Risk of Cardiovascular Death in Osteosarcoma
By Yukun Jia, Yu Xiong, Zhan Peng, Guangye WangAffiliations
doi: 10.29271/jcpsp.2023.03.266ABSTRACT
Objective: To assess the risk of cardiovascular mortality (CVM) in patients with osteosarcoma.
Study Design: Descriptive study.
Place and Duration of the Study: Department of Orthopaedics, The People’s Hospital of Baoan, Shenzhen, Guangdong, China, from 1st January 2019 to 1st January 2022.
Methodology: Data on patients diagnosed with osteosarcoma, between 1975 and 2019, were obtained from the surveillance, epidemiology, and end results (SEER) database. Using the Nelson-Aalen cumulative risk curve to assess the risk of CVM in patients with osteosarcoma. Competing risk models were used for identifying and analysing independent risk factors for CVM in the patients.
Results: Data from a total of 1335 patients with osteosarcoma were obtained from the SEER database. The characteristics of patients with osteosarcoma independently related with a high risk of CVM were age over 65 years (HR: 2.528; 95% CI: 1.156 - 5.527), race of other categories (HR: 1.498; 95% CI: 1.044 - 2.151), and exposure radiotherapy (HR: 0.493; 95% CI: 0.244 - 0.998). Receiving chemotherapy was independently associated with a low risk of CVM (HR: 1.911; 95% CI: 1.016 - 3.593).
Conclusion: Cardiovascular disease death from osteosarcoma was significantly associated with older age at diagnosis, race other class, receiving radiation therapy, and not undergoing chemotherapy.
Key Words: Osteosarcoma, Cancer risk factors, Epidemiology.
INTRODUCTION
Osteosarcoma is the most common primary malignant tumour of bone and is characterised by poor prognosis and high disability rates.1,2 The cause of death in patients with osteosarcoma include cancer-related and non-cancer-related causes. The leading cause of non-cancer death in patients with osteosarcoma is cardiovascular death (CVD).3 Early prevention and control of CVD may help improve survival in patients with osteosarcoma, and it is particularly important to identify risk factors for CVD.
Studies have found a link between lifestyle factors, such as smoking, unhealthy diet, excessive alcohol consumption and a lack of exercise, and both cardiovascular disease and cancer.4 Social and psychological factors, such as marital status, have also been found to be associated with cardiovascular mortality in cancer patients in recent years.5 In addition, the cancer treatment process may induce endothelial dysfunction, causing myocardial damage and altering cardiac conduction,6 and radiotherapy and chemotherapy are associated with the risk of CVD in cancer patients.7
Identifying the characteristics of cancer patients presenting with CVD and preemptively identifying those at risk allows for early targeting, which is valuable in reducing the risk of CVD in cancer patients.
However, no studies have reported on the factors influencing cardiovascular mortality in patients with osteosarcoma. Therefore, the aim of this study was to explore the factors influencing cardiovascular mortality associated with patients with osteosarcoma.
METHODOLOGY
Data were obtained from the surveillance, epidemiology, and end results (SEER) database, extracted from patients with primary osteosarcoma between 1975 and 2019. Inclusion criteria were patients aged ≥14 years with a histological diagnosis of osteosarcoma. The histological code was International Classification of Diseases of Oncology, Third Revision (9180-9187.9192-9194).
The screening variables included, age at diagnosis, gender, race, marital status, average annual household income, and SEER histological staging (local, regional, and distant), and histological grading (well-differentiated: grade I; moderately differentiated: grade II; poorly differentiated: grade III; undifferentiated: grade IV), whether to receive surgery, radiotherapy and chemotherapy, cause of death (COD), survival status, and survival time.
The outcome of interest is death from cardiovascular disease (CVD). According to the international classification of diseases-10 (ICD-10) codes, CVD includes heart disease, hypertension without heart disease, atherosclerosis, cerebrovascular disease, aortic aneurysm and coarctation, and other diseases of other arteries, small arteries, and capillaries.8
Given that death from non-cardiovascular disease is a competing risk, Fine-Gray competitive risk analysis was performed to determine the risk associated with CVM, and cumulative fractional risk curves were used to assess the risk of CVM in different subgroups of patients with osteosarcoma. Further multivariate competing risks regression analysis was performed to avoid false positive results. Afterwards, clinical variables significantly associated with CVD were screened to construct competing risk nomograms for assessing the likelihood of CVD occurrence in patients with osteosarcoma. The C-index is used to assess the discriminative power of the model. Data for categorical variables are expressed as percentages. All the tests were two-sided, and p-value <0.05 was the criterion of significance. R software version 4.0.3 and SPSS version 25.0 (Chicago, IL, USA) were used.
