Role of Ondansetron in Reducing Methotrexate Intolerance in Patients with Inflammatory Arthritis
By Saba Saif1, Spenta Kakalia2, Rizwana Kitchlew1, Haseeb Ahmed Khan3, Samina Fida1, Muhammad Siddique1Affiliations
doi: 10.29271/jcpsp.2022.10.1308ABSTRACT
Objective: To determine the frequency of intolerance to Methotrexate (MTX) in patients with inflammatory arthritis by using MTX intolerance severity score, and evaluate the effects of Ondansetron in reducing MTX intolerance.
Study Design: Interventional study.
Place and Duration of Study: Rheumatology clinic, Combined Military Hospital (CMH), Lahore, from 1st November 2021 to 30th April 2022.
Methodology: Patients with inflammatory arthritis taking methotrexate regularly for >3 months participated in the study. The patients’ age, gender, education level, marital status and smoking history were documented. The disease duration and disease activity was also recorded. Dose/duration/route/frequency and timing of MTX were noted. MTX intolerance was calculated with the use of the Methotrexate intolerance severity score (MISS) questionnaire. Those MTX intolerant patients who reported nausea and vomiting were prescribed ondansetron along with MTX and were followed up for the next 3 consecutive months.
Results: Out of 181 patients, 48(26.5%) showed methotrexate intolerance. The predominant symptom was nausea after taking MTX reported in 93.8% of the MTX intolerant patients followed by behavioural symptoms including restlessness and irritability reported among 79% and 77% of intolerant patients respectively. Those methotrexate intolerant patients who mainly had complaints of nausea and vomiting were started on ondansetron on the day of methotrexate and showed a significant reduction in the median of MISS score in the following two consecutive months (p <0.05) while at 3 months the median did not show further reduction as compared to second month (p=0.12).
Conclusion: Ondansetron prescribed along with methotrexate in patients having complaints of nausea and vomiting with MTX, reduces the intolerance significantly.
Key Words: Rheumatoid arthritis, Methotrexate, Ondansetron, Nausea, Arthritis juvenile.
INTRODUCTION
Rheumatoid arthritis (RA), is the most frequently occurring autoimmune chronic polyarticular disease found in 0.5 to 1% of the population worldwide. The prevalence of RA in developing countries is variable; in urban Karachi, it is about 0.142%, in Northern Pakistan 0.55%, and in India, it is 0.75%.1
Since 1980s, the treatment of choice for inflammatory arthritis has been Methotrexate (MTX), because of its potent efficacy, low-cost and good tolerability.1
It may be used alone or in combination with other DMARDS both conventional and biologics in patients who do not have a sufficient response to methotrexate.2 MTX stops the radiographic development in almost 33% of patients with inflammatory arthritis by taking regular low dose MTX (10-25mg/week) after 1 year of treatment.3
The disagreeable troublesome symptoms mostly, nausea and vomiting, experienced by some patients using MTX are referred to as MTX intolerance. Frequency of MTX Intolerance in RA is 11-33% and the incidence is much higher in children reaching up to 50% for juvenile idiopathic arthritis (JIA).4,5
There are two mechanisms of GI intolerance with MTX. Firstly, the oral and intestinal epithelium is sensitive to MTX, which increases with the accumulation of MTX over time, irrespective of the folate deficiency. Secondly, stimulation of the chemotactic trigger zone (CTZ) occurs. These symptoms of intolerance can also be triggered by associated stimuli, like the smell of alcohol cleansing swabs, or exposure to yellow-coloured liquid. This is called anticipatory nausea which is thought to be mainly due to a Pavlovian phenomenon.6 MTX intolerance usually evolves earlier within a year of treatment initiation but how it progresses over time is unknown.7
To assess MTX intolerance, different scoring systems are developed like Gastrointestinal Score for Kids (GISSK), Methotrexate Intolerance Severity Score (MISS)6 and Methotrexate Intolerance and Severity assessment in Adults (MISA)8 questionnaire. MISS was mainly validated in children but has been used in adults. Recently a patient-centred perspective scale known as the patient-perceived methotrexate intolerance scale (PPMIS) has been validated in RA patients which includes treatment benefits and risks, disease risks, and willingness to take MTX regardless of risks.9
Various strategies have been tried to overcome GI intolerance to MTX. This includes the use of folate, various anti-emetics, switching from oral to subcutaneous MTX and behavioural therapy. Anti-emetics have been used to treat anticipatory nausea. In RA and JIA, ondansetron is effective in treating nausea associated with MTX.10 It decreases the duration and intensity of nausea from the very first week of initiation and improvement is maintained during follow-up.11
The aim of this study was to evaluate the utility of ondansetron in subjects who have GI intolerance to MTX.
