Short-term Reproductive Outcomes of Nulliparous Women with Previous Ectopic Pregnancy
By Yildiz Akdas Reis, Erol Nadi Varli, Murat Levent Dereli, Turkan Dikici Aktas, Harun Egemen Tolunay, Salim ErkayaAffiliations
doi: 10.29271/jcpsp.2022.08.987ABSTRACT
Objective: To determine the characteristics and subsequent pregnancy outcomes in patients with a previous ectopic pregnancy (EP).
Study Design: Descriptive-cross sectional study.
Place and Duration of Study: Department of Obstetrics-Gynaecology, Etlik Zubeyde Hanim Maternity and Women’s Health Teaching and Research Hospital, Ankara, Turkey, between January 2014 and December December 2018.
Methodology: The data of nulliparous patients diagnosed with tubal ectopic pregnancy (EP) was analysed retrospectively. Reproductive outcomes within the first two years after ectopic pregnancy diagnosis were used as “short-term” reproductive outcomes. Their EP treatment and pregnancy outcome were determined.
Results: Expectant management was chosen in 5.8% of the patients, while the surgical intervention was 32.3%. Medical therapy involving methotrexate (MTX) was given to the remaining patients (61.9%). The tubal rupture was confirmed in 12% of the cases that received MTX. In the 2-year follow-up period after the ectopic event, the most common outcome of the subsequent pregnancies was a live birth (47.7%). Recurrent EP occurred in 4.6%.
Conclusion: The subsequent short-term pregnancy outcomes in this study were not related to the chosen treatment modality.
Key Words: Ectopic pregnancy, Nulliparity, Reproductive outcomes, Treatment modalities, Expectant management.
INTRODUCTION
Ectopic pregnancy (EP) occurs when the embryo implants outside the uterine cavity with a incidence between 1-2% in all the pregnancies.1,2 Tubal EP is the most common form affecting 95-99% of all the cases.3 Non-tubal EPs such as cervical, cesarean scar, cornual, ovarian, and abdominal pregnancies account for approximately 5% of all the cases. Furthermore, heterotopic pregnancies, a rare complication mostly related to the increasing use of assisted reproductive techniques in which both ectopic and intrauterine pregnancies occur simultaneously, can be seen in approximately 0.09% of the pregnancies.4 EP is the most common cause of maternal deaths in the first trimester.5,6
It may also play a causal role in reproductive morbidity and may influence future reproductive success rates.7 Although up to half of the patients do not have risk factors for EP, various risk factors such as advancing age, previous history of EP, pelvic infection, tubal surgery, smoking, and infertility have been identified.8-10 The diagnosis of EP usually relies on a combination of ultrasound scanning and serial measurement of serum human chorionic gonadotropin (hCG) levels. The main treatment options, expectant management, medication, and surgery can be applied considering multiple factors and conditions such as patient characteristics, hemodynamic stability, and the presence of comorbidities.
Since increasingly most women tend to conceive in their first pregnancy at an older age, particularly in well-developed countries, EPs are more common in later life.11 Besides, a sharp decline is seen in pregnancy rates of women, particularly at the age of their 40s.12 Therefore optimal management and the treatment of choice become even more important.
The aim of this study was to evaluate the characteristics of nulliparous women diagnosed with EP and their short-term reproductive outcomes after the chosen treatment modality.
