Role of r-irisin in Nicotine-induced Oxidative Stress and Endothelial Dysfunction in BALB/c mice
By Madiha Sarwar, Sadia Ahsin, Gule Naghma Saeed, Hira AshrafAffiliations
doi: 10.29271/jcpsp.2022.09.1175ABSTRACT
Objective: To determine the protective role of irisin in attenuating nicotine-induced oxidative stress in vascular tissue in mice.
Study Design: Experimental study.
Place and Duration of Study: Foundation University, Islamabad, Pakistan, from January 2019 to June 2020.
Methodology: Thirty healthy BALB/c mice were divided into 3 groups. Group 1 was control, group II received nicotine 2 mg/Kg body weight intraperitoneally for 28 days, and group III, in addition, received r-irisin 0.5 μg/g body weight /day via tail vein, for the last 14 days. The tissue anti-oxidant enzymes (SOD, CAT, and GR) and lipid peroxidation marker (TBARS) were estimated. Aortic endothelium was analysed for atherosclerotic changes. The significant difference across groups was calculated using ANOVA.
Results: Group II showed statistically significant increase in lipid peroxidation marker (TBARS) levels (1059.04±32.31 ng/ml, p<0.001) and reduction in anti-oxidative enzymes (SOD, CAT and GR) levels (5479.24±25.38 pg/ml, 11.51±0.24 ng/ml and 1924.88±31.23 ng/ml, p<0.001) in aortic tissue homogenate as compared to group I. In Group III, with co- administration of r-irisin, significant improvement in antioxidant enzymes i.e. SOD, CAT, and GR levels (7958.70±110.54 pg/ml, 20.86±0.57 ng/ml, and 2897.18±52.93 ng/ml) and reduction in TBARS levels (239.14±19.90 ng/ml) was observed as compared to Group II (p<0.001). Endothelial damage manifested to type IV on histological examination. Co-administration of r-irisin in group III showed significant improvement in histological grading (only Type I and II lesions were seen).
Conclusion: Exogenous administration of irisin improves anti-oxidant enzyme levels, ameliorates nicotine-induced oxidative stress, and endothelial dysfunction in the BALB/c mice.
Key Words: Irisin/FNDC-5, Oxidative stress, Anti-oxidant enzymes, Endothelial dysfunction, Atherosclerosis.
INTRODUCTION
The byproducts of oxidation, such as hydrogen peroxide (H2O2), hydroxyl radical (OH-), and superoxide anion (O2-), are produced in body as a result of normal aerobic metabolism. These molecules are highly reactive with other biological molecules and are referred as reactive oxygen species (ROS).1 Under physiological conditions, an appropriate balance exists between the production and neutralisation of ROS. Mitochondrial antioxidant enzymes such as superoxide dismutase, catalases, glutathione peroxidases, and reductases, which are capable of alleviating oxidative stress by scavenging ROS.2 Oxidative stress is characterised by overwhelming production of ROS and decreased antioxidant defence mechanism leading to the disturbance in cellular homeostasis. It influences various aspects of cell physiology resulting in tissue damage, hence contributing in the pathogenesis of a wide variety of disease states.3,4