RESULTS
A total of 1135 eligible patients with osteosarcoma were included in the follow-up analysis, of which 44 were included due to CVD. The mean age at diagnosis was 14-65 years in 972 (85.6%) individuals and greater than or equal to 65 years in 163 individuals. Only 473 (41.7%) patients had annual household incomes greater than or equal to $70,000. The majority of patients were females (57.9%), white (76.5%), and widowed (65.8%), SEER stage regional (39.4%), and histologic grade II (62.5%). A total of 904 patients (79.6%) underwent surgery, 836 patients (73.7%) received chemotherapy, and only 126 patients (11.1% %) received radiation therapy. Baseline characteristics are detailed in the Table I.
The results of all cumulative fractional risk curves for patients with osteosarcoma using competing risk models are presented in Figure 1. The following subgroups were related with risk of CVM: marital status, age, stage, and whether or not they received surgery, radiation therapy, or chemotherapy.
The authors used a multivariate competing risk model to identify indicators associated with CVM in patients with osteosarcoma (Table II). The authors found that the following patient characteristics were independently associated with a higher risk of CVM: age over 65 years (HR: 2.528; 95% CI: 1.156 - 5.527), ethnicity in other categories (HR: 1.498; 95% CI: 1.044 - 2.151), and receiving radiotherapy (HR: 0.493; 95% CI: 0.244 - 0.998). The authors also found that receiving chemotherapy was independently associated with a low risk of CVM. (HR: 1.911; 95% CI: 1.016 - 3.593).
Based on independent risk factors obtained from multivariate competing risk model analysis, a nomogram was constructed to predict the probability of cardiovascular death in patients with osteosarcoma (Figure 2). The overall C-index predicted by the model was 0.664, indicating that the model has good results.