METHODOLOGY
This research project was conducted in the Rheumatology OPD of Combined Military Hospital (CMH), Lahore, from 1st November to 30th April 2022. The sample size was calculated using the frequency of MTX intolerance as 21.6%, CI as 95% and margin of error as 6%.12 Total 181 patients between 14-60 years with inflammatory arthritis and using MTX (oral or parenteral) regularly for >3 months were included in this interventional study. MTX dose was as prescribed by the rheumatologist. These patients were labelled as having JIA as per ILAR criteria and RA as per 2010 ACR/EULAR criteria.
Written informed consent of the subjects or guardians where applicable, were obtained. The patient’s demographic details were collected. The dose/duration/route of MTX, and frequency and timing of MTX were noted. Non-compliant patients were excluded from the study. These are the patients who have been started on any medication for a specific indication by a doctor but discontinued it on their own without a reason and this has happened twice or more in the past.
Similarly, patients with history of peptic ulcer disease or GI complaints prior to starting MTX or having impaired cognitive function/ or psychiatric illness were excluded. Those who had known Ondansetron allergy or congenital long-QT syndrome were also excluded from the study. Baseline ECG was done in all patients to avoid any complications.
Patient pain visual analogue score (VAS) was noted and clinical examination was performed to ascertain disease activity and MISS was calculated using MISS questionnaire. The questionnaire is made up of four domains; including GI and behavioural issues. The MISS questionnaire collects information on whether the GI symptoms occurred before or after taking or upon thinking of MTX. For each of these items, the intensity could range from 0 (none) to 3 (severe). The duration of symptoms, could last from a few hours to more than a day and constantly. The minimum score is 0 and the maximum is 36 points. To be labelled as MTX intolerant, the patient has to have a score of >6, with no less than one anticipatory, associative, or behavioural manifestation.12
Out of these MTX intolerant patients, only those patients who complained of nausea and vomiting were prescribed premedication with oral Ondansetron 8mg (4 mg if less than 30 kg) 1 hour before taking MTX and then repeat dose 8 hourly (maximum 2 doses) if the patient was awake. The patients were followed up every 4 weeks for 3 visits and on each visit, their MISS was calculated. The patients were assessed by their treating rheumatologist and monitored for any drug side effects. Complete blood picture, baseline profile, ESR, and urine routine were done every four weeks.
Data were analysed using SPSS-26. Age was presented as means with standard deviation. Disease duration and duration on MTX were presented as median. Variables like gender, dose and route of MTX and adverse effects experienced by patients were presented as frequency and percentages. Baseline MISS was compared with the total MISS calculated over the following next 3months using a Mann-Whitney test. The level of significance was p ≤0.05.
RESULTS
Total subjects included were 181. The mean age was 43.18 + 12.3 years. Most patients were females 139 (76.8%) while only 42 (23.2%) were male (Table I). The median duration of illness was 6 years (range=1-40) while the median duration of MTX intake was 12 months, ranging from 3 to120 months. Out of 181 patients, 76 (42%) were using other DMARDS as well.
Median of the total MISS score was 6 (0-20). MTX intolerance started after 6.05+11.3 months. The frequency of MTX intolerance was 48(26.5%). Behavioural symptoms were one of the major adverse effects experienced by the patients followed by nausea, abdominal pain, and vomiting (Figure 1).
