Starting Antrectomy in Less than 2 cm from Pylorus at Laparoscopic Sleeve Gastrectomy
By Burhan MayirAffiliations
doi: 10.29271/jcpsp.2022.06.701ABSTRACT
Objective: To evaluate the effect of antrectomy in which resection was started from 2 cm or closer to the pylorus on % excess weight loss (EWL), nausea, vomiting, and complication rates.
Study Design: Comparative study.
Place and Duration of Study: Antalya Training and Research Hospital, from April 2018 to December 2018.
Methodology: Patients in whom laparoscopic sleeve gastrectomy (LSG)were done starting at a level of 2 cm or closer to pylorus were included in the study. Patients were divided into one of the two groups based on the distance between the pylorus and the resection margin: group 1 having resection ≤10 mm and group 2 at 11-20 mm. Above mentioned parameters were compared in both groups.
Results: Ninety-two patients were included. Postoperative nausea and vomiting rates were similar in both groups. At the end of the first year, % EWL was 82.9% and 73.5% in groups 1 and 2 (p=0.003).
Conclusion: Starting antrectomy at a distance of 2 cm or less from the pylorus is safe and effective. Starting antrectomy at a distance of 1 cm or less from the pylorus in LSG provides effective weight loss without increasing complications.
Key Words: Bariatric surgery, Antrectomy, Laparoscopic sleeve gastrectomy, Complications.
INTRODUCTION
With the increase in obesity and related metabolic problems in the world, the rate of bariatric-metabolic surgery applied to these patients is increasing. Although there are different types of procedures, nowadays the most common surgical procedure is laparoscopic sleeve gastrectomy (LSG).1 LSG is a restrictive surgery type which is an effective surgical method not only for the treatment of obesity but also for the treatment of obesity-related comorbid diseases.2
LSG is an effective method that has been used for a long time in the surgical treatment of morbid obesity. Despite its established efficacy and safety, controversies about optimal bougie size, distance of resection margin from the pylorus, the shape of section at the gastroesophageal junction, stapler line reinforcement, and intraoperative leak test are still ongoing.3,4
Sleeve gastrectomy is restrictive surgery and therefore, the smaller the operated stomach volume, the more weight loss can be considered. However, it can be thought that too much decrease in stomach volume can disrupt food tolerance and increase complications such as leaks by increasing intra-gastric pressure.5 According to the resection distance from the pylorus, different authors have adopted a resection distance of 2 to 8 cm from the pylorus.6-9
There are many studies about LSG in which antrectomy started from a distance of 2 cm close to the pylorus, but there is no study about distance less than 2 cm yet. In some survey studies, it was reported that some surgeons performed antrectomy starting less than 2 cm from pylorus, but there are no data about the outcomes of these.10,11 The aim of his study was to determine the effect of antrectomy in which resection was started from 2 cm or closer to the pylorus on % excess weight loss (%EWL) and frequency of complications were evaluated.
METHODOLOGY
Consecutive patients, who were treated for morbid obesity by laparoscopic sleeve gastrectomy during the period at Antalya Training and Research Hospital from April to December 2018, were eligible for the study. Patients’ files were retrospectively searched. Patients in whom gastrectomy had been started at a level of 2 cm or closer to pylorus were included in the study. Patients meeting the criteria of the 1991 National Institue of Health consensus for bariatric surgery were accepted for surgery, these criterias were a body mass index (BMI) higher than 40 Kg/m2 or BMI of 35–40 Kg/m2 with a major comorbid disease (type 2 diabetes, hypertension, dyslipidemia, obstructive sleep apnea). Patients who have gastroesophageal reflux or hiatal hernia detected in an endoscopic examination, patients undergoing additional surgical interventions with bariatric surgery, patients undergoing re-sleeve gastrectomy, and patients who did not admit for follow-up controls regularly for one year were excluded. Ethics Committee approval was obtained for the study.