Table I: Baseline characteristics among osteosarcoma patients.
|
Variables |
Osteosarcoma patients |
Cause of death |
|
|
CVD |
Others |
||
|
Total |
1135 |
44 |
523 |
|
Age |
|
|
|
|
14-65 years |
972 |
25 |
403 |
|
≥65 years |
163 |
19 |
120 |
|
Sex |
|
|
|
|
Female |
657 |
26 |
304 |
|
Male |
478 |
18 |
219 |
|
Race |
|
|
|
|
White |
868 |
28 |
393 |
|
Black |
127 |
7 |
59 |
|
Other |
140 |
9 |
71 |
|
Marital status |
|
|
|
|
Married |
389 |
25 |
204 |
|
D/S/S |
697 |
14 |
287 |
|
Widowed |
49 |
5 |
32 |
|
SEER stage |
|
|
|
|
Localised |
385 |
7 |
119 |
|
Regional |
447 |
16 |
177 |
|
Distant |
263 |
19 |
203 |
|
Unknown |
40 |
2 |
24 |
|
Histologic grade |
|
|
|
|
Grade I |
132 |
3 |
25 |
|
Grade II |
709 |
27 |
344 |
|
Grade III |
294 |
14 |
154 |
|
Surgery |
|
|
|
|
Yes |
904 |
27 |
353 |
|
No |
231 |
17 |
170 |
|
Radiotherapy |
|
|
|
|
Yes |
126 |
12 |
86 |
|
No |
1009 |
32 |
437 |
|
Chemotherapy |
|
|
|
|
Yes |
836 |
22 |
383 |
|
No |
299 |
22 |
140 |
|
Household income |
|
|
|
|
35000-70000 |
662 |
21 |
291 |
|
≥70000 |
473 |
23 |
232 |
|
D/S/S: Divorced/Single (never married)/Separated; CVD: cardiovascular disease. |
|||
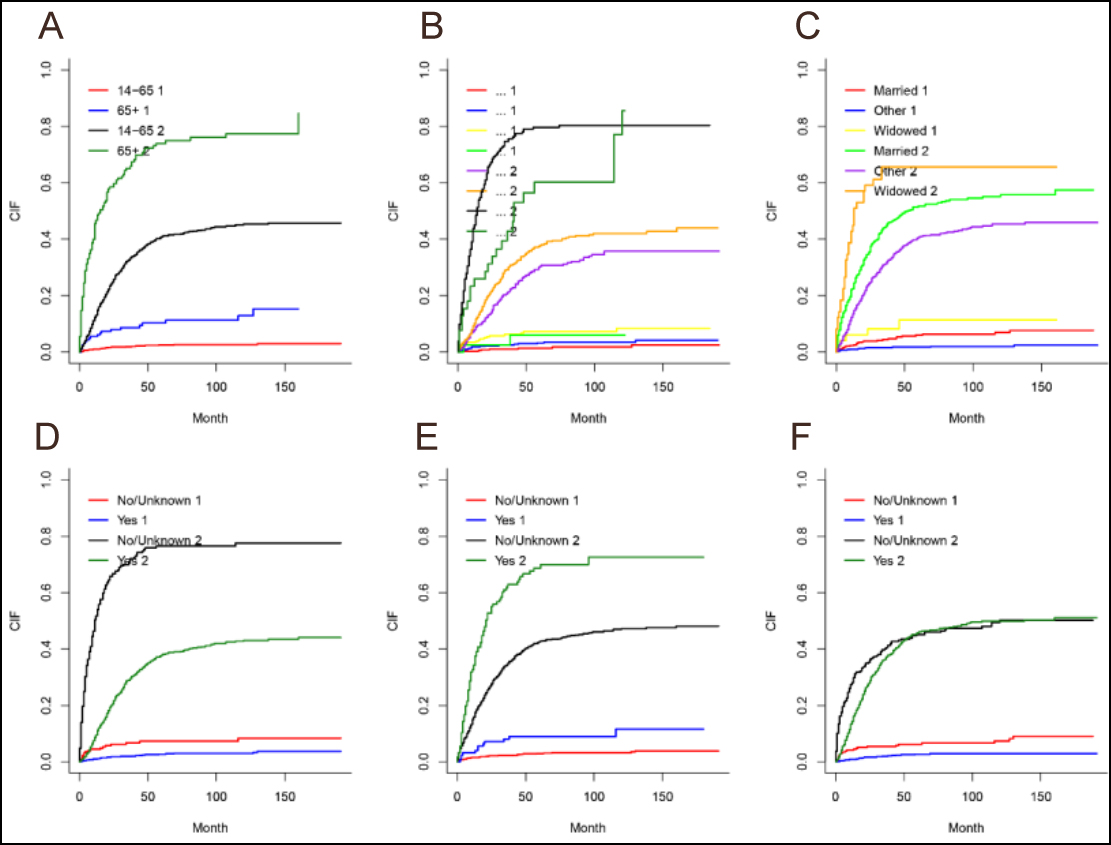 Figure 1: Cumulative incidence of cardiovascular death by different factors. (a) age. (b) SEER stage, (c) marital stage, (d) whether to receive surgery, (e) whether to receive radiotherapy, (f) whether to receive chemotherapy.
Figure 1: Cumulative incidence of cardiovascular death by different factors. (a) age. (b) SEER stage, (c) marital stage, (d) whether to receive surgery, (e) whether to receive radiotherapy, (f) whether to receive chemotherapy.
1 represents the cause of death as cardiovascular disease; 2 represents the cause of death as other.