Various risk factors responsible for causing cardiovascular diseases, induce oxidative stress, and alter vascular endothelial cells capacity to maintain homeostasis, resulting in endothelial dysfunction.1 Vascular endothelial dysfunction (VED) is considered as an independent risk factor in initiation and progression of atherosclerotic disease. It is characterised by vasoconstriction, platelet aggregation, and oxidation of low density lipoproteins which influence monocyte adhesion and migration into the sub-endothelial space, their modification into macrophages and subsequent formation of foam cells.5
Irisin, a newly discovered novel adipo-myokine, is a peptide hormone known to be secreted by the skeletal muscle and adipose tissue in response to the exercise by proteolytic cleavage of transmembrane protein fibronectin type III (FNDC5).6 It has been hypothesised that irisin increases mitochondrial enzymes’ expression and improves pro oxidant-antioxidant balance, thus alleviates oxidative stress.7,8 Irisin recently gained great interest as a potential target to combat obesity and obesity-related metabolic disorders.9 Substantial high levels of circulating irisin have shown to enhance thermogenesis and increase glucose uptake by the skeletal muscles, thus alleviating hyperlipidemia and hyperglycemia.10
Table I: Comparison of aortic tissue superoxide dismutase (SOD), Catalase (CAT) glutathione reductase (GR), and TBARS levels among the three study groups along with pairwise comparisons.|
Variables |
Groups |
p-value (ANOVA) |
p-value (pairwise comparison) |
||||
|
Control Group (n=10)
|
Nicotine Group II (n=10) |
Nicotine + Irisin |
Control vs Nicotine I vs. II |
Control vs Nicotine + Irisin I vs. III |
Nicotine vs. Nicotine + Irisin II vs. III |
||
|
Tissue SOD pg/ml |
8044.60± 106.49 |
5479.24± 25.38 |
7958.70± 110.54 |
<0.001* |
<0.001* |
0.10 |
<0.001* |
|
Tissue CAT ng/ml |
21.44± 0.27 |
11.51± 0.24 |
20.86± 0.57 |
<0.001* |
<0.001* |
0.07 |
<0.001* |
|
Tissue GR ng/ml |
2960.00± 89.07 |
1924.88± 31.23 |
2897.18± 52.93 |
<0.001* |
<0.001* |
0.08 |
<0.001* |
|
Tissue TBARS ng/ml |
258.43± 8.44 |
1059.04± 32.21 |
239.14± 19.90 |
<0.001* |
<0.001* |
0.15 |
<0.001* |
|
The results are given in mean + SD. *p ≤0.05 considered significant; The groups’ mean was compared using ANOVA while comparison between the groups was done using post hoc Tukey’s HSD test. |
|||||||
|
Atherosclerotic lesion According to AHA |
Group I (n=10) |
Group II (n=10) |
Group III (n=10) |
|
Type 1 Initial lesion |
25% |
40% |
27% |
|
Type 2 Fatty streaks |
Not seen |
13% |
6% |
|
Type 3 Pre-atheroma |
Not seen |
20% |
3% |
|
Type 4 Atheroma |
Not seen |
26% |
Not seen |
|
Type 5 Fibro- atheroma |
Not seen |
Not seen |
Not seen |
|
Type 6 Thrombus |
Not seen |
Not seen |
Not seen |
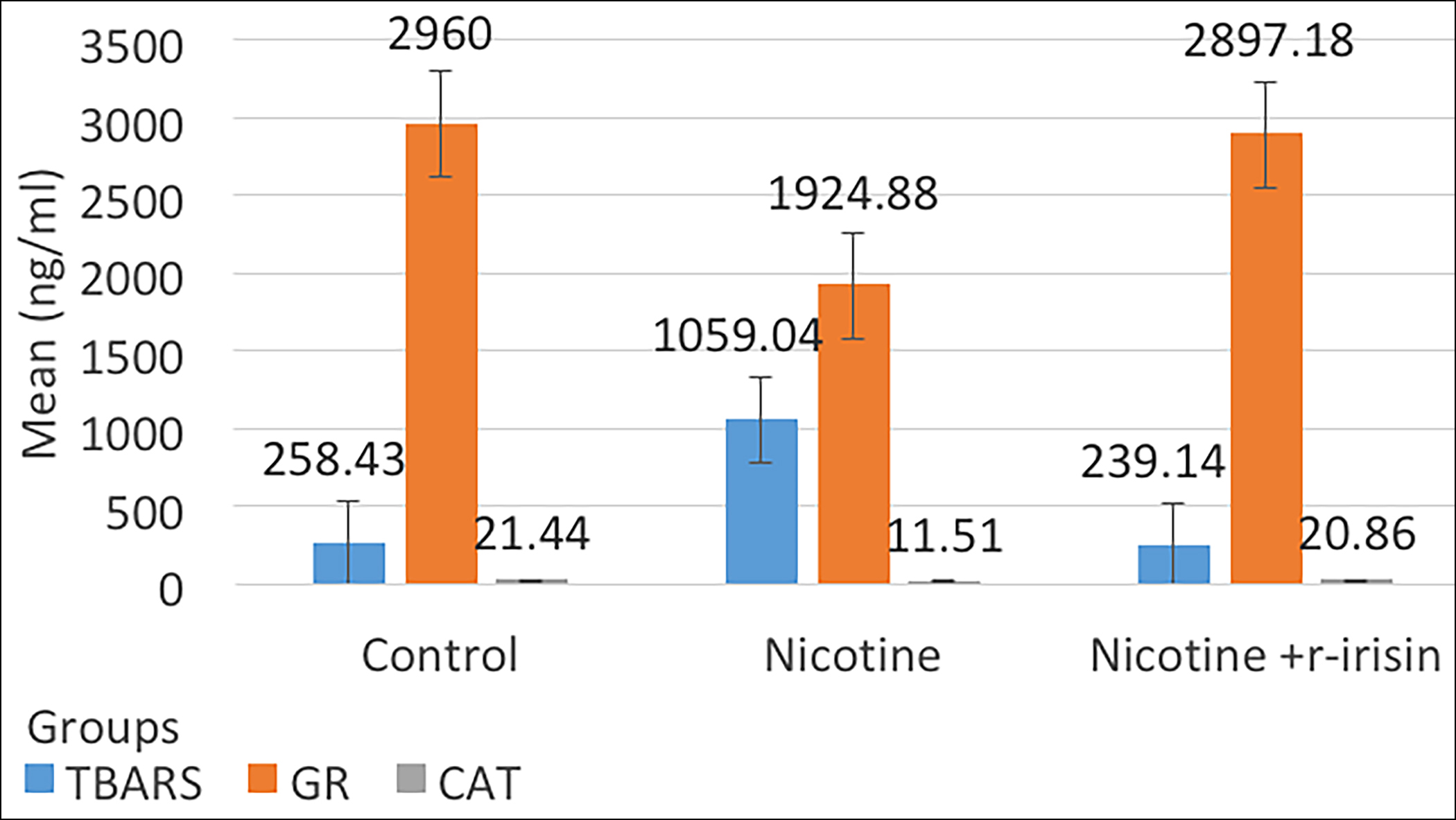 Figure 1: Comparison of mean values of aortic tissue levels of TBARS, GR, and CAT among three groups.