Table I: Demographic data.
|
Characteristics |
|
N (%) |
|
Gender |
Female |
139 (76.8%) |
|
Male |
42 (23.2%) |
|
|
Age group |
<30yrs |
23 (12.7%) |
|
30yrs-50yrs |
87 (48%) |
|
|
>50yrs |
71 (39%) |
|
|
Education |
Matric or less |
71 (39.3%) |
|
Inter or more |
110 (60.8%) |
|
|
Disease duration |
<3years |
61 (33.7%) |
|
>3years |
120 (66.3%) |
|
|
MTX route |
Oral |
159 (87.8%) |
|
Injectable |
22 (12.2%) |
|
|
MTX dose |
<10mg/wk |
83 (46%) |
|
>10-<20mg/wk |
44 (24.3%) |
|
|
>20mg/wk |
54 (29.8%) |
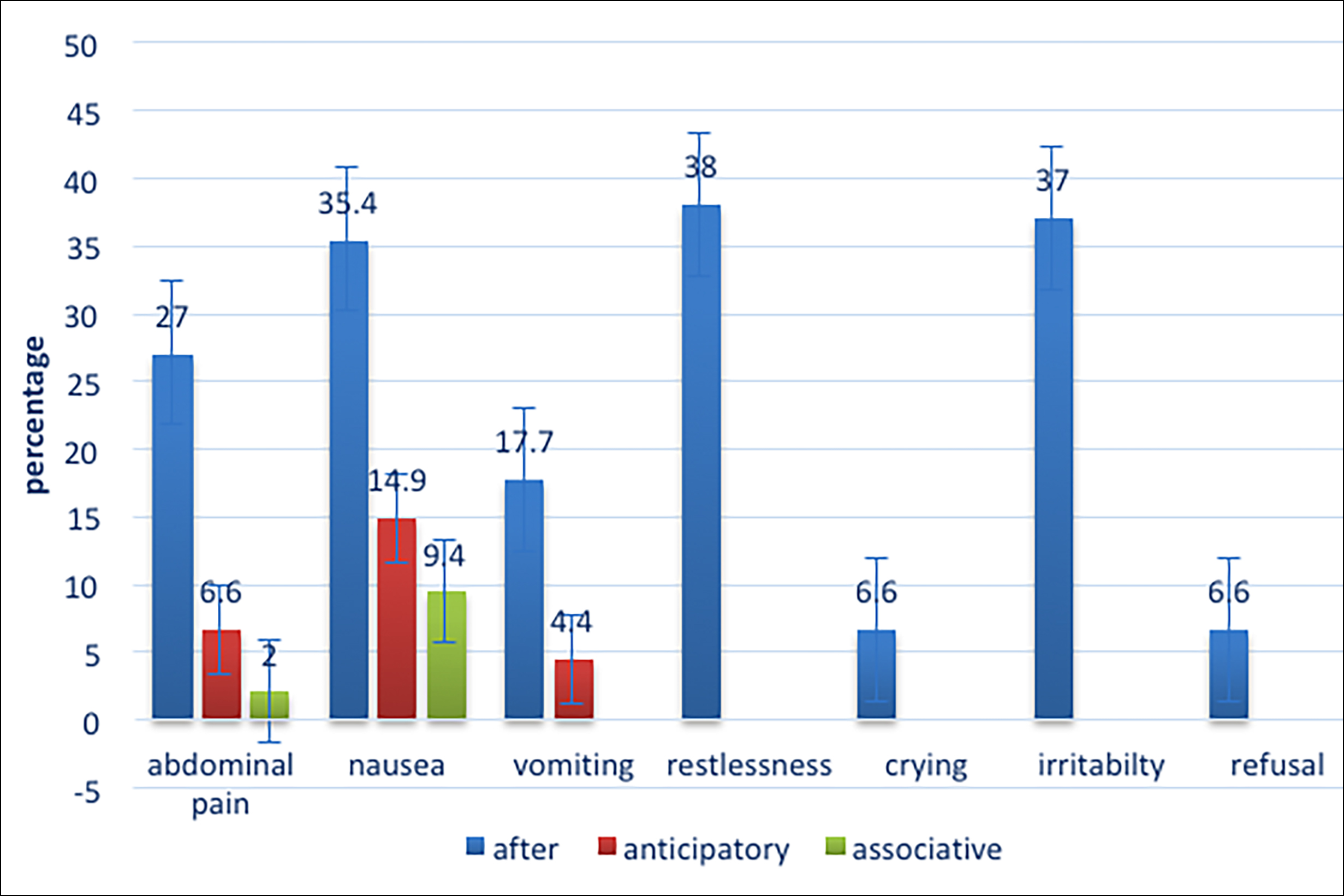 Figure 1: Frequency of adverse effects experienced by MTX users.
