Relationship between Systemic Immune-inflammation Index and Mortality in Intensive Care Patients Diagnosed with Crimean-Congo Hemorrhagic Fever
By Oguz Gundogdu, Onur AvciAffiliations
doi: 10.29271/jcpsp.2022.12.1538ABSTRACT
Objective: To evaluate the power of the systemic immune-inflammation index in the prediction of mortality in severe Crimean-Congo hemorrhagic fever patients.
Study Design: Observational study.
Place and Duration of Study: Department of Anaesthesiology and Reanimation, Sivas Cumhuriyet University, Sivas, Turkey, from January to June 2022.
Methodology: Intensive care patients diagnosed with Crimean-Congo hemorrhagic fever, between January 2012 and January 2022, were included. Demographic data, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index were recorded. Receiver operating characteristic analysis, Cox regression analysis and Kaplan-Meier mortality analyses were done.
Results: A cut-off value <1.85 for neutrophil to lymphocyte ratio showed 41.67% sensitivity and 97.06% specificity. A cut-off value <80.75 for the systemic immune-inflammation index showed 84.72% sensitivity and 76.47% specificity. A cut-off value <37.86 for platelet-to- lymphocyte ratio showed 84.72% sensitivity and 73.53% specificity. In patients with systemic immune-inflammation index value <80.75, the mortality rate increased 2.549 times and 3.732 times in patients with a platelet-to-lymphocyte ratio value <37.86.
Conclusion: Similar sensitivity and specificity levels were found for systemic immune-inflammation index and platel-to-lymphocyte ratio regarding the mortality prediction power and impact on mortality. Both tests can be used for the prediction of mortality during the hemorrhagic period in patients with severe Crimean-Congo Hemorrhagic Fever.
Key Words: Crimean-congo hemorrhagic fever, Systemic immune inflammation index, Mortality, Platelet-to-lymphocyte ratio.
INTRODUCTION
Crimean-Congo hemorrhagic fever (CCHF) is an acute viral infection disease, which progresses with findings like ecchymosis, visceral bleeding, and hepatic dysfunction. Although the mean mortality rate of the disease is between 10% and 40%, it may increase up to 60%-80% depending on the region.1,2 CCHF virus, which belongs to the Nairovirus genus, is responsible for the disease and can be transmitted by several types of ticks.3
CCHF has four clinical periods: incubation, prehemorrhagic, hemorrhagic, and convalescence periods. The prehemorrhagic period is characterised by non-specific infection symptoms and findings like sudden onset of fever, headache, myalgia, and asthenia.
Fever lasts approximately 4-5 days and may be accompanied by diarrhoea, nausea, and vomiting. The duration of the prehemorrhagic period ranges between 1 and 7 days. The hemorrhagic period develops rapidly and lasts usually 2-3 days. Epistaxis, vaginal bleeding, gingival bleeding, and cerebral haemorrhage were reported in some patients during the hemorrhagic period. The convalescence period develops in the surviving patients and lasts 10-20 days.4,5
Bleeding emerging during the hemorrhagic period may require hospitalisation in ICU. These bleedings cause hemodynamic instability and may lead to death due to organ failure. Shock and coma may emerge as a result of alveolar haemorrhage in vital organs and cerebral haemorrhage and may lead to death.
Currently, mortality is predicted in certain medical conditions with the help of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SIII).6-9 It is believed that the mortality increases as a result of the deterioration of the balance between the increased inflammation and the counteracting anti-inflammatory mechanism.
As CCHF progresses with thrombocytopenia and inflammation, the authors believe that the SII index might be a useful tool for predicting mortality in CCHF patients. In the literature search, the authors did not find any study focused on the relationship between the SII index and mortality in CCHF cases. This study is important in this respect. The objective of this retrospective study was to evaluate the power of SIII in the prediction of mortality in severe CCHF patients.
METHODOLOGY
This study was approved by the Ethics Committee (Date: 13/01/2022; No. 2022-01/04). Written informed consent was obtained from all participating patients. This study is designed in line with the principles of the Helsinki Declaration. A total of 140 patients, who had a positive CCHF-polymerase chain reaction (PCR) test and followed in the intensive care unit of Sivas Cumhuriyet University Hospital between January 2012 and January 2022, were included in this single-centre retrospective study. All enrolled patients had been transferred from the infectious disease clinic to the intensive care unit and completed a routine ribavirin treatment (30 mg/Kg initial loading dose; 15 mg/Kg 4 times daily for 4 days; 7.5 mg/Kg 3 times daily for 6 days).
