Surgery for an Isolated Adrenal Metastasis of Gastric Cancer
By Ali Alemdar1, Seracettin Egin1, Ismayil Yilmaz1, Selma Sengiz Erhan2, Recep Yilmaz Bayraktarli3Affiliations
doi: 10.29271/jcpsp.2022.12.1632ABSTRACT
Adrenal metastasis is considered a rare hematogenous metastasis that develops after gastric cancer surgery. The chances of curative surgery are very low. It is usually unresectable. We aim to present a case of isolated adrenal metastasis that developed in a patient, who underwent a total gastrectomy with the diagnosis of gastric cancer approximately 26 months back. Left adrenalectomy was planned with curative intent. R0 resection was performed. The patient was followed up for one year after surgery. The option of surgical treatment is recommended for isolated metachronous adrenal metastases. Curative surgical resection may positively impact the prognosis of patients in selected cases.
Key Words: Gastric cancer, Adrenal metastasis, Surgical resection.
INTRODUCTION
Gastric cancer is a disease with a poor prognosis. Despite the development of neoadjuvant and adjuvant therapies, recurrences are still common after curative treatment. These recurrences are mostly systemic metastases, involving multiple organs, and are responsible for poor prognosis. Systemic chemotherapy is still the standard treatment for metastatic gastric cancer.1 However, good results have been reported with curative surgical treatment added to systemic therapy in metastases involving different organs, including isolated liver metastases.2,3 Isolated adrenal metastases in stomach cancer have been reported in a small number of cases.4,5 We aim to present a case of isolated metachronous adrenal metastasis that developed in a patient, who underwent a total gastrectomy for gastric cancer 26 months back.
CASE REPORT
A 52-year female patient was admitted through the outpatient clinic with a diagnosis of gastric cancer. The patient had a mass from the cardia to the corpus on gastroduodenoscopy. The biopsy from the mass was reported as an adenocarcinoma.
Computed tomography (CT) scanning showed a gastric tumour that measured 1.5 cm in thickness, with serosal invasion, and a lymph node in lesser curvature measuring 0.8 cm in the short axis (Figure 1a). In the positron emission tomography (PET)-CT, fluorodeoxyglucose (FDG) uptake measured in standardised uptake value (SUVmax) of 25.8 was seen in the cardia and lesser curvature regions (Figure 1b). The patient underwent staging laparoscopy. During exploration, there was no implantation of tumour in the abdominal cavity. Peritoneal liquid for cytology examination was negative for malignancy. The patient was staged as cT4N+M0 and neoadjuvant therapy was planned. Three cycles of epirubicin, cisplatin, and 5 fluorouracil (ECF) were given to the patient. Total gastrectomy and D2 dissection were performed in March 2017. The patient was postoperatively discharged without any problem. Out of 78 lymph nodes dissected, only one was positive for metastasis. The tumour showed invasion of the subserosal layer. Therefore, it was evaluated as stage II = (ypT3N1M0) according to tumour node metastasis 8th (TNM) 8th staging.6 After 3 additional postoperative ECF cycles, patient follow-up was performed once every 3 months in the outpatient clinic in the first 2 years. Follow-ups were conducted with clinical examination, biochemical parameters, tumour markers, and CT scans. In the routine follow-up of the patient in April 2019, carbohydrate antigen 19-9 (CA 19-9) increased, and a mass was observed in the left adrenal on CT (Figure 1c). FDG uptake with SUVmax of 12 in the left adrenal was detected in PET-CT (Figure 1d). In magnetic resonance imaging (MRI), the mass in the left adrenal was reported as an adrenal metastasis (Figure 1e, f, g, h). The patient, without any other known organ metastasis, was considered to have an isolated metachronous left adrenal metastasis. The patient was discussed in the tumour board and the decision of surgery was finalised. After obtaining the patient consent, R0 left adrenal resection was performed in May 2019. There were no tumour implants in the abdomen. The patient was discharged without any problem on 5th postoperative day. On histopathological examination, metastatic adenocarcinoma compatible with gastric cancer metastasis was reported (Figure 2a, b). In a one-year follow-up, there was no recurrence of tumour on CT scanning (Figure 1i).