Figure 1: Frequency of adverse effects experienced by MTX users.
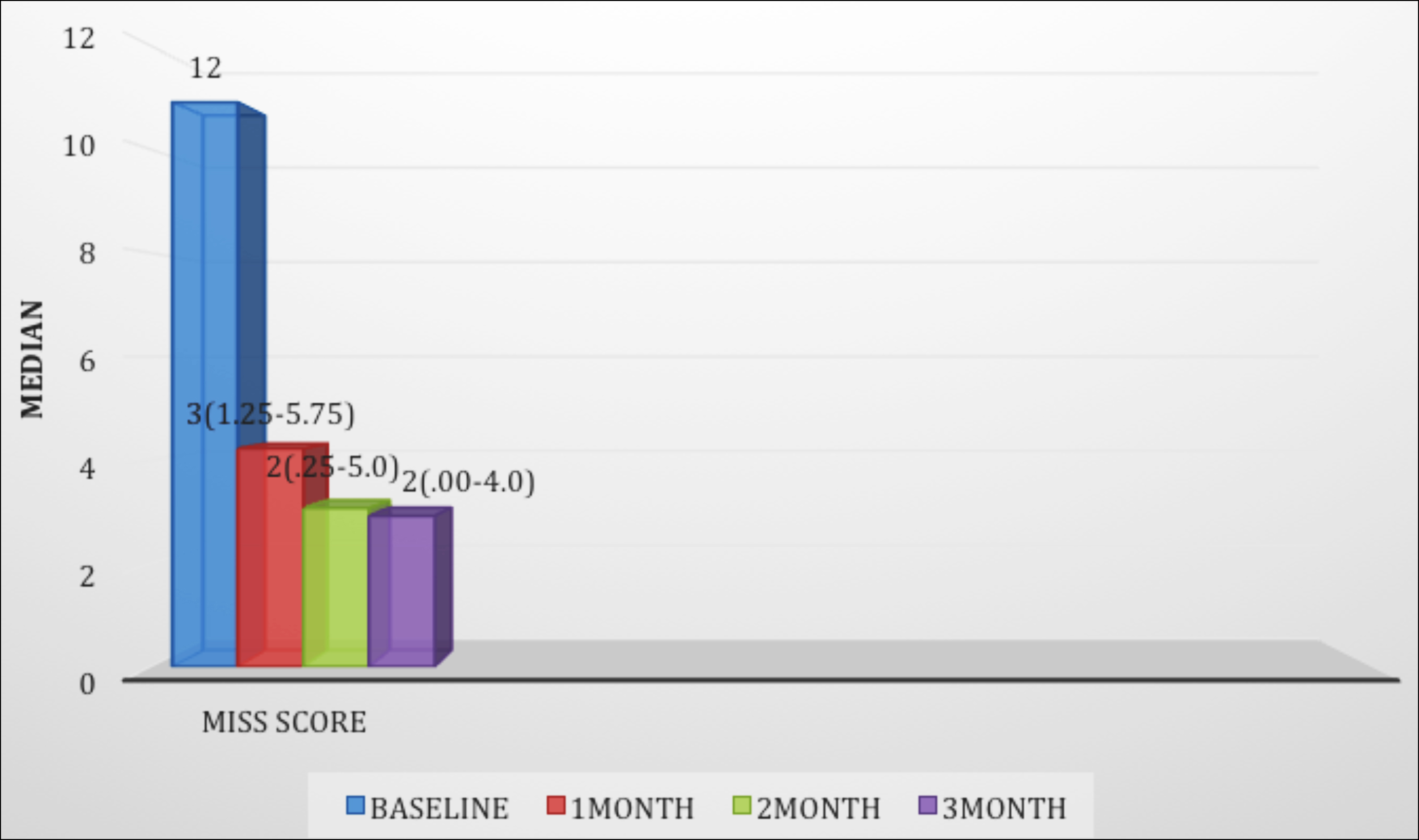 Figure 2: Reduction in the median of MISS score while using ondansetron with methotrexate.