METHODOLOGY
All the nulliparous women diagnosed with EP who attended the Department of Obstetrics-Gynaecology, Etlik Zubeyde Hanim Maternity and Women’s Health Teaching and Research Hospital, between January 2014 and December 2018, were identified. The local Ethics Committee granted its approval for the study's conduct, protocol, and procedures. After the approval, the medical files and computer-based data of the nulliparous patients treated for tubal EP between 2014 and 2018 were retrospectively evaluated. All the information, including sociodemographic variables, clinical, and medical characteristics such as the size of the adnexal mass, amount of the intraperitoneal free fluid in the pouch of Douglas, treatment modalities, and subsequent pregnancy outcomes were gathered. The study included a total of 260 nulliparous patients diagnosed with a tubal EP and investigated with short-term reproductive results defined as pregnancies after EP occurring within 18-24 months after the treatment.13 Pregnancy outcome measures that assess short-term reproductivity were recurrent EP, failure to achieve pregnancy, pregnancy loss, and live birth rate. The subsequent pregnancy outcomes were obtained from the patient files, using computer-based data and national health system data. Exclusion criteria was the patients who have already given birth, non tubal ectopic pregnancies, patients using post-treatment contraception, and patients whose medical records could not be reached or missing.
Before the data analyses, objective data cleaning criteria was applied such as range checks to detect anomalies and inaccuracies. Descriptive statistics are expressed using standard deviation, median, minimum and maximum for continuous variables, frequencies, and percentages for categorical variables. After the data were entered in the Excel file, transferred to IBM SPSS.23 program, and evaluated by statistical analyses. A p-value of less than 0.05 was considered to be statistically significant. The Kolmogorov-Smirnov test was used to assess normality assumption of continuous variables. The Kruskal-Wallis test and Mann-Whitney test were conducted to determine the statistical difference of the study variables according to the groups. In the event of a significant difference, the Mann-Whitney U-test with Bonferroni correction was used to reveal which groups differed. The chi-square was used to assess the relationship between categorical variables.
RESULTS