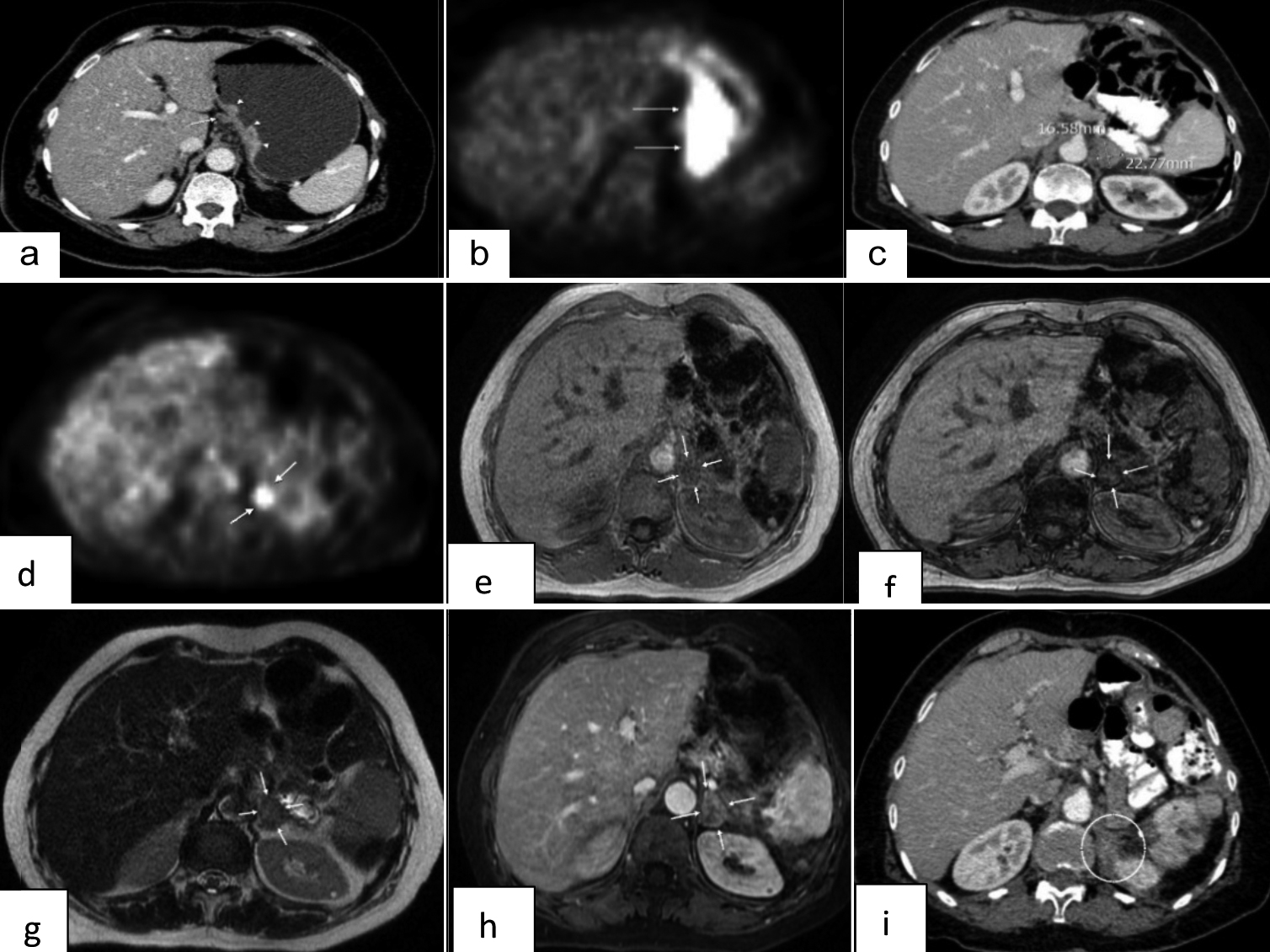 Figure 1: (a) The mass reached to serosa in contrast-enhanced CT (arrowhead) and lymph node (arrows) in small curvature. (b) FDG uptake in small curvature on PET-CT. (c) The left adrenal mass on abdominal CT. (d) The left adrenal FDG uptake on PET-CT. (e) In the adrenal contrast MRI examination, the in-phase sequence lesion is evident. (f) No suppression of the lesion in the out-phase sequence. (g) The left adrenal lesion is heterogeneous and mildly hyperintense in T2-weighted axial examination. (h) In T1-weighted axial examination, heterogeneous contrast enhancement has been observed. (i) No mass lesion on abdominal CT one year after adrenal metastatectomy.
Figure 1: (a) The mass reached to serosa in contrast-enhanced CT (arrowhead) and lymph node (arrows) in small curvature. (b) FDG uptake in small curvature on PET-CT. (c) The left adrenal mass on abdominal CT. (d) The left adrenal FDG uptake on PET-CT. (e) In the adrenal contrast MRI examination, the in-phase sequence lesion is evident. (f) No suppression of the lesion in the out-phase sequence. (g) The left adrenal lesion is heterogeneous and mildly hyperintense in T2-weighted axial examination. (h) In T1-weighted axial examination, heterogeneous contrast enhancement has been observed. (i) No mass lesion on abdominal CT one year after adrenal metastatectomy.