Figure 2: Reduction in the median of MISS score while using ondansetron with methotrexate.
Among those 48 patients who were MTX intolerant nausea was present in majority 45 (93.8%) of patients after taking MTX while anticipatory and associative nausea was reported in 24 (50%) and 16 (33%) respectively. Vomiting was present in 26 (54.2%) patients after taking MTX and in 8 (16.7%) anticipatory. Abdominal pain after taking MTX was present in (19/48) 39.6% of the patients. These intolerant patients mainly having the symptoms of nausea and vomiting (n=40/48, 83%) were prescribed ondansetron along with MTX and their MISS score was calculated again at monthly intervals for 3 consecutive months. Patients maintained a daily diary to report GI-symptoms and side effects related to ondansetron. There was a significant reduction in MISS Score while taking ondansetron with MTX (Figure 2). Intensity and duration of nausea and frequency of vomiting started improving after the first week of therapy and remained stable during the 12 weeks of follow-up.
A Mann-Whitney test indicated that the baseline MISS was greater for MISS at 1 month (Median 3 Q1-Q3 (1.25-5.75) p=0.036) than for MISS at two months (Median 2 Q1-Q3 (0.25-5.00) p=.034) and three months (Median 2 Q1-Q3 (.00-4.00) p=0.12).
At the end of the study, GI-symptoms continued in 6(15%) of patients although 3 patients demonstrated reduced duration of nausea. There were no major adverse effects of Ondansetron except a few reported mild headaches occasionally. The dose of MTX was reduced in these patients but still 3 opted to discontinue MTX.
DISCUSSION
Rheumatic and MSK diseases are reported by WHO as the leading cause of disability worldwide. According to a local study, the estimated prevalence of rheumatic diseases in this set-up is 17.3%.13 It was found that 26.5% of patients with inflammatory arthritis developed MTX intolerance.
In a study conducted in Saudi Arabia, the frequency of MTX intolerance in rheumatoid arthritis patients was found to be 39.5%.14 In various other studies, it was reported to be between 21.7% and 55.9%.15,16
In MTX intolerant patients’ nausea was the commonest complaint and moderate to severe nausea was present in 35 out of 64 patients. Other pre-dominant symptoms after taking MTX were vomiting followed by abdominal pain. Anticipatory vomiting was less common. Behavioural symptoms were commonly noted as restlessness in 79% and irritability in 77% patients.
In a study by Amara et al., symptoms in patients on MTX included nausea as the most common, at 92.3%. Abdominal pain occurred in 42.3% and vomiting in 30.7%. For nausea prior to treatment, there were associative symptoms in 84.6% and 69.2% had anticipatory symptoms. There were behavioural symptoms in 96.1%.15
In another study done on Moroccan RA patients, at least one GI symptom was observed in 74.5% patients while on MTX. After MTX was administered, 93% patients with GI intolerance reported nausea, 73.7% abdominal pain, and, vomiting in 57.9%. These symptoms occurred both when thinking about MTX and prior to taking it. There was anticipatory nausea in 45.6% and associative nausea in 54.5%. In persons with anticipatory nausea, 22.8% had abdominal pain, while abdominal pain occurred in 42.1% patients with associative nausea. Only 8.8% had anticipatory vomiting. There were behavioural symptoms in 87.7%, of which restlessness was pre-dominant occurring in 71.9% patients.16
In another study, it was noted that nausea was more common in adolescents. Compared with adults, 36 of 49 (73%) adolescent patients reported nausea, while 20 out of 57 (35%) were reported by adults (p<0.001). Of these, only 10% adults and 22% adolescents had been prescribed anti-emetics.17 The present authors found similar results in this study with 56.5% of patients less than 30 years of age reporting nausea (p = 0.022).
In patients with rheumatic diseases, the major reason for non-adherence with treatment is GI intolerance which is usually reported within the first year of treatment. The authors observed that MTX intolerance started in less than 24 months of starting the treatment in 30/48(62.5%) of patients.