After applying exclusion criteria, 260 nulliparous patients with EP were included in this study. The patients were divided into three groups based on their final treatment modalities (expectant management, methotrexate therapy, and salpingectomy). Methotrexate (MTX) was used in 161 patients (61.9%). A total of 84 patients (32.3%) underwent salpingectomy, while the remaining 15 patients (5.8%) were managed expectantly (Figure 1).
Among 183 patients treated with MTX, 22 (12%) underwent urgent surgery who developed signs or symptoms of hemodynamic instability and ongoing intraperitoneal bleeding that suggest tubal rupture. Among patients in the MTX treatment group, 44 patients (24.1%) required a second dose of MTX, while only three patients (1.7%) needed a third dose (Table I).
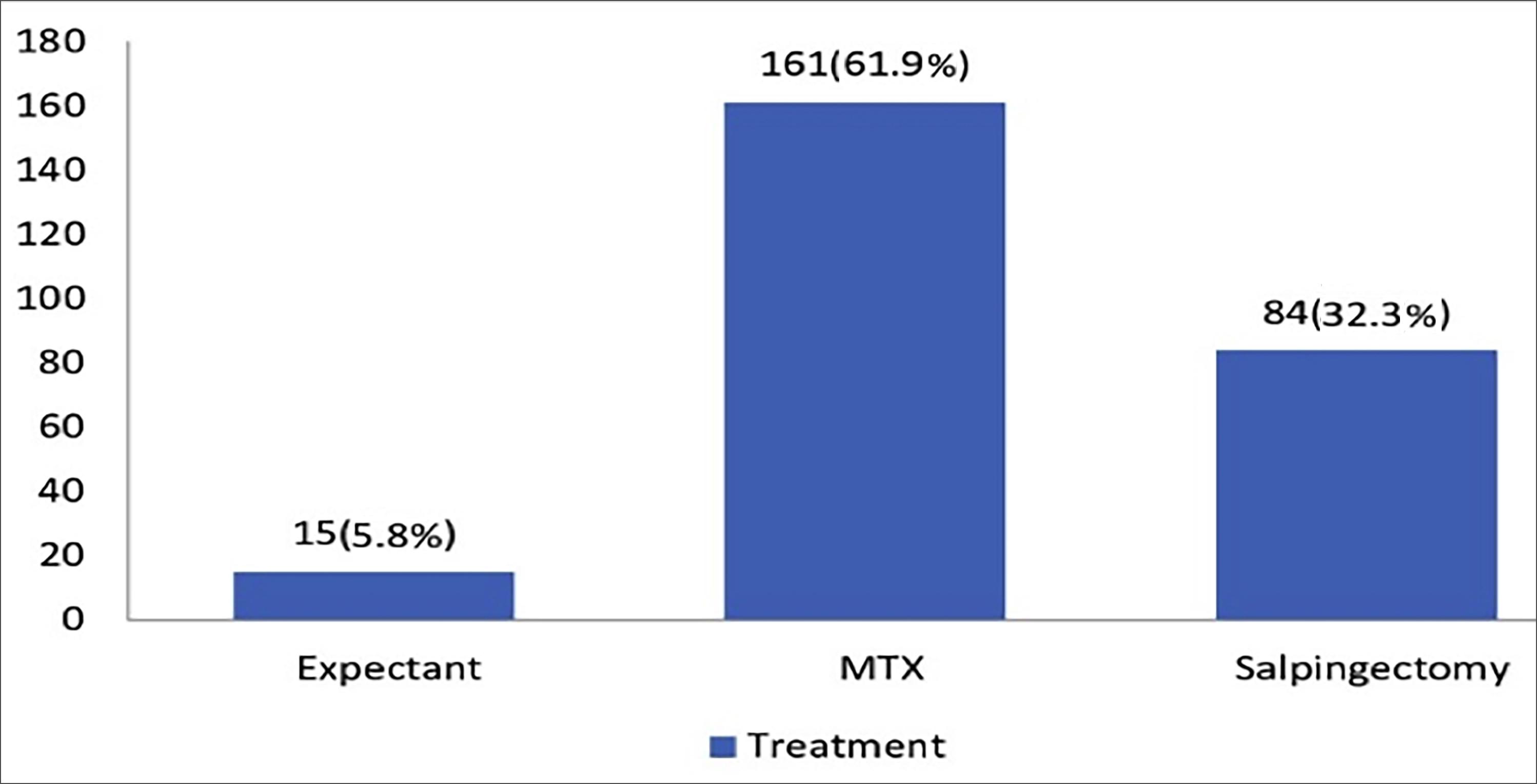 Figure 1: Frequency and percentage of treatment types.
Figure 1: Frequency and percentage of treatment types.
Table I: Frequency and percentage of methotrexate treatment results.
|
All patients |
Frequency |
Percentage (%) |
|
Methotrexate (MTX) |
||
|
No |
77 |
29.6 |
|
Yes |
183 |
70.4 |
|
Rupture after MTX |
|
|
|
No |
161 |
88 |
|
Yes |
22 |
12 |
|
2. Dose MTX |
|
|
|
No |
139 |
75.9 |
|
Yes |
44 |
24.1 |
|
3. Dose MTX |
|
|
|
No |
180 |
98.3 |
|
Yes |
3 |
1.7 |
A Kruskal–Wallis test indicated that the maximum adnexal mass diameter in patients of the three groups differed significantly, p <.001. Using a Bonferonni corrected p-value, post hoc Mann–Whitney tests exposed that the median maximum adnexal mass diameter in the salpingectomy group (n = 23) was higher than in the MTX group (16, p <.001).
The median amount of free intraperitoneal fluid differed significantly among the groups, p <.001. After using the Bonferonni p-value correction, Post hoc Mann–Whitney test indicated that the median amount of intraperitoneal free fluid in the surgery group (42) was significantly higher than the other two groups (p <.001, Table II). As seen in Table III, the subsequent pregnancy outcome was not related to treatment modalities (p = 0.134).
DISCUSSION
The choice of treatment in ectopic pregnancies has been determined by many studies and guidelines in the literature.14,15 Moreover, advances in diagnostic methods such as ultrasonography and laboratory measurements have enabled earlier and more accurate diagnosis which allows earlier treatment, thus reducing serious side effects and the need for the surgery.16,17 Besides, an earlier diagnosis gives practitioners more time to consider optimal treatment planning that may have minimal impact on future fertility.