Figure 1: Comparison of mean values of aortic tissue levels of TBARS, GR, and CAT among three groups.
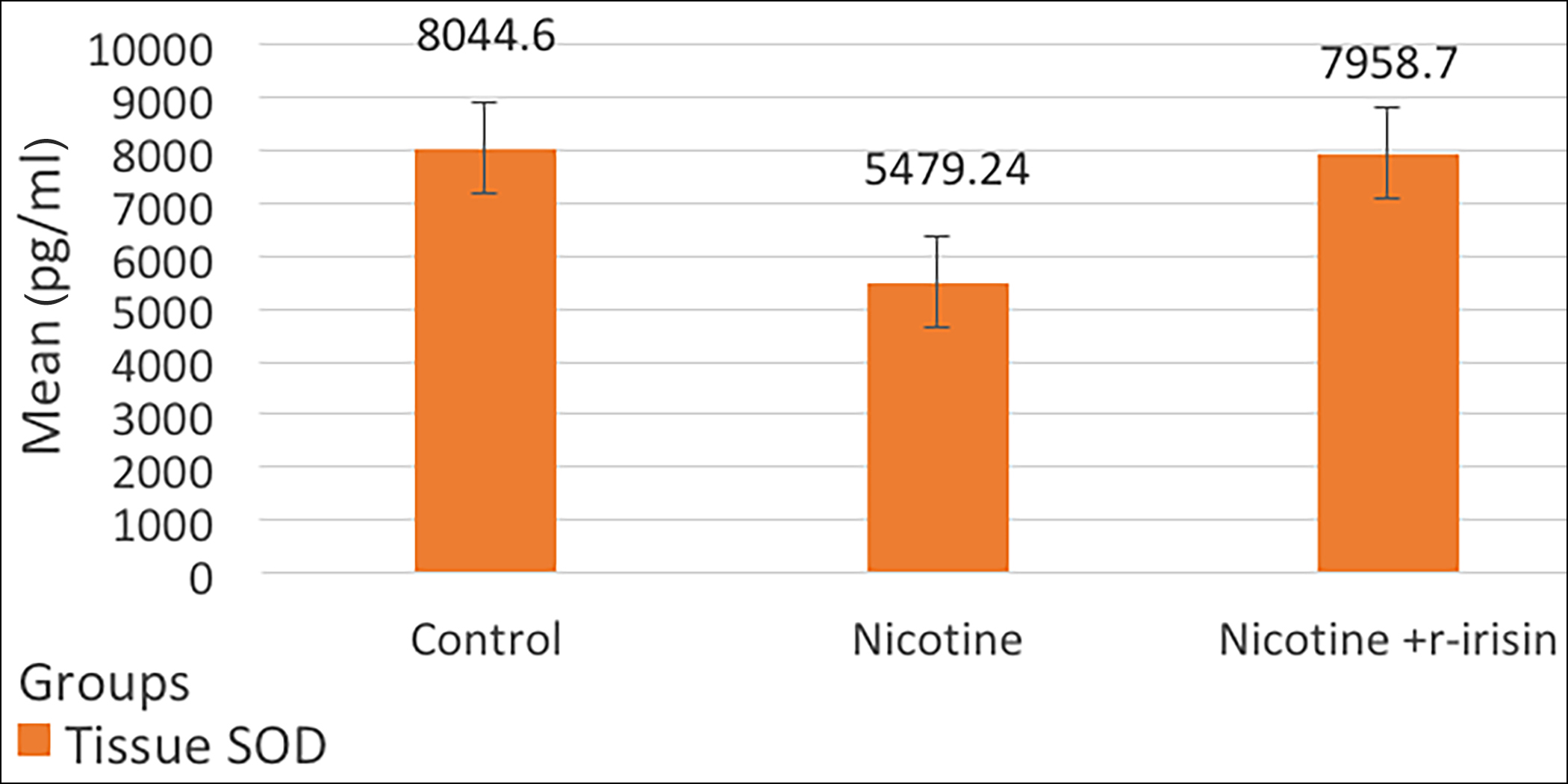 Figure 2: Comparison of mean values of aortic tissue SOD levels among three groups.
Figure 2: Comparison of mean values of aortic tissue SOD levels among three groups.