Similar results were reported by Dalkilic. In his study symptoms were reported in the first three months of starting treatment and 28.6% of patients discontinued MTX secondary to GI intolerance, at a mean duration of 8.1 ± 11.5 months.18
Ondansetron significantly reduced MTX intolerance in patients with main complaints of nausea and vomiting as indicated by the reduction in the MISS score over the following 3 months (p <0.001). Blanco et al. studied the role of ondansetron in MTX intolerance in RA patients. In this study, the patients had severe nausea at baseline, with an average duration of 6.8±1.5 hours which significantly decreased, both in intensity and duration after starting ondansetron. The improvement in nausea lasted through follow-up of 24 weeks.11
In the study done in the pediatric group, by Kempinska ondansetron was given prior to MTX administration in 50 patients, and within the first 12 weeks after starting treatment only one patient reported nausea. When compared to the group without ondansetron 6 of 10 (60%) reported nausea (p<0.001). After discontinuation of ondansetron, when the subsequent MTX dose was administered, nausea was reported by 10% patients, who responded well to restarting ondansetron. Anticipatory nausea was experienced by 6 out of 10 (10%) children, while 3 responded to ondansetron premedication.19 Although, there is evidence that antiemetic’s play an important role in alleviating or decreasing GI symptoms of MTX, other studies have also refuted this. Scheuern et al. used the MISS questionnaire in children with JIA to evaluate GI symptoms associated with MTX and 46% reported intolerance; however, there was no beneficial effect of antiemetics.20 Similarly, children with Crohn’s Disease on MTX were evaluated for GI symptoms. Of the 102 patients evaluated, 31% were found to have GI intolerance to MTX using the MISS questionnaire. Prophylactic antiemetic’s, did not have any beneficial effect on the GI symptoms in these patients.19
Ondansetron is used by some pediatric rheumatologists as the first choice among anti-emetics. According to the United Kingdom provider survey, to combat MTX-associated GI symptoms, greater than 10% patients with rheumatologic issues were shifted from MTX to a biologic agent. Upon initiating MTX therapy, 21% pediatric rheumatologists agreed upon almost always concurrently prescribing anti-emetiscs.21 The Ontario rheumatology association (ORA) EAP Committee recommends ondansetron funding for pediatric patients with rheumatic diseases who are receiving regular weekly methotrexate (oral or s/c) for treating nausea and vomiting caused by MTX.22
The beneficial effect of anti-emetics varies for different indications. For chemotherapy- induced nausea/vomiting serotonin and neurokinin antagonists (ondansetron and aprepitant) works better while metoclopramide and anti-histamines are preferred for pregnancy-related symptoms.23 In this study, the authors focused on the use of ondansetron for MTX-associated GI symptoms. Other anti-emetics have also been used with varying success. Dupont-Lucas et al. evaluate children with inflammatory bowel disease on MTX for GI symptoms. Prophylactic antiemetic’s, with included but was not limited to Ondansetron were studied in these patients.24 Delvin et al., in 1999 were already trying to find ways to combat MTX associated GI symptoms, using 5-HT3 receptor antagonists. Using Granisteron compared with Prochlorperazine, in patients with inflammatory arthritis, they were able to demonstrate that all patients on Granisteron were able to complete the 8-week duration of MTX, while only one out of six patients on Prochlorperazine were able to do so, because of MTX-induced GI symptoms. These patients, when subsequently switched to Granisteron, had significant improvement in symptoms (p<0.001).25
There were certain limitations of the study. First, a limited number of participants with the majority of the patients being females. Second, most of the patients were receiving oral MTX because of patient preference and low compliance with the sub-cutaneous route. Those limited numbers of patients taking sub-cutaneous MTX were also not started from the very beginning but usually shifted in between when they developed symptoms of intolerance to the oral form. Third, the number of patients with JIA was limited. It was a single-centred study.
During the study, intolerant patients were not switched from oral to injectable and were not tried on other anti-emetics before prescribing ondansetron.