Patients were divided into two groups based on the distance between the pylorus and the resection margin: group 1 had the resection started at a level less than or equal to 10 mm and group 2 longer than 10 mm from the pylorus. All patients were evaluated by taking history and clinical examination and laboratory investigations including basic preoperative investigations, thyroid, and suprarenal hormonal tests. Preoperative evaluation of the stomach with endoscopy was applied to rule out the presence of hiatal hernia or intrinsic lesions of the stomach or duodenum by the surgeon. Low molecular weight heparin was routinely administered subcutaneously 12 hours before the operation.
All patients underwent sleeve gastrectomy by the same general surgeon experienced in bariatric-metabolic surgery. The operations were performed under general anaesthesia. The patient was placed in a supine position and in reverse Trendelenburg with splitting legs. Five ports were placed into the abdominal cavity. Pneumoperitoneum was achieved with carbondioxide with a pressure of 13 mmHg. Dissection started with the opening of the greater curvature using a 5 mm vessel sealing device. The dissection then continued towards the gastroesophageal junction. Afterward greater curvature is dissected until the pylorus. After insertion of 38 French gastric calibration tubes into the stomach, the gastric division was started from a point as much as possible in proximity to pylorus using a 60-mm endo-stapler, and resection was completed (Figure 1). The transected stomach was then removed through the 12-mm left midclavicular port. The distance between the first stapler line and pylorus was measured with a marked grasper and recorded. Stapler line reinforcement was not used in any patient. A drain was placed next to the stapler line routinely.All patients were mobilised 6-8 hours after surgery. All patients were started a clear liquid diet at the first postoperative day. The drain was withdrawn at postoperative 5 to 7 days. Low molecular weight heparin was applied to the patients during hospitalisation and for another 10 days after discharge.
Patients were called for follow-up at the postoperative 1st, 3rd, 6th, and 12th months. At follow-up, weights were measured, and gastrointestinal complaints of patients such as nausea and vomiting were questioned and recorded. The %EWL was defined as lost weight / (preoperative weight–ideal body weight).
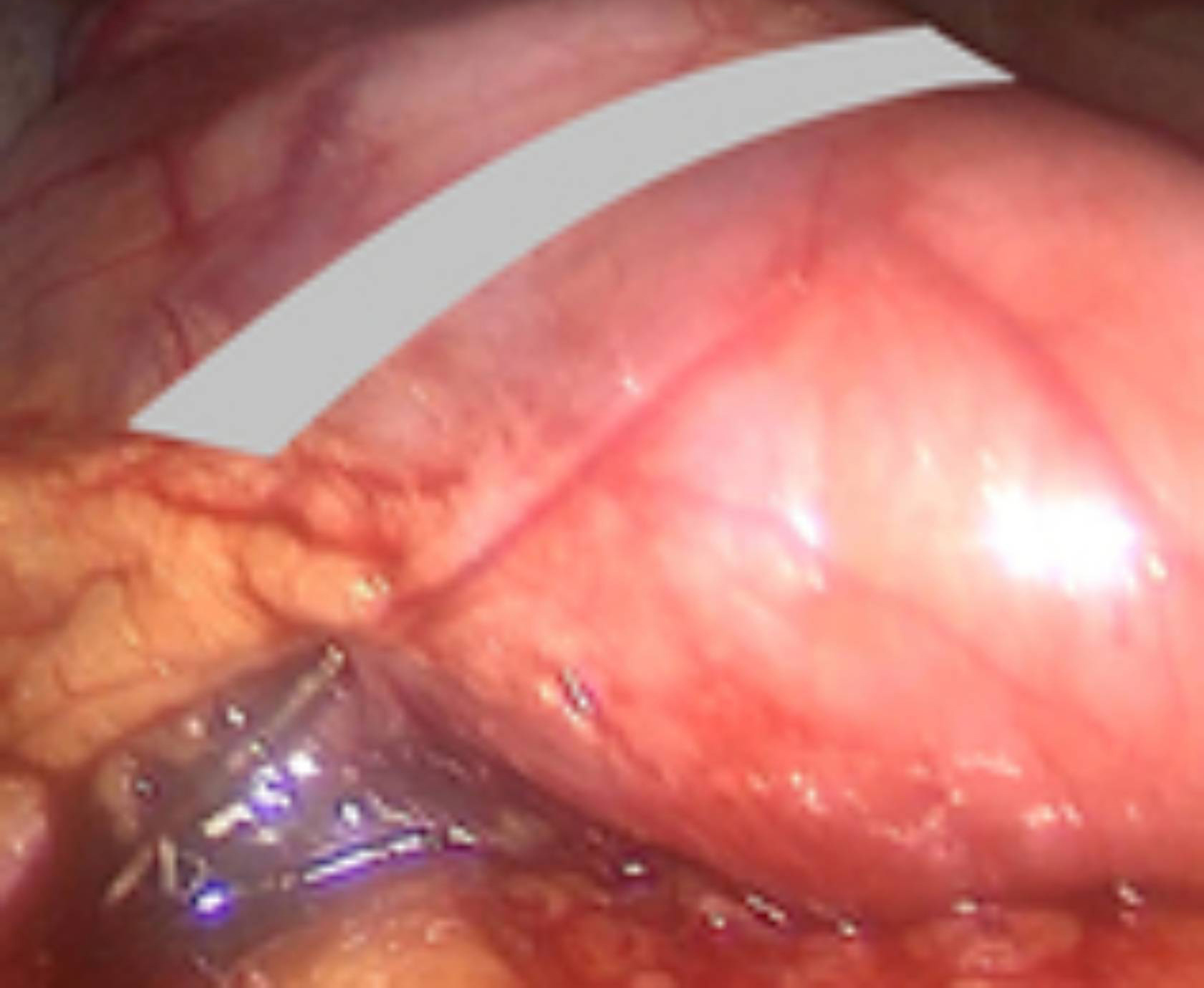 Figure 1: View of pylorus and stapler line. The gray area shows the location of the pylorus.
Figure 1: View of pylorus and stapler line. The gray area shows the location of the pylorus.
Preoperative data included age, gender, initial height, weight, initial BMI, operative data included duration of operation, intraoperative complications, length of hospitalisation, and postoperative follow-up data were recorded retrospectively from the patient files.