It has been presumed that irisin may play a role in promoting endothelial cell proliferation so it may have therapeutic effects in atherosclerotic diseases.11
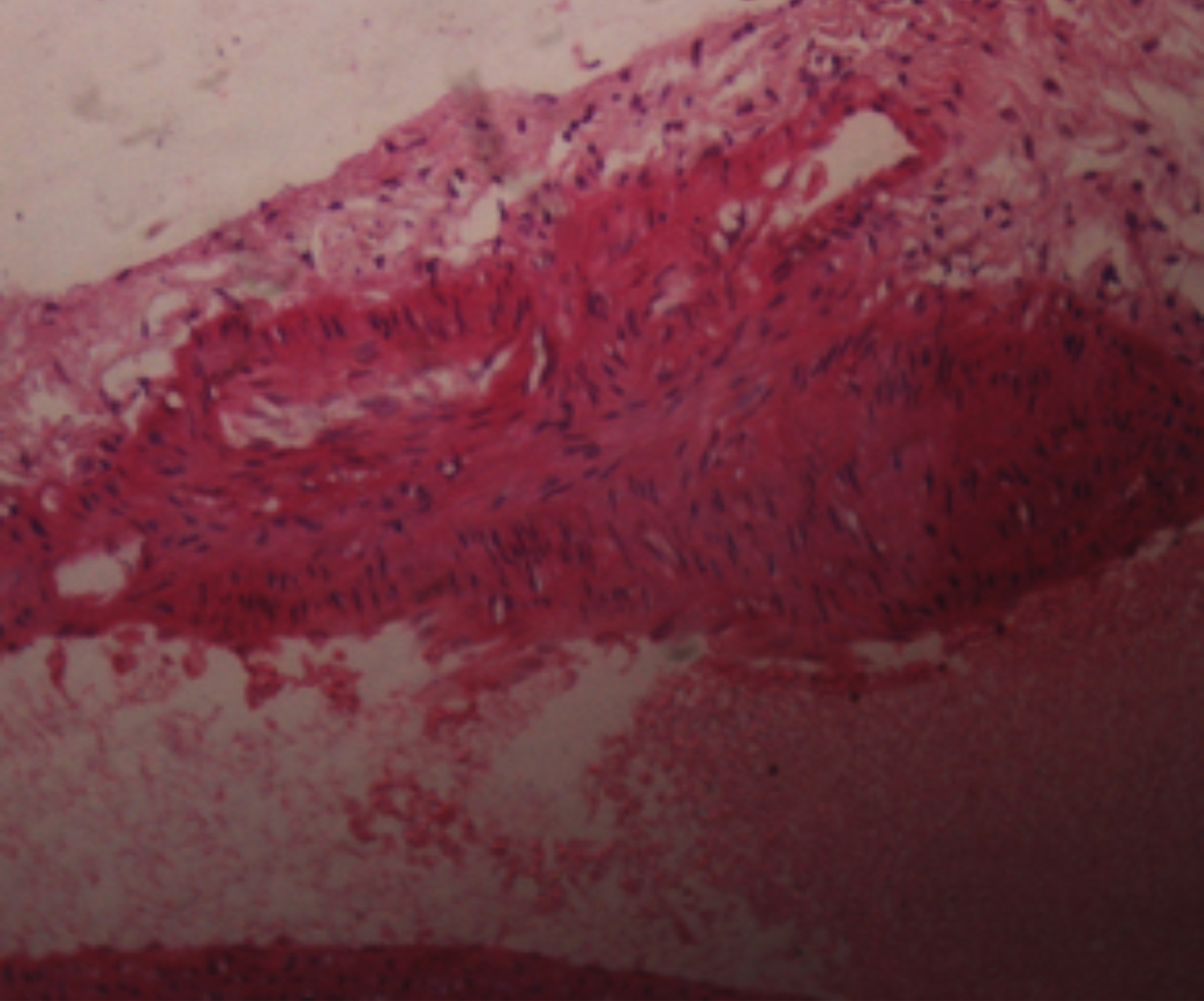 Figure 3: H and E staining (40X) nodular thickening of the vessel wall, smooth muscle hyperplasia, inflammatory cells.
Figure 3: H and E staining (40X) nodular thickening of the vessel wall, smooth muscle hyperplasia, inflammatory cells.
Nicotine exposure is associated with the multiple vascular complications resulting in cardiovascular pathologies, including coronary and peripheral vascular diseases. It enhances production of ROS, which triggers vascular endothelial damage by depleting antioxidant enzymes that result in accumulation of cytotoxic ROS and contribute towards VED. The prolonged administration of nicotine at a steady non-lethal low-dose in the rats has been shown to induce vascular endothelial damage.12 It impairs endothelium-dependent vasodilation, increase intima-media thickness, and proliferation of elastic lamellae leading to the endothelial dysfunction and atherogenesis.13
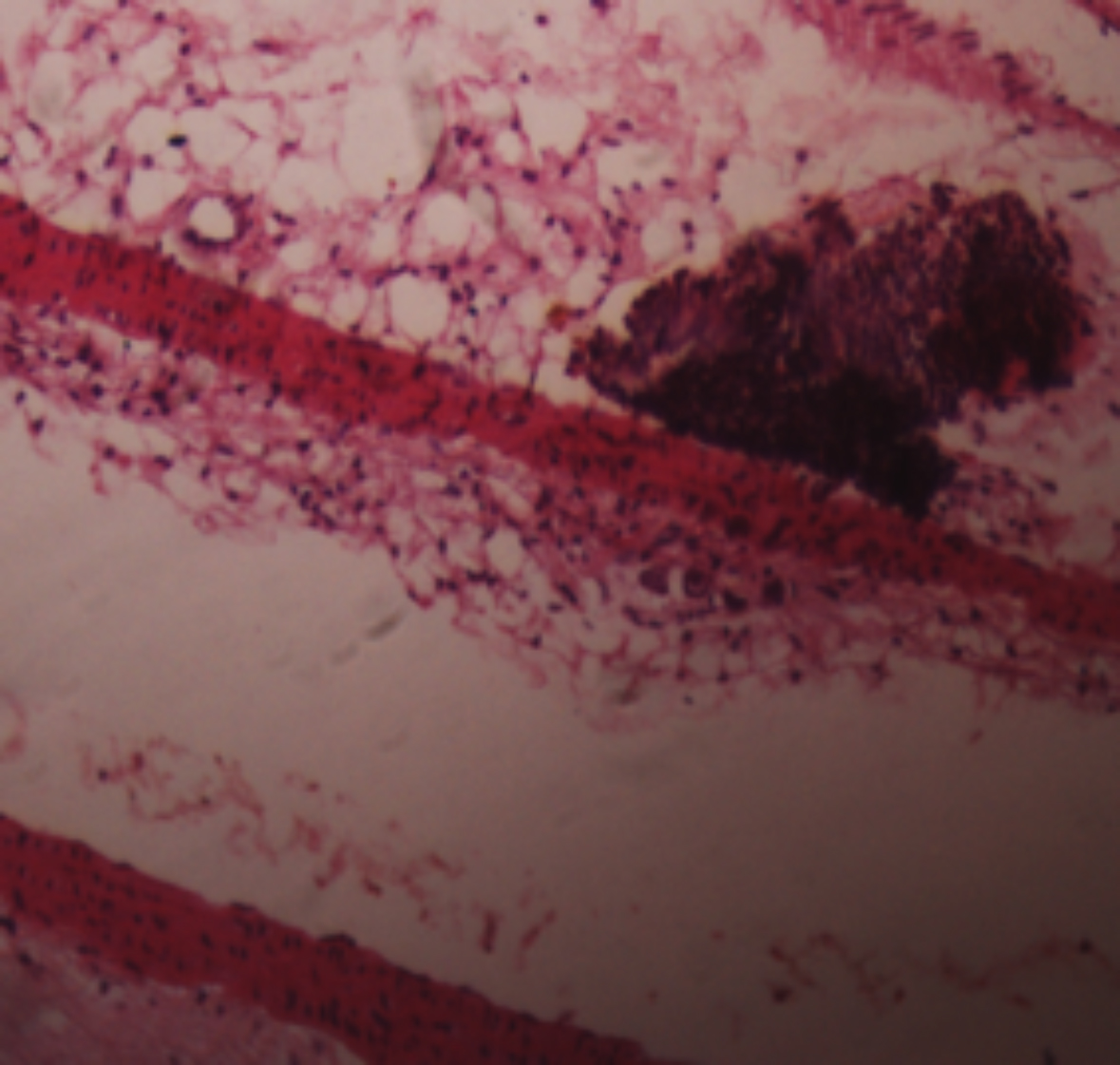 Figure 4: H and E staining (40X): Raised lesion, atheroma with foam cell histocytes, smooth muscles migration with increased EC matrix.
Figure 4: H and E staining (40X): Raised lesion, atheroma with foam cell histocytes, smooth muscles migration with increased EC matrix.
However, the antioxidant role of irisin in mitigating ischemic and inflammatory vascular injury has been sparsely studied. Therefore, this study was planned to evaluate the role of exogenous irisin in improving the redox state by assessing the aortic tissue antioxidant enzyme levels, along with the histological examination of aortic tissue in nicotine-induced oxidative stress and vascular endothelial damage in BALB/c mice.
METHODOLOGY
After the approval of synopsis from Research Evaluation Unit (REU) of CPSP and Ethical Review Committee of Foundation University Islamabad (FUI), an experimental study was carried out at the Physiology department FUI in collaboration with the National Institute of Health (NIH), Islamabad, from January 2019 to June 2020.