Having a baby can interfere with women’s career and vice versa. Several factors such as financial security, travel, and not being emotionally and psychologically readiness discourage maternity and lead to late marriages, so the women become mothers later in life.
Table II: Values of some medical and sociodemographic parameters of patients according to the groups.|
Parameter |
Expectant n=15 Median (Min.-Max.) |
MTX n=161 Median (Min.-Max.) |
Salpingectomy n=84 Median (Min.-Max.) |
p-value |
|
Age* |
24(20-29) |
26(17-43) |
26(15-38) |
.265* |
|
BMI* |
22(17-36) |
24(15-37) |
22(16-35) |
.611* |
|
Adnexal mass* (max. diameter mm) |
18(5 -33) |
16(0-60) |
23(1-55) |
<.001* |
|
Intraperitoneal free fluid* (max. diameter mm) |
0(0-85) |
0 (0-211) |
42(0-130) |
<.001* |
|
*Kruskal-Wallis test. BMI: Body mass ındex, MTX: Methotrexate. |
||||
Table III: The frequency and percentages of subsequent pregnancy outcomes of the groups.
|
Subsequent pregnancy outcomes |
Expectant n=15 |
MTX n=161 |
Salpingectomy n=84 |
p-value |
|
None |
7 (46.7%) |
55 (34.2%) |
41 (48.8%) |
0.134 |
|
Live birth |
7 (46.7%) |
79 (49.1%) |
38 (45.2%) |
|
|
Abortion |
1 (6.7%) |
16 (9.9%) |
4 (4.8%) |
|
|
Ectopic pregnancy |
0 (0%) |
11 (6.8%) |
1 (1.2%) |
|
As a consequence of this trend, pregnancy incidence in advanced maternal age (35 years) has increased. Therefore, the risk of EP rises with the advancing age. On the other hand, assisted reproductive technologies, a risk factor for EP, also increased in this group. Many women facing these challenging circumstances may not have a chance to get pregnant again.
In this study, a total of 103 patients (39.6%) did not get pregnant within two years. The subsequent intrauterine pregnancy rate of patients with EP is about 60% in the literature.16 Besides, 12 patients (4.6%) out of 260 had a repeat EP, while 47.7% of the patients experienced a live birth in their subsequent pregnancies. In a cohort study, 69% of 2917 patients whose first pregnancy was ectopic had a live birth in their subsequent pregnancies.18 The absolute contraindications for conservative therapy (expectant management and MTX treatment) are the evidence of rupture with hemodynamic instability and patient preference. On the other hand, surgical options for tubal EPs include salpingostomy and salpingectomy, depending on the patient's current hemodynamic status, fertility expectations, and presence of tubal damage. Salpingectomy is the chosen surgical method particularly, in cases of tubal gestational sac greater than or equal to 5 cm, extensive tubal damage or rupture, ongoing bleeding, or prior tubal ligation for permanent contraception.19,20
In this study, the vast majority of the patients (61.9%) received MTX therapy, while only 5.8% of patients were managed expectantly. Surgery was performed in 32.3% of the patients. In the MTX treatment group, tubal rupture developed in 12% of the patients following MTX therapy. The risk of tubal rupture after MTX treatment ranges from 7-14%. There is no accurate tool to predict tubal rupture in patients receiving MTX therapy. Moreover, most but not all the patients with unruptured EP feel pelvic pain, and it is difficult to identify cases of ruptured EP based only on symptoms such as pelvic pain.20,21
Since medical and/or surgical intervention is required in most EPs, it is thought that future pregnancy outcomes can be affected by the chosen treatment approach. According to the results, the risk of EP recurrence in subsequent pregnancies was not related to the chosen treatment modality. In the literature, a study has shown that the risk of adverse pregnancy outcomes in salpingectomy is slightly increased than in salpingostomy.22 However, many studies show that patients receiving salpingostomy versus salpingectomy have comparable intrauterine pregnancy rates.23 Besides, persistent EPs may arise from the trophoblastic tissue due to the residual trophoblasts left after salpingostomy.24
Moreover, the choice of surgical procedure can also be determined by the surgeon's preference. At the study centre, salpingectomy was chosen for all the patients with EP who needed surgery after evaluating the advantages and disadvantages of all the methods described in the literature.