Table II: Results of the multivariate competing risk analysis of the risk of cardiovascular death in patients with osteosarcoma.
|
Characteristics |
HR |
95% CI |
p-value |
|
Age |
2.528 |
1.156 - 5.527 |
0.020 |
|
Gender |
0.847 |
0.428 - 1.675 |
0.630 |
|
Race |
1.498 |
1.044 - 2.151 |
0.028 |
|
Histologic grade |
1.129 |
0.679 - 1.876 |
0.640 |
|
SEER stage |
0.2751 |
0.904 - 1.918 |
0.150 |
|
Surgery |
1.073 |
0.453 - 2.542 |
0.870 |
|
Radiation |
0.493 |
0.244 - 0.998 |
0.049 |
|
Chemotherapy |
1.911 |
1.016 - 3.593 |
0.044 |
|
Marital status |
0.722 |
0.418 - 1.250 |
0.240 |
|
Income |
1.263 |
0.679 - 2.348 |
0.460 |
|
HR: Hazard ratio; CI: Confidence interval.
|
|||
DISCUSSION
The risk of cardiovascular disease was comprehensively assessed in patients with osteosarcoma. The cumulative survival curves showed that age, marital status, stage, and whether or not they received surgery, radiation, or chemotherapy were associated with the risk of CVM. The interesting finding was that patients with osteosarcoma who received surgery and chemotherapy had a lower risk of cardiovascular disease death than those who did not receive surgical treatment. These findings should be interpreted with caution, and the present study can be used as a reference.
The reasons for the increased risk of CVD in patients with osteosarcoma are varied. In this study, multivariate competing risks were used to determine the factors associated with CVM in patients with osteosarcoma. The authors found that CVM was higher in elderly patients than in younger patients. Several studies have pointed out that age is a risk factor for cancer and cardiovascular disease, which may be related to the decreased physical function and various physiological imperfections in elderly patients.9 In addition, the current study found that white patients had a lower risk of CVM compared to patients of other races. There are differences in cancer treatment in the United States among patients of different races.10 This difference alone does not explain the differences in deaths from non-cancer causes among cancer patients [20]. Therefore, further investigation of this topic is necessary.
The majority of patients with osteosarcoma (79.6%) in this study underwent surgery, 73.7% underwent chemotherapy, and only 11.1% underwent radiotherapy. Notably, multivariate analysis showed a reduced risk of CVM in patients who received chemotherapy compared to those who did not. This finding does not seem to be consistent with the cardiotoxic effects of chemotherapy reported in the literature.11,12 However, in agreement with the results reported by Low et al.13 and Sun et al.,14 one possible explanation is that patients receiving chemotherapy may not have sufficient life expectancy to experience a CVD event. Regarding radiation therapy, A recent study reported that radiation-induced macrovascular damage accelerates microvascular damage as well as atherosclerosis.15 In this study, patients who received radiotherapy had an increased risk of CVM compared to patients who did not receive radiotherapy. Most studies reported an increased risk of CVM in patients, who did not undergo surgery than in those who underwent surgery.14,16 Notably, in this study, surgery was not an independent predictor of CVM. This finding is inconsistent with the results of previous studies,16 thus necessitating further validation of the relationship between surgery and patients with osteosarcoma.
To the authors knowledge, this is the first nomogram to predict the risk of cardiovascular death in patients with osteosarcoma. In this work, the traditional Cox regression was replaced with a competitive risk model. In contrast to traditional survival analysis, competing event models ensure that the influences selected are most directly related to cardiovascular mortality prognosis.17 This facilitates the identification of patients at high risk for CVD death. However, the C-index of the nomogram is 0.664, which means that the model needs to be further refined in the future to improve its predictive power.
There are still certain limitations to this study. First, the following information related to CVM is missing from the SEER database: for example, whether the patient smokes and drinks alcohol, and the dose of radiation and chemotherapy drugs. Second, because the study was retrospective, the presence of participant selection bias could not be avoided. Third, the causes of death identified from death certificates may be misclassified. Finally, the causes of death determined from death certificates may be misclassified.
CONCLUSION
Age, race, and receipt of radiotherapy were independent risk factors for cardiovascular mortality. This study has the potential to help clinicians determine which patients with osteosarcoma are at higher risk for CVM and who would benefit more from preventive measures.
ETHICAL APPROVAL:
The Ethics Committee of Shenzhen Baoan District People’s Hospital considered this study was ethically exempted because it was a retrospective case series study and used only raw data that was completely de-identified and anonymous.
PATIENTS’ CONSENT
It was a retrospective study and used only raw data that was completely de-identified and anonymous were included in this study.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
YJ: Formal analysis, data curation, writing original draft, and reviewing.