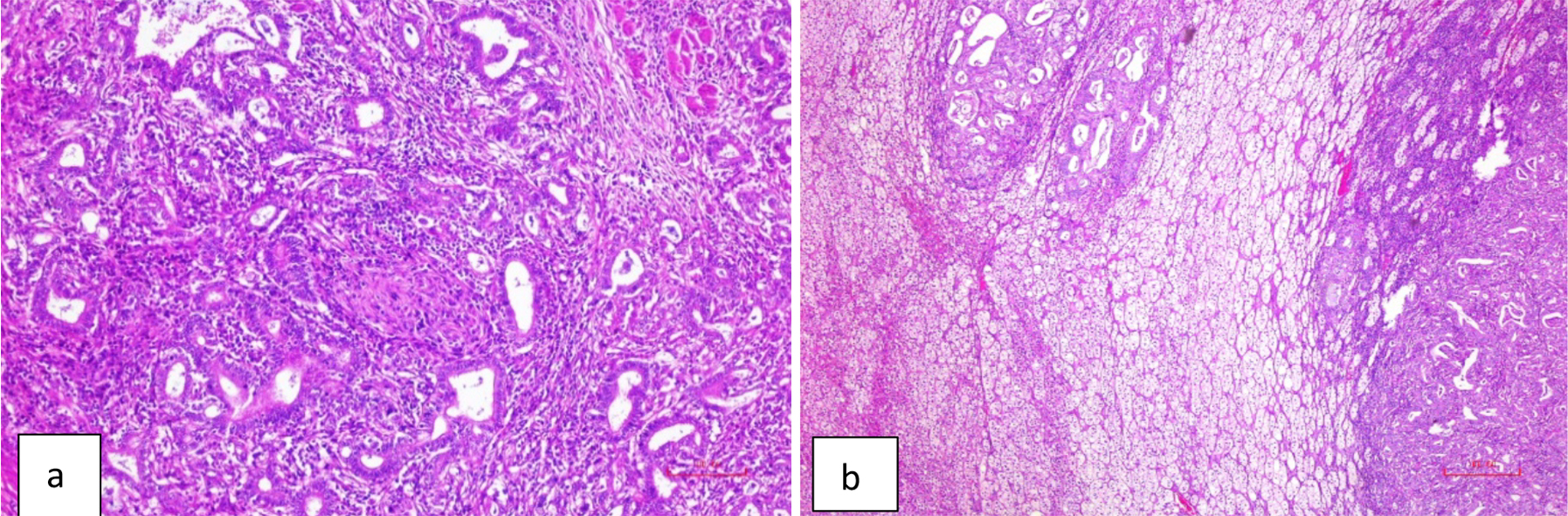 Figure 2: (a) The gastric adenocarcinoma. (H&E × 100). (b) The section from left adrenal showing neoplastic lesion with similar appearance as in Figure 2a. (H&E × 40).
Figure 2: (a) The gastric adenocarcinoma. (H&E × 100). (b) The section from left adrenal showing neoplastic lesion with similar appearance as in Figure 2a. (H&E × 40).
DISCUSSION
Isolated adrenal metastasis of gastric cancer is a rare form of metastasis. Adrenal metastasis is usually seen in disseminated metastasis. Isolated adrenal metastasis in this patient developed by hematogenous spread without extensive metastasis. Surgical treatment in metastatic gastric cancer is limited and palliative chemotherapy treatments are standards of care. To be able to succeed in treatment, it is necessary to understand whether the disease is disseminated and to evaluate the patient with a multidisciplinary approach and determine treatment strategy according to the circumstances.1,7 In the surgical approach of isolated adrenal metastasis, the target should be curative resection. In disseminated disease, the chance of curative resection is low. Extensive surgical resection may be required with multiorgan spread of tumour. Curative R0 resection is the most important prognostic factor.8 The target in similar isolated liver metastasis should be curative resection too.2,3 The widespread use of CT scanning in routine follow-up after gastric cancer treatment has increased the detection rates for early diagnosis of metastatic disease and isolated adrenal metastasis.9 An adrenal mass of approximately 1.5×2.2 cm was observed in the abdominal CT scanning during routine follow-ups of our patient. FDG uptake was observed in the left adrenal gland by PET-CT. MRI is known to have high sensitivity and specificity in the differential diagnosis of adrenal masses. MRI confirmed that the patient had an adrenal metastasis. Adrenal metastasis does not manifest symptoms like Cushing's syndrome. Fine needle aspiration biopsy (FNAB) could be used to increase the sensitivity of the diagnosis and differential diagnosis.5 Because this patient had known primary gastric cancer in addition to metastatic adrenal tumour on clinical and imaging findings, we did not perform FNAB. Because of the increase in CA19-9, FDG uptake in PET-CT, and no mass in abdominal CT during previous follow-ups, we strongly thought that there was an isolated metachronous adrenal metastasis after primary gastric cancer. The patient was discussed in the tumour board by the multidisciplinary team after all these results.