The primary outcome of the study was % EWL and the presence of nausea and vomiting. Secondary outcomes were duration of operation, length of postoperative hospitalisation period, mortality rate, reexploration rate, BMI, and presence of postoperative complications including gastric leakage, haemorrhage.
All obtained data were analysed via the statistical package for social science (SPSS version 22 (IBM Corporation, Chicago, IL) software program. Categorical varaibles were expressed as n (%). Comparisons of categorical variables between groups were conducted using the Pearson’s chi-square test. The normality of data distribution was determined by Kolmogorov-Smirnov test. Continuous variables with normal distribution were expressed as mean and SD and non-normally distributed variables were expressed as median and range. Differences between groups in normally distributed continuous variables were tested using the independent samples t-test, and the Mann-Whitney U-test was used for non-normally distributed variables. The p-value <0.05 was accepted as statistically significant.
RESULTS
Ninety-two patients meeting the criteria for the study and attending the follow-up regularly for a postoperative one-year period were included in the study. There were 44 patients in group 1 and 48 patients in group 2. The demographic characteristics of the patients are shown in Table I. All operations were completed laparoscopically. The duration of operation in group 1 was longer than in group 2 and the length of hospitalisation was similar in both groups (p=0.02, Table II). Bleeding, leak, and any other complications or mortality were not observed during hospitalisation period. Re-operation was not required during the postoperative period for any of the patients. Postoperative nausea and vomiting rates were similar in both groups. Nausea and vomiting in the early postoperative period were observed in 10 (22.7%) of patients in group 1 and 11 (22.9%) of patients in group 2 (p>0.99). Nausea had been continued at 2 patients in each group for 3 months and at just one patient from the second group after 6 months. Nausea and vomiting were recovered in other patients. In group 2, one patient was re-hospitalized two months after surgery, because of nausea and vomiting leading to nutritional insufficiency. Endoscopy was performed to a patient and the pathological finding was not detected. Nausea and vomiting completely resolved at the end of the 6th month in this patient.At the end of one year, BMI was lower in group 1, but this difference was not statistically significant (p=0.64). The %EWL in group 1 was statistically significantly greater than in group 2 (p=0.003, Table III).