YX: Data curation and writing original draft.
ZP: Resources, supervision, drafting, and reviewing of the manuscript.
GW: Conceptualisation, methodology, and project administration, drafting and revising of the manuscript.
All the authors have agreed to publish the final version of the manuscript to be published.
REFERENCES
- Whelan J, Seddon B, Perisoglou M. Management of osteosarcoma. Curr Treatment Options Oncol 2006; 7(6):444-55. doi:10.1007/s11864-006-0020-y.
- Kumar R, Kumar M, Malhotra K, Patel S. Primary osteosarcoma in the elderly revisited: Current concepts in diagnosis and treatment. Curr Oncol Rep 2018; 20(2):13. doi:10.1007/s11912-018-0658-1.
- Youn P, Milano MT, Constine LS, Travis LB. Long-term cause-specific mortality in survivors of adolescent and young adult bone and soft tissue sarcoma: A population-based study of 28,844 patients. Cancer 2014; 120(15): 2334-42. doi:10.1002/cncr.28733.
- Sanderson SC, Waller J, Jarvis MJ, Humphries SE, Wardle J. Awareness of lifestyle risk factors for cancer and heart disease among adults in the UK. Patient Edu Coun 2009; 74(2):221-7. doi:10.1016/j.pec.2008.08.003.
- Guan T, Wang Y, Li F. Association of marital status with cardiovascular outcome in patients with breast cancer. J Thoracic Dis 2022; 14(4):841-50. doi:10.21037/jtd- 21-1261.
- Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy: Best practices in diagnosis, prevention, and management: Part 1. J Am Coll Cardiol 2017; 70(20):2536-51. doi:10.1016/ j.jacc.2017.09.1096.
- Guan T, Qiu Z, Su M. Cardiovascular death risk in primary central nervous system lymphoma patients treated with chemotherapy: A registry-based cohort study. Frontiers Oncol 2021; 11:641955. doi:10.3389/fonc.2021.641955.
- Fung C, Fossa SD, Milano MT, Sahasrabudhe DM, Peterson DR, Travis LB. Cardiovascular disease mortality after chemotherapy or surgery for testicular nonseminoma: A Population-based study. J Clin Oncol 2015; 33(28):3105-15. doi:10.1200/jco.2014.60.3654.
- Carioli G, Malvezzi M, Bertuccio P. Cancer mortality in the elderly in 11 countries worldwide, 1970-2015. Ann Oncol 2019; 30(8):1344-55. doi:10.1093/annonc/mdz178.
- Gad MM, Saad AM, Al-Husseini MJ. Temporal trends, ethnic determinants, and short-term and long-term risk of cardiac death in cancer patients: A cohort study. Cardiovasc Pathol 2019; 43:107147. doi:10.1016/j.carpath.2019.08.001.
- 11van Dalen EC, van der Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: A long-term follow-up study. Eur J Cancer (Oxford, England : 1990) 2006; 42(18):3191-8. doi:10. 1016/j.ejca.2006.08.005.
- Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nature reviews Cancer 2009; 9(7):463-75. doi:10.1038/nrc2656.
- Low SK, Giannis D, Bahaie NS, Trong BLH, Moris D, Huy NT. Competing mortality in patients with neuroendocrine tumour s. American J Clin Oncol 2019; 42(8):668-74. doi:10. 1097/coc.0000000000000575.
- Sun S, Wang W, He C. Cardiovascular mortality risk among patients with gastroenteropancreatic neuroendocrine neoplasms: A registry-based analysis. Oxidative Medicine Cellular Longev 2021; 2021:9985814. doi:10.1155/2021/ 9985814.
- Darby SC, Cutter DJ, Boerma M. Radiation-related heart disease: current knowledge and future prospects. Int J Radiation Oncol Biol Physics 2010; 76(3):656-65. doi:10.1016/j.ijrobp.2009.09.064.
- Du B, Wang F, Wu L. Cause-specific mortality after diagnosis of thyroid cancer: A large population-based study. Endocrine 2021; 72(1):179-89. doi:10.1007/ s12020-020-02445-8.
- Cooke RM, Morales-Napoles OJJosp, inference. Competing Risk Cox Proportional Hazard Model. J Statistical Plan Inference 2006; 136(5):1621-37.