Adrenal metastasis is a disease with a poor prognosis. Average survival varies between 13 to 21 months. Surgical resection is rarely performed because of the rate of multi-organ metastasis during the period when adrenal metastasis is diagnosed. Some publications reported that the disease-free survival of at least 6 to 12 months after the patient's first surgery positively affected the prognosis.8 The general and nutritional conditions of the patient are important for postoperative morbidity. In synchronous or metachronous adrenal metastases, curative R0 resection with low morbidity is important for prognosis. Some publications reported that the primary disease detected as adenocarcinoma affected the prognosis positively.8 There are case reports about adrenal metastasis of gastric cancer reporting long survival with curative R0 resection.4
Disseminated metastasis in gastric cancer patients with adrenal metastasis reduces the chance of curative surgery. Palliative chemotherapy is mostly applied to these patients.1,7 This condition negatively affects patient prognosis and survival expectations.
In conclusion, adrenal metastasis in gastric cancer poses treatment challenge for clinicians and should be handled with a multidisciplinary approach. As in this case, a curative resection is a treatment option for an isolated metachronous adrenal metastasis of gastric cancer. However, patient selection should be carried out carefully. There should be no other metastases apart from adrenal metastasis, the tumour should be resectable, and the patient's condition should be stable. In addition, the experience of the surgical team is important for R0 resection. Curative surgical resection can provide a positive contribution to patient survival in selected patients.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
AA: Surgical and medical practices, literature search, conception or design of the work, data collection, data analysis and interpretation, drafting the article.
SE: Data analysis and interpretation, critical revision of the article, final approval of the version to be published.
IY: Surgical and medical practices, data collection, data analysis and interpretation.
SSE: Pathological evaluation and interpretation.
RYB: Radiological evaluation and interpretation.
All the authors have approved the final of the manuscript to be published.
REFERENCES
- Ali G, Yildirim R. Surgical management of metastatic gastric cancer: Moving beyond the guidelines. Transl Gastroenterol Hepatol 2019; 4:58. doi: 10.21037/tgh.2019.08.03.
- Kwahara K, Makino H, Kametaka H, Hoshino I, Fukada T, Seike K, et al. Outcomes of surgical resection for gastric cancer liver metastases: A retrospective analysis. World J Surg Oncol 2020; 18:41. doi: 10.1186/s12957-020-01816-9.
- Montagnani F, Crivelli F, Aprilr G, Vivaldi C, Pecora I, De Vivo R, et al. Long-term survival after liver metastasectomy in gastric cancer: Systematic review and meta-analysis of prognostic factor. Cancer Treat Rev 2018; 69:11-20. doi: 10.1016/j.ctrv.2018.05.010.
- Kim DJ, Lee JH, Kim W. Surgical treatment for late-appearing adrenal metastasis from gastric cancer: Report of two cases. World J Surgical Oncol 2014; 12:116. doi: 10.1186/1477-7819-12-116.
- Xue W, Li Y, Zhao Z, Li W, Wang S, Zhang M, et al. Solitary adrenal metastasis from advanced gastric cancer invading duodenal bulb with situs inversus totalis. Medicine 2019; 98:15(e15244). doi: 10.1097/MD.0000000000015244.
- Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK (eds), et al. AJCC Cancer Staging Manual. ed. 8 th, Chicago: Springer; 2017.
- Mihmanli M, Ilhan E, Idiz UO, Alemdar A, Demir U. Recent development and innovations in gastric cancer. World J Gastroenterol 2016; 22(17):4307-20. doi: 10.3748/wjg.v22. i17.4307.
- Hwang EC, Hwang I, Junk SII, Kang TW, Kwon DD, Heo SH, et al. Prognostic factors for recurrence-free and overall survival after adrenalectomy for metastatic carcinoma: a retrospective cohort pilot study. BMC Urology 2014; 14:41. doi: 10.1186/1471-2490-14-41.
- Lee S, Choi KD, Hong SM, Park SH, Gong EJ, Na HK, et al. Pattern of extragastric recurrence and the role of abdominal computed tomography in surveillance after endoscopic resection of early gastric cancer: Korean experiences. Gastric Cancer 2017; 20(5):843-52. doi: 10. 1007/s10120-017-0691-z.