Sample size was calculated using G*Power method. Thirty healthy, non-diabetic, 08-10 weeks old male BALB/c mice weighing 20-25gms, were included in the study. Animals that developed diabetes or any disease during the course of study, were excluded from the study. Animals were divided into three groups. According to the ARRIVE guidelines for animal research, mice were housed in standard cages at the animal house of NIH in properly ventilated, controlled temperature conditions, and light/dark cycle.14
They were fed normal diet ad libitum prepared according to the standard laid down by Universities Federation for Animal Welfare.15
Group I mice received normal saline (1ml/Kg body weight) intra-peritonealy (I.P) daily for 28 days. Group II received nicotine (2 mg/Kg body weight) (I.P) for 28 days to induce oxidative stress12,16 obtained from the ‘Alfa Aesar, Johnson Matthey Company, Great Britain. Group III mice, in addition to nicotine for 28 days, also received r-irisin 0.5 μg/g body weight/day (Abbexa Ltd. Cambridge, UK) injected via tail vein injection, for the last 14 days.9,11,17 On 29th day, mice were euthanised and dissected to obtain aortic tissue. A vertical midline incision was made, skin was reflected laterally and abdominal cavity was opened up. Viscera were gently separated, rib cage was opened, and aorta was exposed and removed. Aortas were washed with cold saline, and were saved for the histological examination. Aortic tissue sections were made and stained with haematoxylin and eosin. The histological analysis of aortic endothelium for grading of atherosclerosis was done using the American Heart Association (AHA) classification of atherosclerosis.18
Aortic tissue homogenate was prepared using phosphate buffer saline. The tissue homogenate samples were centrifuged, supernatant was used for the analysis of antioxidant enzyme levels [superoxide dismutase (SOD) reduced glutathione (GR), and catalases (CAT)], and lipid peroxidation marker; thiobarbituric acid-reactive substances (TBARS)] using enzyme-linked immunosorbent assay (ELISA). The statistical analysis was done using SPSS version 24. Values were expressed as means ± SD. The significant difference in parameters across the groups was calculated using one-way ANOVA, followed by the post hoc Tukey’s HSD (Honestly Significant Difference) test for the multiple comparisons. A p-value of <0.05 was considered statistically significant.