CONCLUSION
The most important thing that women, who diagnosed with EP and have no previous live birth, worry about whether they can get pregnant again. The short-term pregnancy outcomes were not related to the chosen treatment modality in the present study.
ETHICAL APPROVAL:
According to the Declaration of Helsinki, this study was conducted in compliance with the ethical principles, and the local Institutional Review Board approved it.
PATIENTS’ CONSENT:
Since it was designed as a retrospective study, the data were collected from the Hospital archive after approval of the Ethics Committee.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
YAR: Data acquisition and analysis, interpretation, drafting, and final approval.
ENV: Conception and design, interpretation, critical revision, and final approval.
MLD: Design, critical revision, and final approval.
TDA: Analysis and interpretation, drafting, and final approval.
HET: Interpretation, critical revision, and final approval.
SE: Interpretation, critical revision, and final approval.
REFERENCES
- Farquhar CM, Ectopic pregnancy. Lancet 2005. 366(9485):583-91. doi: 10.1016/S0140-6736(05)67103-6.
- ACOG Practice Bulletin No. 191: Tubal Ectopic Pregnancy. Obstet Gynecol 2018; 131(2): 65-77. doi: 10.1097/AOG. 0000000000002464.
- Bouyer J, Coste J, Fernandez H, Pouly JL, Job-Spira N. Sites of ectopic pregnancy: A 10 year population-based study of 1800 cases. Hum Reprod 2002; 17(12):3224-30. doi: 10. 1093/humrep/17.12.3224.
- Perkins KM. Boulet SL, Kissin DM, Jamieson DJ. Risk of ectopic pregnancy associated with assisted reproductive technology in the United States, 2001-2011. Obstetrics Gynecol 2015; 125(1):70-8 doi: 10.1097/AOG.0000000 000000584.
- Berg CJ, Chang J, Callaghan WM, Whitehead SJ. Pregnancy-related mortality in the United States, 1991-1997. Obstet Gynecol 2003; 101(2):289-96. doi: 10.1016/s0029- 7844(02)02587-5.
- Stulberg DB, Cain L, Dahlquist IH, Lauderdale DS. Ectopic pregnancy morbidity and mortality in low-income women, 2004-2008. Hum Reprod 2016; 31(3):666-71. doi: 10.1093/ humrep/dev332.
- Banz C, Chalvatzas N, Kelling K, Beyer D, Hornemann A, Diedrich K, et al. Laparoscopic management of ectopic pregnancy during a 9-year period. Fertil Steril 2010; 94(7):2780-2. doi: 10.1016/j.fertnstert.2010.04.051.
- Practice Committee of American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: A committee opinion. Fertil Steril 2013; 100(3):638-44. doi: 10.1016/j.fertnstert.2013.06.013.
- Barnhart KT, Clinical practice. Ectopic pregnancy. N Engl J Med 2009; 361(4):379-87. doi: 10.1056/NEJMcp0810384.
- Tsakiridis I, Giouleka S, Mamopoulos A, Athanasiadis A, Dagklis T. Diagnosis and management of ectopic pregnancy: A comparative review of major national guidelines. Obstet Gynecol Surv 2020; 75(10):611-23. doi: 10.1097/ OGX.0000000000000832.
- Goisis A, Remes H, Barclay K, Martikainen P, Myrskyla M. Advanced maternal age and the risk of low birth weight and preterm delivery: A within-family analysis using finnish population registers. Am J Epidemiol 2017; 186(11):1219- 26. doi: 10.1093/aje/kwx177.