Larger studies are needed for further confirmation of these findings. Long-term follow-ups of patients on MTX is recommended to determine the number of patients who would remain on MTX without intolerance one year after starting MTX.
CONCLUSION
Ondansetron is useful and safe in reducing nausea and vomiting related to MTX. It significantly reduces the symptoms of nausea and vomiting secondary to methotrexate intake in arthritis patients, hence increasing compliance with treatment.
ETHICAL APPROVAL:
This study was approved by the ethical review committee of CMH Lahore Medical College (case#635/ERC/CMH/LMC) prior to the initiation of the research work.
PATIENTS’ CONSENT:
Informed consent were obtained from the patients or their guardians where applicable, prior to the writing of this manuscript to publish their data while maintaining anonymity.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
SS, RK, HAK: Concept or design of the work.
SS, SK, RK, HAK, MS: Data collection, literature review, data analysis or interpretation.
SS, SK, SF, MS: Proofreading.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Alam SM, Kidwai AA, Jafri SR, Qureshi BM, Sami A, Qureshi HH, et al. Epidemiology of rheumatoid arthritis in a tertiary care unit, Karachi, Pakistan. J Pak Med Assoc 2011; 61(2): 123-6.
- Bedoui Y, Guillot X, Sélambarom J, Guiraud P, Giry C, Jaffar-Bandjee MC, et al. Methotrexate an old drug with new tricks. Int J Mol Sci 2019; 20(20):5023. doi: 10.3390/ijms 20205023.
- Weinblatt ME. Methotrexate in rheumatoid arthritis: A quarter century of development. Trans Am Clin Climatol Assoc 2013; 124:16-25. PMID: 23874006; PMCID: PMC 3715949.
- Salt E, Lohr K, Edward J. Methotrexate intolerance: A complex belief system. Orthop Nurs 2021; 40(5):316-21. doi: 10.1097/NOR.0000000000000792.
- Khan S, Mancini J, Hopper C, Rennick JE. Perceptions of methotrexate intolerance and its impact on daily life in school-age children with juvenile idiopathic arthritis. J Pediatr Nurs 2019; 48:49-54. doi: 10.1016/j.pedn.2019. 06.004.
- Fatimah N, Salim B, Nasim A, Hussain K, Gul H, Niazi S. Frequency of methotrexate intolerance in rheumatoid arthritis patients using methotrexate intolerance severity score (MISS questionnaire). Clin Rheumatol 2016; 35(5): 1341-5. doi: 10.1007/s10067-016-3243-8.
- van Dijkhuizen EH, Bulatović Ćalasan M, Pluijm SM, de Rotte MC, Vastert SJ, Kamphuis S, et al. Prediction of methotrexate intolerance in juvenile idiopathic arthritis: a prospective, observational cohort study. Pediatr Rheumatol Online J 2015; 13:5. doi: 10.1186/s12969-015-0002-3.
- Vijaykumar D, Dhir V, Jain S, Pai V, Kaur J, Naidu GS, et al. Assessing methotrexate intolerance and its prevalence in rheumatoid arthritis: Development and validation of the MISA questionnaire. Int J Rheum Dis 2021; 24(10):1294- 1301. doi: 10.1111/1756-185X.14207.
- Salt E, Wiggins A, Lohr K, Crofford L, Studts J, Nowell WB, Cappelleri JC, et al. The development and validation of a patient-perceived methotrexate intolerance scale for use in adult rheumatoid arthritis patients. Musculoskeletal Care 2021. doi: 10.1002/msc.1606.
- Falvey S, Shipman L, Ilowite N, Beukelman T. Methotrexate-induced nausea in the treatment of juvenile idiopathic arthritis. Pediatr Rheumatol Online J 2017; 15(1):52. doi: 10.1186/s12969-017-0180-2.
- Blanco R, González-Gay MA, García-Porrúa C, Ibañez D, García-Pais MJ, Sánchez-Andrade A, et al. Ondansetron prevents refractory and severe methotrexate-induced nausea in rheumatoid arthritis. Br J Rheumatol 1998; 37(5):590-2. doi: 10.1093/rheumatology/37.5.590.