Table I: Baseline characteristics of the two studied groups.
|
|
Group 1 |
Group 2 |
p |
|
Age (year) A |
34.7±11.1 |
39.5±11.6 |
0.049 a |
|
Sex (F/M) (n, %) |
34/10, 77.3/22.7 |
41/7, 85.4/14.6 |
0.315 b |
|
Height (cm) A |
166.8±9.5 |
161.1±7.1 |
0.002 a |
|
Weight (kg) B |
117 (94-216) |
132 (87-147) |
0.181 c |
|
BMI (Kg/m2) B |
43 (37.3-73.9) |
48.4 (37.7-58.9) |
0.462 c |
|
aIndependent t test / bPearson Chi Square test / cMann-Whitney U-test, AData are presented as mean ± standart deviation, BData are presented as median (ranges). |
|||
Table II: Perioperative data of the two studied groups.
|
|
Group 1 |
Group 2 |
p |
|
Hospital stay (day) A |
3 (2-5) |
3 (1-3) |
0.102 a |
|
Operation time (min) A |
50 (32-113) |
44 (28-118) |
0.020 a |
|
Distance between starting point of antrectomy and pylorus (mm) A |
10 (5-10) |
20 (12-20) |
0.001 a |
|
aMann-Whitney U test, AData are presented as median (ranges). |
|||
Table III: Postoperative changes in body weight, BMI, and %EWL at 1 year.
|
|
Group 1 |
Group 2 |
P |
|
Weight at 1 year (kg) A |
68 (50-115) |
70 (51-99) |
0.710 a |
|
BMI at 1 year (kg/m2) B |
25.9±4.6 |
27.6±4 |
0.064 b |
|
%EWL at 1 year B |
82.9±15.8 |
73.5±14.2 |
0.003 b |
|
aMann-Whitney U test / bIndependent t test, AData are presented as median (ranges), BData are presented as mean±standart deviation). |
|||
DISCUSSION
The purpose of LSG is to provide volume restriction by reducing the stomach volume as much as possible. On the other hand, short-term complications such as leak or long-term complications such as nutritional problems should be avoided. Over the years, technical procedures in sleeve gastrectomy have been changed. Today, many studies have shown that antrectomy performed during sleeve gastrectomy increases weight loss.8-12,13 There are different approaches about how far must be the distance between pylorus and antral division starting point.
In a survey study, Adil et al. showed that 73% of surgeons prefer a distance of 3–5 cm from the pylorus for the beginning of gastric transection.11 There is no sufficient data about how this distance is determined. Since it is demonstrated in the literature that antrectomy provides more weight loss in LSG. The authors changed the surgical technique over time, considering that LSG with a more radical antrectomy than the current application will provide more effective weight loss so then the author started gastric division as close as possible to the pylorus. This study is the first to reveal the results of patients who underwent LSG in which gastric division started at a distance of 2 cm or closer to pylorus. In this study, efficient weight loss was achieved without an increase in nausea, vomiting, and other complication rates.
Some authors stated that starting antrectomy very close to the pylorus will disrupt pylorus functions, therefore, increasing complications.14 However, there is not enough scientific data supporting this statement. In some studies examining gastric motility after LSG, motility changes were observed in patients who underwent antrectomy, but there were not any unfavorable effects on patient outcomes.9,15 In a randomised study, Abdallah et al. compared the patients in whom antral resection started at a distance of 2 cm and 6 cm from the pylorus and showed that weight loss was significantly more when antral resection starts 2 cm from the pylorus without a significant increase in the complication rate and they stated that antral resection is associated with better weight loss.16 In this study, nausea and vomiting rates in patients in whom gastrectomy had been started at a distance of 2 cm or more close to pylorus were found to be the same as in other studies. Postoperative complications and nausea and vomiting rates were not increased in patients who have had antrectomy started at a distance of 1 cm and closer from the pylorus, however, more weight loss was observed after 12 months in these patients.