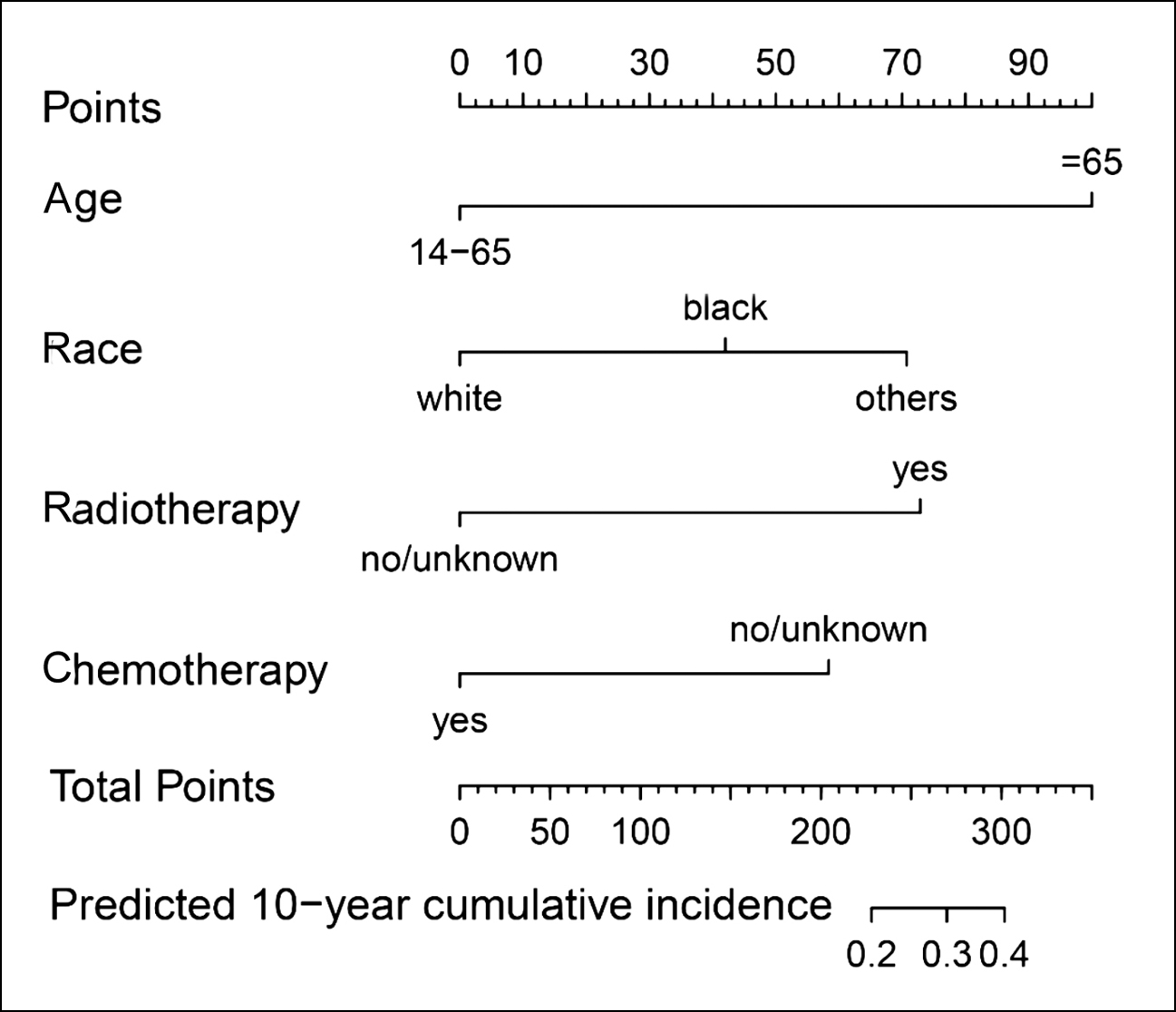 Figure 2: Nomogram for predicting the probability of cardiovascular death in patients with osteosarcoma.
Figure 2: Nomogram for predicting the probability of cardiovascular death in patients with osteosarcoma.