RESULTS
The effect of nicotine and combined administration of nicotine and r-irisin on antioxidant enzymes and lipid peroxidation markers in aortic tissue is shown in Table I, and Figures 1 and 2. In group II, significant decrease in antioxidant enzymes levels was observed as compared to group I. Aortic tissue antioxidant enzyme levels i.e. SOD, CAT, and GR levels were significantly declined (p ≤0.001) in group II as compared to the group I. Oxidative stress was evident with the significant increase in aortic tissue lipid peroxidation marker (TBARS) levels (p≤0.001). Whereas, in group III, mice receiving r-irisin, antioxidant enzyme levels were significantly increased and TBARS levels were significantly reduced as compared to the group II. Statistically, there was no significant difference between group I and group III.
Endothelial dysfunction was characterised by the presence of inflammatory cells, advance lesion is characterised by the formation of foam cells, fatty streaks, and smooth muscle infiltration and atheroma.
Atherosclerotic lesions’ type among three groups according to the AHA classification is shown in Table II. In group I, 25% aortic sections showed Type I minimal change with isolated intimal scattered macrophage foam cells in sub-endothelial space indicating initial inflammatory response of endothelium. Whereas in aortic sections of group II, 40% exhibited Type I lesion, 13% showed Type II lesion characteried by the layers of macrophage foam cells, lipid-laden vascular smooth muscle cells with intracellular lipid accumulation forming fatty streaks. Type III lesion described as scattered collection of extracellular lipid droplets without a well-defined core and disruption of intimal smooth muscle cells, known as preatheroma, was seen in 20% cases. In 26% cases disruptive atheroma with well-defined lipid core characteristic and luminal surface covered by the normal intima, known as atheroma (Type IV), were seen (Figures 3 and 4). Histologically, partial reversal of atherosclerotic changes was observed in group III. Scattered foam cells were seen in the 27% cases. Fatty streaks and Type III lesions were only 6% and 3%, respectively. Based on AHA grading, group III had predominantly Type I lesions. Type V and Type VI lesions were not observed in any of the study groups.
DISCUSSION
Physically active individuals have lower rates of cardiovascular events due to the reduction in modifiable risk factors.19 The current study has evaluated potentially beneficial role of r-irisin in ameliorating nicotine-induced oxidative stress and endothelial dysfunction in mice due to oxidative stress, and endothelial damage caused by nicotine. Results showed r-irisin treatment up-regulated antioxidant enzymes’ levels, attenuated oxidative stress, and minimised oxidative stress-induced tissue damage by preventing atherosclerotic plaque formation.
Antioxidant enzyme assays of group III mice receiving r-irisin showed statistically significant improvement in SOD, CAT, and GR levels in aortic tissue homogenate and reduction in oxidative stress marker TBARS as compared to the group II mice receiving nicotine only. This anti-oxidative activity of irisin is probably a consequence of increased expression of key anti-oxidative enzymes which counteracted the harmful effects of ROS by up-regulating mitochondrial biogenesis. Bi et al., found antioxidant role of irisin in relieving oxidative stress. The treatment with irisin promoted mitochondrial biogenesis, decreased the levels of ROS and MDA, while increased levels of SOD and GSH-Px along with significant reduction in the levels of pro-inflammatory cytokines, relieving oxidative stress in hepatic tissue following ischemia/reperfusion (I/R) injury.20
Ren et al. assessed the protective role of irisin in attenuating oxidative stress in intestinal cells. Intra-peritoneal injection of irisin significantly attenuated oxidative stress by reducing intestinal MDA and increasing SOD and GSH levels, along with the effective reduction in inflammatory mediators such as IL-6 and TNF-α, thus protected against multiple organ damage.21 Wang et al., found that intravenous irisin treatment restored mitochondrial SOD activity in cardiomyocytes and alleviated oxidative stress.22 Similarly, Ho et al., showed that the irisin treatment improved mitochondrial function, increased SOD expression, and reduced myocardial infarct size.23 These studies strongly support this study results, where improvement in antioxidant enzyme activity and alleviation in oxidative stress were observed with exogenous irisin. The tissue used in this study was different as the authors used aortic tissue and oxidative stress was induced with parenteral nicotine. Other researches measured only SOD and lipid peroxidation markers while the present researches measured all the enzymatic antioxidants which are considered as major cellular defence against the free radicals.