In this study, starting antrectomy at a point 1 cm or closer to pylorus provided more efficient weight loss without causing an increase in complications determined in the comparative analysis. It may be considered that the restriction provided by more gastric resection through starting gastrectomy at a distance less than 2 cm from the pylorus will not make so much difference. The %EWL difference between groups may not be related to only increased restriction. Although LSG is a restrictive surgery, it is known that its effect on weight loss is not only with restriction but also by changing stomach motility and changing the secretion of various intestinal hormones. These changes also contribute to the remission of comorbid diseases.17,18 Therefore, this surgery provided weight loss not only with restriction but also due to some motility changes and gut hormone changes that occur after surgery. In this study, changes in postoperative gastric motility or intestinal hormones were not studied. Further studies about these subjects will reveal physiological changes occurring after surgery in patients in whom antrectomy was started 2 cm or more closely to the pylorus. In this study, it was observed that the ages of the patients were different in the two groups. Older patients may have different results.19 Age difference may be the reason for different weight loss between groups. To overcome this limitation, randomized studies are needed. As a surgical approach, it was aimed to start antrectomy as close as possible to pylorus, but technically this was not possible in every patient. In some patients dissection of the great curvature until the pylorus might be technically difficult. In such cases, we avoided additional dissections that would prolong the operation time and increase morbidity. The presence of previous intraabdominal surgery can make dissection more complicated near the pylorus likewise some of the patients in the study. Sometimes, even though dissection could be made up to the pylorus, resection from the aimed point could not be achieved due to trochar placement resulting inability to use the stapler efficiently. In some cases, anatomical thickening of the antral wall close to pylorus made the resection point far from pylorus because of concern about insufficient compression by stapler. For these reasons, although we always want to start antrectomy as close as possible to the pylorus, it may not be technically possible in some cases. This study has some limitations. It has some unfavorable effects due to being a retrospective study. We did not evaluate nutritional complications, resolutions of comorbid diseases. The follow-up period is restricted to one year in this study. So it is not possible to predict whether these results will reflect long-term results or not. For this purpose, the author continue to follow up with the present patient group. Randomised controlled trials and longer-term results will better demonstrate the effectiveness of this surgical approach. This study is the first to reveal mid-term results of starting antrectomy at a distance less than 2 cm from the pylorus in LSG. CONCLUSION Starting antrectomy at a distance of 2 cm or less from the pylorus is safe and effective. Starting antrectomy at a distance of 1 cm or less from the pylorus in LSG provides effective weight loss without increasing complications.ETHICAL APPROVAL:
Ethics committee approval was obtained for the study.
PATIENTS' CONSENT:
Informed and written consents were obtained from the patients to publish the data.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHOR’S CONTRIBUTION:
BM: Contribution to the design of the work, the acquisition, analysis / interpretation of data for the work, drafting the work, revising it critically for important intellectual content; final approval of the version to be published.
- English WJ, DeMaria EJ, Hutter MM, Kothari SN, Mattar SG, Brethauer SA, et al. American society for metabolic and bariatric surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg Obes Relat Dis 2020; 16(4):457-63. doi: 10.1016/j.soard. 2019.12.022.
- Schauer P, Bhatt D, Kirwan J. Bariatric surgery versus intensive medical therapy for diabetes. N Engl J Med 2014; 371(7):680-2. doi: 10.1056/NEJMc1407393.
- Ferrer-Marquez M, Belda-Lozano R, Ferrer-Ayza M. Technical controversies in laparoscopic sleeve gastrectomy. Obes Surg 2012; 22(1):182-7. doi: 10.1007/s11695-011-0492-0.
- Mayir B. Practices concerning sleeve gastrectomy in Turkey: A survey of surgeons. World J Gastrointest Surg 2021; 13(5):452-460. doi: 10.4240/wjgs.v13.i5.452.
- Avlanmis O, Isil RG, Burcu B. Effect of resection distance from pylorus on weight loss outcomes in laparoscopic sleeve gastrectomy. Obes Surg 2019; 29(9):2731-8. doi: 10.1007/s11695-019-03923-3.
