC is a disease that is mostly sensitive to CRT. SCLC commonly shows recurrence within one to two years and may become resistant to treatment. In the last three decades, a significant increase in response status has been achieved mainly due to the combined use of CRT regimen in these patients.14
PCI has been shown in clinical trials to reduce the incidence of brain metastasis in SCLC. PCI provides long-term survival benefits to patients with LS-SCLC, who show a complete or near-complete response after CRT induction. In this study, as above mentioned, PCI was found to be statistically significant in univariate analysis in terms of OS and PFS.15,16
National Clinical Research Network's concurrent CRT clinical trials for LS-SCLC reported that elderly patients compared to younger patients had worse OS and PFS, similar to results of this study.17 Similar to OS results obtained in this study, Valan et al. reported that OS of early-stage (Stage I-II), Stage IIIA, and Stage IIIB LS-SCLC patients were 33.8 months, 33.0 months, and 18.8 months, respectively.18 Contrary to the present study, no difference was found between stages in terms of OS according to TNM staging in another study.19 Go et al. found that complete response after CRT was a good independent risk factor in PFS and OS in patients with LS-SCLC, which is consistent with the present results.20 In patients with the partial response after CRT, additional treatments may be required due to the high risk of relapse. Many studies of chemotherapy or surgical resection after CRT have been performed. However, the role of consolidation treatments in such patients has not been proven.21,22
The strength of the current study was that all patients completed chemotherapy and radiotherapy. The major limitation of this study was its retrospective data collection. Another limitation was that although the total or partial response was obtained in 88.7% of the patients, only 50.6% received PCI.
CONCLUSION
Younger age, better ECOG status, stage I-II disease, and complete response after the combined modality treatment had a favourable impact on OS and PFS in LS-SCLC. In addition, this study found that complete response after CRT was a good independent risk factor in terms of PFS and OS in patients with LS-SCLC.
ETHICAL APPROVAL:
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Institutional Review Board (IRB approval No. 48670771-514.10/150). The ethical approval was obtained prior to initiation of the present study.
PATIENTS’ CONSENT:
Informed consent was not required as it is a retrospective study and patient identity was kept anonymous.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
MMA, AS: Conception, design, analyses, interpretation, drafting, final approval.
EU, OS, OC: Acquistion, drafting, final approval, revision.
FA: Analyses, drafting, final approval.
REFERENCES
- American Society of Clinical Oncology. The state of cancer care in America, 2014: A report by the American society of clinical oncology. J Oncol Pract 2014; 10(2):119-42. doi: 10.1200/JOP.2014.001386.
- National, Comprehensive Cancer Network. Small cell lung cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw 2004; 2:134-57. doi: 10.6004/jnccn.2004.0012.
- Hanna NH, Einhorn LH. Small-cell lung cancer: State of the art. Clin Lung Cancer 2002; 4:87-94. doi: 10.3816/clc.2002. n.018.
- Carter BW, Glisson BS, Truong MT, Erasmus JJ. Small cell lung carcinoma: Staging, imaging, and treatment considerations. Radiographics 2014; 34:1707-21. doi: 10.1148/rg.34614 0178.
- Noronha V, Sekhar A, Patil VM, Menon N, Joshi A, Kapoor A, et al. Systemic therapy for limited-stage small-cell lung carcinoma. J Thorac Dis 2020; 12:6275-90. doi: 10.21037/jtd-2019-sclc-11.
- Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992; 10:890-5. doi: 10.1200/JCO.1992.10.6.890.
- Pignon JP, Arriagada R, Ihde DC, Johnson DH, Perry MC, Souhami RL, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992; 327:1618-24. doi: 10.1056/NEJM199212033272302.
- Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: Results of the Japan clinical oncology group study 9104. J Clin Oncol 2002; 20:3054-60. doi: 10.1200/JCO.2002.12.071.
- Salem A, Mistry H, Hatton M, Locke I, Monnet I, Blackhall F, et al. Association of chemoradiotherapy with outcomes among patients with stage I to II vs. Stage III small cell lung cancer: Secondary analysis of a randomised clinical trial. JAMA Oncol 2019; 5:e185335. doi: 10.1001/jamaoncol. 2018.5335.
- Xie D, Marks R, Zhang M, Jiang G, Jatoi A, Garces YI, et al. Nomograms predict overall survival for patients with small-cell lung cancer incorporating pretreatment peripheral blood markers. J Thorac Oncol 2015; 10:1213-20. doi: 10.1097/JTO.0000000000000585.
- Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalised” approach to cancer staging. CA Cancer J Clin 2017; 67:93-9. doi: 10.3322/caac.21388.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European organisation for research and treatment of cancer, national cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92:205-216. doi: 10.1093/ jnci/92.3.205.
- The National Cancer Institute. Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3.0).9.2009.
- Alvarado-Luna G, Morales-Espinosa D. Treatment for small cell lung cancer, where are we now?-a review. Transl Lung Cancer Res 2016; 5:26-38. doi: 10.3978/j.issn.2218-6751. 2016.01.13.
- Aupérin A, Arriagada R, Pignon JP, Le Péchoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic cranial irradiation overview collaborative group. N Engl J Med 1999; 341:476-84. doi: 10.1056/NEJM199908123 410703.
- Cao KJ, Huang HY, Tu MC, Pan GY. Long-term results of prophylactic cranial irradiation for limited-stage small-cell lung cancer in complete remission. Chin Med J (Engl) 2005; 118:1258-62.
- Stinchcombe TE, Fan W, Schild SE, Vokes EE, Bogart J, Le QT, et al. A pooled analysis of individual patient data from national clinical trials network clinical trials of concurrent chemoradiotherapy for limited-stage small-cell lung cancer in elderly patients versus younger patients. Cancer 2019; 125:382-90. doi: 10.1002/cncr.31813.
- Valan CD, Slagsvold JE, Halvorsen TO, Herje M, Bremnes RM, Brunsvig PF, et al. Survival in limited disease small cell lung cancer according to N3 lymph node involvement. Anticancer Res 2018; 38:871-876. doi: 10.21873/anticanres.12296.
- Su J, Zhu S, Liu Z, Jin S, Shen W, Li J. Prognostic analysis of limited-stage small-cell lung cancer after chemoradiotherapy. Onkologie 2012; 35:362-7. doi: 10.1159/00033 8962.
- Go SI, Keam B, Kim TM, Lee SH, Kim DW, Kim HJ, et al. Clinical significance of downstaging in patients with limited-disease small-cell lung cancer. Clin Lung Cancer 2014; 15:e1-6. doi: 10.1016/j.cllc.2013.09.003.
- Rossi A, Garassino MC, Cinquini M, Sburlati P, Di Maio M, Farina G, et al. Maintenance or consolidation therapy in small-cell lung cancer: A systematic review and meta-analysis. Lung Cancer 2010; 70:119-28. doi: 10.1016/j.lungcan.2010.02.001.
- Veronesi G, Scanagatta P, Leo F, De Pas T, Pelosi G, Catalano G, et al. Adjuvant surgery after carboplatin and VP16 in resectable small cell lung cancer. J Thorac Oncol 2007; 2:131-4. PMID: 17410028.