- Hourvitz A, Machtinger R, Maman E, Baum M, Dor J, Levron J, et al. Assisted reproduction in women over 40 years of age: How old is too old? Reprod Biomed Online 2009; 19(4):599-603. doi: 10.1016/j.rbmo.2009.04.002.
- Bouyer J, Job-Spira N, Pouly JL, Coste J, Germain E, Fernandez H. Fertility following radical, conservative-surgical or medical treatment for tubal pregnancy: A population-based study. BJOG 2000; 107(6):714-21. doi: 10.1111/j.1471-0528.2000.tb13330.x.
- ACOG practice bulletin No. 193: Tubal ectopic pregnancy. Obstetrics Gynecol 2018; 131(3):91-103. doi: 10.1097/AOG. 0000000000002560.
- Naveed AK, Anjum MU, Hassan A, Nasir Mahmood S. Methotrexate versus expectant management in ectopic pregnancy: A meta-analysis. Arch Gynecol Obstet 2022; 305(3):547-53. doi: 10.1007/s00404-021-06236-y.
- Gervaise A, Masson L, de Tayrac R, Frydman R, Fernandez H. Reproductive outcome after methotrexate treatment of tubal pregnancies. Fertil Steril 2004; 82(2):304-8. doi: 10.1016/j.fertnstert.2004.04.023.
- Lou T, Gao Y, Feng Y, Lu J, Zhang Z, Bai H, et al. Reproductive outcomes of cesarean scar pregnancies pretreated with methotrexate and uterine artery embolisation prior to curettage. Taiwan J Obstet Gynecol 2020; 59(3):381-6. doi: 10.1016/j.tjog.2020.03.008.
- Karhus LL, Egerup P, Wessel Skovlund C, Lidegaard O. Long-term reproductive outcomes in women whose first pregnancy is ectopic: A national controlled follow-up study. Hum Reprod 2013; 28(1):241-6. doi: 10.1093/humrep/ des375.
- Alkatout I, Honemeyer U, Strauss A, Tinelli A, Malvasi A, Jonat W, et al. Clinical diagnosis and treatment of ectopic pregnancy. Obste Gynecol Surv 2013; 68(8):571-81. doi: 10.1097/OGX.0b013e31829cdbeb.
- Alur-Gupta S, Cooney LG, Senapati S, Sammel MD, Barnhart KT. Two-dose versus single-dose methotrexate for treatment of ectopic pregnancy: A meta-analysis. Am J Obstet Gynecol 2019; 221(2):95-108.e2. doi: 10. 1016/j.ajog.2019.01.002.
- Dudley PS, Heard MJ, Sangi-Haghpeykar S, Carson SA, Buster JE. Characterising ectopic pregnancies that rupture despite treatment with methotrexate. Fertil Steril 2004; 82(5):1374-8. doi: 10.1016/j.fertnstert.2004.03.066.
- Chouinard M, Mayrand MH, Ayoub A, Healy-Profitós J, Auger N. Ectopic pregnancy and outcomes of future intrauterine pregnancy. Fertil Steril 2019; 112(1):112-9. doi: 10.1016/j. fertnstert.2019.03.019.
- Cheng X, Tian X, Yan Z, Jia M, Deng J, Wang Y, et al. Comparison of the fertility outcome of salpingotomy and salpingectomy in women with tubal pregnancy: A systematic review and meta-analysis. PLoS One 2016; 11(3):0152343. doi: 10.1371/journal.pone.0152343.
- Zhang Y, Chen J, Lu W, Li B, Du G, Wan X. Clinical characteristics of persistent ectopic pregnancy after salpingostomy and influence on ongoing pregnancy. J Obstetrics Gynaecol Res 2017; 43(3):564-70. doi: 10. 1111/jog.13251.