- Amaral JM, Brito MJM, Kakehasi AM. High frequency of methotrexate intolerance in longstanding rheumatoid arthritis: using the methotrexate intolerance severity score (MISS). Adv Rheumatol 2020; 60(1):43. doi: 10.1186/ s42358-020-00145-5.
- Mohsin Z, Asghar AA, Faiq A, Khalid I, Ul-Haque I, Rehman S, et al. Prevalence of rheumatic diseases in a tertiary care hospital of karachi. Cureus 2018; 10(6):2858. doi: 10.7759/ cureus.2858.
- Albaqami J, Alshalhoub R, Almalag H, Dessougi M, Al Harthi A, Bedaiwi MK, et al. Prevalence of methotrexate intolerance among patients with rheumatoid arthritis using the Arabic version of the methotrexate intolerance severity score. Int J Rheum Dis 2019; 22(8):1572-7. doi: 10.1111/ 1756-185X.13637.
- Martins AJ, Maria KA, Brito, Menezes MJ. “First use of the methotrexate intolerance severity score (miss) portuguese version: age and glicocorticoid are associated to methotrexate intolerance in rheumatoid arthritis (RA)". p. 449. In: Anais do 36o Congresso Brasileiro de Reumatologia. [ISBN 978-85-212-1892-0]. São Paulo: Blucher, 2019. doi: 10.5151/sbr2019-449.
- Mahroug M, Azzouzi H, Boutaibi H. AB0274 Methotrexate intolerance in Moroccan rheumatoid arthritis patients. Ann Rheumatic Diseases 2021; 80:1162-3.
- Patil P, Parker RA, Rawcliffe C, Olaleye A, Moore S, Daly N, et al. Methotrexate-induced nausea and vomiting in adolescent and young adult patients. Clin Rheumatol 2014; 33(3):403-7. doi: 10.1007/s10067-013-2389-x.
- Dalkilic E, Sahbazlar M, Gullulu M, Yavuz M, Dilek K, Ersoy A, et al. The time course of gastric methotrexate intolerance in patients with rheumatoid arthritis and psoriatic arthritis. Mod Rheumatol 2013; 23(3):525-8. doi: 10.1007/s10165-012-0685-y.
- Kempinska A, Benchimol EI, Mack A, Barkey J, Boland M, Mack DR. Short-course ondansetron for the prevention of methotrexate-induced nausea in children with Crohn disease. J Pediatr Gastroenterol Nutr 2011; 53(4):389-93. doi: 10.1097/MPG.0b013e31822855e7.
- Scheuern A, Tyrrell PN, Haas JP, Hügle B. Counter-measures against methotrexate intolerance in juvenile idiopathic arthritis instituted by parents show no effect. Rheumatology (Oxford) 2017; 56(6):901-6. doi:10.1093/ rheumatology/kew507.
- Amin TS, Shenton S, Mulligan K, Wedderburn LR, Wood M, VanRooyen V, et al. Strategies for the prevention and management of methotrexate-related nausea and vomiting in juvenile idiopathic arthritis: Results of a UK paediatric rheumatology prescriber survey. Rheumatology (Oxford) 2015; 54(11):2108-9. doi: 10. 1093/rheumatology/kev259.
- https://ontariorheum.ca/ondansetron-for-methotrexate-induced-nausea-vomiting.jan31 2019.
- Athavale A, Athavale T, Roberts DM. Antiemetic drugs: what to prescribe and when. Aust Prescr 2020; 43(2): 49-56. doi:10.18773/austprescr.2020.011.
- Dupont-Lucas C, Grandjean-Blanchet C, Leduc B, Tripcovici M, Larocque C, Gervais F, et al. Prevalence and risk factors for symptoms of methotrexate intolerance in pediatric inflammatory bowel disease. Inflammatory Bowel Diseases 2017; 23(2):298-303. doi: 10.1097/MIB.000000000000 1014.
- Devlin J, Wagstaff K, Arthur V, Emery P. Granisetron (Kytril) suppresses methotrexate-induced nausea and vomiting among patients with inflammatory arthritis and is superior to prochlorperazine (Stemetil). Rheumatology (Oxford, England) 1999; 38(3):280-2. doi: 10.1093/rheumatology/ 38.3.280.