One of the major manifestations of oxidative stress is lipid peroxidation. TBARS, end product of lipid peroxidation, is considered as a potent biomarker for oxidative stress and cellular damage due to ROS. In this study, administration of r-irisin in Group III significantly decreased TBARS levels, ameliorated lipid peroxidation and endothelial dysfunction, thus reinforcing protective nature of irisin against the oxidative injuries, as depicted by Schaalan et al. through exercise-medicated increased irisin levels with subsequent reduction in TBARS and improvement in oxidative status.24
Zhu et al. showed improvement in vascular endothelium with IP administration of irisin by relieving oxidative and nitrative stresses in type 2 Diabetes. In-vitro treatment of vascular endothelium with irisin, reduced oxidative stress by decreasing superoxide generation highlighting the protective role of irisin on endothelium in oxidative stress.9 In this study, the authors investigated in vivo effects of r-irisin on oxidative stress induced by the nicotine on aortic endothelium. The treatment with parenteral r-irisin for two weeks in the experimental animals relieved oxidative stress and decreased atherosclerotic lesions thus, suggesting a potential role of exogenous irisin in endothelial dysfunction and vascular complications.
In this study, AHA classification was used for the assessment of inflammatory response and grading of atherosclerotic lesion. Disruptive atheromas with well-defined lipid cores (26%) along with foam cells (40%) and lipid-laden vascular smooth muscle cells (13%) along with scattered extracellular lipid droplets were evident with nicotine. These changes were markedly reduced in group III animals who were injected r-irisin. They exhibited mainly type I (27%) lesion with scattered macrophages and foam cells on the histological examination of aortic tissue and only 9% exhibited type II and III lesions in comparison to group II where 33% mice exhibited type II and III lesions. These findings strongly establish the protective role of irisin in preserving endothelial function. The absence of advanced lesions like fibro-atheroma in any of these experimental animals could be due to the short duration of the study.
The results of this study are comparable with the study conducted by Zhu et al. on the diabetic rats. They analysed the role of irisin on endothelial progenitor cells (EPCs) functions and vascular complications of diabetes on human umbilical vein endothelial cells by culturing them with irisin, concluding that irisin reduced diabetes-induced oxidative stress on endothelial cells.9 They also incubated aortic segments exhibiting oxidative stress with irisin, resulting in endothelium-dependent vaso-relaxation with reduction in oxidative and nitrative stresses due to the decreased production of superoxide and peroxynitrite.9 Likewise, Lu et al. showed the protective role of irisin against endothelial dysfunction and atherosclerosis in diabetic mice, and in human umbilical vein endothelial cells where Irisin improved endothelial function, markedly decreased atherosclerotic plaque, inhibited glucose-induced apoptosis, increased antioxidant enzymes expression, and relieved oxidative stress in both samples.8 Similar findings were observed in 2018 by Deng et al.25
The disorganised, disrupted smooth muscle layer with a marked increase in lipid-laden foam cells were seen with nicotine whereas, administration of r-irisin restored antioxidant enzymes minimised inflammatory changes and improved endothelial histology. Based on this study’s findings and the literature analysis, it can be inferred that irisin has a protective effect on endothelium by curtailing oxidative stress, and if given for a longer duration, it may be more beneficial and may become a potential therapeutic agent for the treatment of atherosclerosis and vascular diseases in future.
The assessment of molecular mechanisms by which irisin regulates the synthesis of antioxidant enzymes at mRNA level, was beyond the scope of this study. Short duration of the study was another limitation.
CONCLUSION
Irisin appeared to ameliorate nicotine-induced oxidative stress and vascular dysfunction by improving antioxidant enzymes’ levels with corresponding decrease in membrane lipid peroxidation and severity of aortic tissue damage in BALB/c mice.
FUNDING:
The project was fully funded by Foundation University, Islamabad (FUI).
ETHICAL APPROVAL:
The study was carried out after formal approval of synopsis from Ethical Review Committee of Foundation University Islamabad (FUI) and Research Evaluation Unit (REU) of CPSP.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
MS: Conception and design of study, acquisition, analysis, interpretation of data, and drafting the manuscript.
SA: Substantial contributions to the conception and design of the work, and final approval of the version to be published.
GNS: Contribution in acquisition and interpretation of the data.
HA: Contribution in drafting and revising the content.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Imlay JA. Pathways of oxidative damage. Ann Rev Microbiol 2003; 57:395-418. doi: 10.1146/annurev.micro.57.030502. 090938.
- Hecksteden A, Wegmann M, Steffen A, Kraushaar J, Morsch A, Ruppenthal S, et al. Irisin and exercise training in humans - Results from a randomised controlled training trial. BMC Med 2013; 11:235. doi: 10.1186/1741-7015- 11-235.
- Polyzos SA, Mathew H, Mantzoros CS. Irisin: A true, circulating hormone. Metabolism 2015; 64(12):1611-8. doi: 10.1016/j.metabol.2015.09.001.
- Soto ME, Soria-Castro E, Lans VG, Ontiveros EM, Mejia BI, Hernandez HJ, et al. Analysis of oxidative stress enzymes and structural and functional proteins on human aortic tissue from different aortopathies. Oxid Med Cell Longev 2014; 2014:760694. doi: 10.1155/2014/760694.