- Hady HR, Olszewska M, Czerniawski M, Groth D, Pawluszewicz P, Diemieszczyk I, et al. Different surgical approaches in laparoscopic sleeve gastrectomy and their influence on metabolic syndrome: A retrospective study. Medicine (Baltimore) 2018; 97(4):e9699. doi: 10.1097/ MD.0000000000009699.
- Obeidat F, Shanti H, Mismar A, Albsoul N, Al-Qudah M. The magnitude of antral resection in laparoscopic sleeve gastrectomy and its relationship to excess weight loss. Obes Surg 2015; 25(10):1928-32. doi: 10.1007/s11695- 015-1642-6.
- Vives M, Molina A, Danús M, Rebenaque E, Blanco S, París M, et al. Analysis of gastric physiology after laparoscopic sleeve gastrectomy (LSG) with or without antral preservation in relation to metabolic response: A Randomised study. Obes Surg 2017; 27(11):2836-44. doi: 10.1007/s11695-017-2700-z.
- Michalsky D, Dvorak P, Belacek J, Kasalicky M. Radical resection of the pyloric antrum and its effect on gastric emptying after sleeve gastrectomy. Obes Surg 2013; 23(4):567-73. doi: 10.1007/s11695-012-0850-6.
- Lin S, Guan W, Hans P, Liang H. Status of laparoscopic sleeve gastrectomy in China: A national survey. Obes Surg 2017; 27(11):2968-73. doi: 10.1007/s11695-017-2727-1.
- Adil MT, Aminian A, Bhasker AG, Rajan R, Corcelles R, Zerrweck C, et al. Perioperative practices concerning sleeve gastrectomy - a survey of 863 surgeons with a cumulative experience of 520,230 procedures. Obes Surg 2020; 30(2):483-92. doi: 10.1007/s11695-019-04195-7.
- Robert M, Pasquer A, Pelascini E, Valette PJ, Gouillat C, Disse E. Impact of sleeve gastrectomy volumes on weight loss results: A prospective study. Surg Obes Relat Dis 2016; 12(7):1286-91. doi: 10.1016/j.soard.2016.01.021.
- McGlone ER, Gupta AK, Reddy M, Khan OA. Antral resection versus antral preservation during laparoscopic sleeve gastrectomy for severe obesity: Systematic review and meta-analysis. Surg Obes Relat Dis 2018; 14(6):857-64. doi: 10.1016/j.soard.2018.02.021.
- Parikh M, Issa R, McCrillis A, Saunders JK, Ude-Welcome A, Gagner M. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: A systematic review and meta-analysis of 9991 cases. Ann Surg 2013; 257(2): 231-7. doi: 10.1097/SLA.0b013e31826cc714.
- Sioka E, Tzovaras G, Perivoliotis K, Bakalis V, Zachari E, Magouliotis D, et al. Impact of laparoscopic sleeve gastrectomy on gastrointestinal motility. Gastroenterol Res Pract 2018; 2018:4135813. doi: 10.1155/2018/4135813.
- Abdallah E, El Nakeeb A, Youssef T, Abdallah H, Abd Ellatif M, Lotfy A, et al. Impact of extent of antral resection on surgical outcomes of sleeve gastrectomy for morbid obesity (a prospective randomised study). Obes Surg 2014; 24(10):1587-94. doi: 10.1007/s11695-014-1242-x.
- Sista F, Abruzzese V, Clementi M, Carandina S, Cecilia M, Amicucci G. The effect of sleeve gastrectomy on GLP-1 secretion and gastric emptying: A prospective study. Surg Obes Relat Dis 2017; 13(1):7-14. doi: 10.1016/j.soard. 2016.08.004.
- Pomerri F, Foletto M, Allegro G, Bernante P, Prevedello L, Muzzio PC. Laparoscopic sleeve gastrectomy- radiological assessment of fundus size and sleeve voiding. Obes Surg 2011; 21(7):858-63. doi: 10.1007/s11695-010-0255-3.
- Aslaner A, Ongen A, Kosar M, Çakır T, Mayir B, Doğan U, et al. Relation between weight loss and age after laparoscopic sleeve gastrectomy. Eur Rev Med Pharmacol Sci 2015; 19(8):1398-402.