- Bonomini F, Tengattini S, Fabiano A, Bianchi R, Rezzani R. Atherosclerosis and oxidative stress. Histol Histopathol 2008; 23(3):381-90. doi: 10.14670/HH-23.381.
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481(7382):463-8. doi: 10.1038/nature10777.
- Raschke S, Elsen M, Gassenhuber H, Sommerfeld M, Schwahn U, Brockmann B, et al. Evidence against a beneficial effect of irisin in humans. PLOS One 2013; 8(9): e73680. doi:10.1371/journal.pone.0073680.
- Lu J, Xiang G, Liu M, Mei W, Xiang L, Dong J. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 2015; 243(2):438-48. doi:10.1016/j.athero sclerosis.2015. 10.020.
- Zhu D, Wang H, Zhang J, Zhang X, Xin C, Zhang F, et al. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol 2015; 87:138-47. doi:10.1016/j.yjmcc.2015.07.015.
- Arhire LI, Mihalache L, Covasa M. Irisin: A hope in understanding and managing obesity and metabolic syndrome. Front Endocrinol (Lausanne) 2019; 10:524. doi:10.3389/fendo.2019.00524.
- Zhang Y, Song H, Zhang Y, Wu F, Mu Q, Jiang M, et al. Irisin inhibits atherosclerosis by promoting endothelial proliferation through microRNA126-5p. J Am Heart Assoc 2016; 5(9):004031. doi.org/10.1161/JAHA.116.004031.
- Si LY, Kamisah Y, Ramalingam A, Lim YC, Budin SB, Zainalabidin S. Roselle supplementation prevents nicotine-induced vascular eothelial dysfunction and remodelling in rats. Appl Physiol Nutr Metab 2017; 42(7):765-72. doi: 10.1139/apnm-2016-0506.
- Zhang S, Day I, Ye S. Nicotine induced changes in gene expression by human coronary artery endothelial cells. Atherosclerosis 2001; 154(2):277-83. doi:10.1016/s0021- 9150(00)00475-5.
- Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The arrive guidelines 2.0. Updated guidelines for reporting animal research. J Cereb Blood Flow Metab 2020; 40(9):1769-77. doi: 10.1371/journal.pbio. 3000410.
- Hubrecht RC, Kirkwood J., editors. The UFAW handbook on the care and management of laboratory and other research animals: John Wiley & Sons; 2010 Jan 19.
- Chakkarwar VA. Fenofibrate attenuates nicotine-induced vascular endothelial dysfunction in the rat. Vascul Pharmacol 2011; 55(5-6):163-8. doi: 10.1016/j.vph.2011. 08.215.
- Zhang Y, Mu Q, Zhou Z, Song H, Zhang Y, Wu F, et al. Protective effect of Irisin on atherosclerosis via suppressing oxidised low density lipoprotein induced vascular inflammation and endothelial dysfunction. PLoS One 2016; 11(6): e0158038. doi: 10.1371/journal.pone.0158038.
- Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, et al. A definition of advanced types of athero-sclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, American heart association. Arterioscler Thromb Vasc Biol 1995; 15(9): 1512-31. doi: 10.1161/01.CIR.92.5.1355.
- D'Agostino RB, Pencina MJ, Massaro JM, Coady S. Cardiovascular disease risk assessment: Insights from framingham. Glob Heart 2013; 8(1):11-23. doi: 10.1016/j. gheart.2013. 01.001.
- Bi J, Zhang J, Ren Y, Du Z, Li Q, Wang Y, et al. Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol 2019; 20:296-306. doi: 10.1016/j.redox.2018.10.019.
- Ren YF, Wang MZ, Bi JB, Zhang J, Zhang L, Liu WM, et al. Irisin attenuates intestinal injury, oxidative and endoplasmic reticulum stress in mice with L-arginine-induced acute pancreatitis. World J Gastroenterol 2019; 25(45):6653-67. doi: 10.3748/wjg.v25.i45.6653.
- Wang Z, Chen K, Han Y, Zhu H, Zhou X, Tan T, et al. Irisin protects heart against ischemia-reperfusion injury through a SOD2-dependent mitochondria mechanism. J Cardiovasc Pharmacol 2018; 72(6):259-69. doi:10.1097/FJC.000000 0000000608.
- Wang H, Zhao YT, Zhang S, Dubielecka PM, Du J, Yano N, et al. Irisin plays a pivotal role to protect the heart against ischemia and reperfusion injury. J Cell Physiol 2017; 232(12):3775-85. doi: 10.1002/jcp.25857.
- Schaalan MF, Ramadan BK, Abd Elwahab AH. Synergistic effect of carnosine on browning of adipose tissue in exercised obese rats; a focus on circulating irisin levels. J Cell Physiol 2018; 233(6):5044-57. doi: 10.1002/jcp.26370.
- Deng X, Huang W, Peng J, Zhu TT, Sun XL, Zhou XY, et al. Irisin alleviates advanced glycation end products-induced inflammation and endothelial dysfunction via Inhibiting ROS-NLRP3 inflammasome signaling. Inflammation 2018; 41(1):260-75. doi:10.1007/s10753-017-0685-3.