Patients, who had coronary artery disease, secondary infectious disease except for CCHF, underwent chemotherapy, and radiotherapy in the last year, had a haematological disease affecting the blood cell count and bone marrow function, were diagnosed with cancer, received corticosteroid treatment, had chronic obstructive lung disease, ulcerative colitis, diseases like Crohn's disease, which progress with chronic inflammation, complications, diabetes mellitus with uncontrolled blood sugar, were excluded from the study.
During the study, the age, gender, duration of the ICU hospitalisation, comorbidities, distribution of the cases according to the years, C-reactive protein (CRP) levels on the first ICU day, neutrophil, lymphocyte, and platelet counts of the patients, whose files were available, were recorded. The systemic immune-inflammation index was calculated according to the following formula and recorded: (platelet count x neutrophil count)/lymphocyte count (109/L). PLR was calculated according to the following formula and recorded: platelet count/lymphocyte count. NLR was calculated according to the following formula and recorded: neutrophil count/lymphocyte count.
The statistical analyses were performed with the SPPSS version 25.0 software package. The normal distribution of the variables was evaluated with the histogram charts and the Kolmogorov-Smirnov test. The descriptive analyses were given in mean, standard deviation, median, minimum, maximum, and IQR values. The categorical variables were compared with Pearson's chi-square test. The intergroup evaluation of the non-parametric variables was done with the Mann-Whitney U test. The spearman correlation test was used in the analysis of the metric data. The significant cut-off values, with which the measured values can predict mortality, were investigated with the ROC analysis. The variables related to survival were evaluated with the Kaplan-Meier analysis and the factors affecting the mortality were assessed with the COX regression analysis. The p-values smaller than 0.05 were considered statistically significant.
RESULTS
A total of 140 patients, age between 18 and 83 years, were included in the study. Forty-nine of them were females and 91 were males. The median hospitalisation time was 5 days. The mean NLR value was 3.83±2.82, mean PLR value was 52.26±60.63, mean SIII value was 123.21±188.10, and mean CRP value was 61.80±63.98 mg/L.
Fifty-four patients had co-morbidities. Forty of them had hypertension, 11 diabetes mellitus, 2 benign prostate hyperplasia, and one epilepsy. Seventy-two patients died.
The correlation of mortality with age, gender, hospitalisation time, comorbidity, neutrophil, lymphocyte and platelet counts, NLR, SIII, PLR, and CRP levels was assessed. According to the analysis, the dead patients were older. The hospitalisation time was shorter in the dead patients. The mortality rate was higher among patients with other comorbidities. In addition, the lymphocyte level was higher in the dead patients, while the levels of platelets, NLR, SIII, and PLR were lower in this group (Table I).
The correlation between hospitalisation time, age, NLR, SIII, PLR, and CRP was investigated in the survived patients. The authors found an inverse correlation between hospitalisation time and NLR, SIII, and PLR. In other words, the hospitalisation time was prolonged with the decrease in NLR (r: -0.307, p: 0.011), SIII (r: -0.419, p: <0.001), and PLR (r: -0.239 p: 0.049) values.
The NLR, SIII, PLR, and CRP levels, which can be useful in predicting mortality, were investigated. A cut-off value <1.85 for NLR showed 41.67% sensitivity and 97.06% specificity with a positive predictive value (PPV) 93.75% and a negative predictive value (NPV) 61.11%, 95% CI, 0.637-0.804, p<0.001. A cut-off value <80.75 for SIII showed 84.72% sensitivity and 76.47% specificity (PPV: 79.22%, NPV: 82.54%, 95% CI, 0.847-0.944, p<0.001). A cut-off value <37.86 for PLR showed 84.72% sensitivity and 73.53% specificity (PPV: 77.22%, NPV: 81.97%, 95% CI, 0.784-0.912, p<0.001). The authors did not detect a significant cut-off value for CRP (Figure 1).
The survival rate was higher in patients without comorbidities. Furthermore, survival was worse in patients with NLR <1.85, SIII <80.75, and PLR <37.86 (Table II, Figures 2 and 3).
The effects of comorbidity, NLR, SIII, and PLR on mortality were also evaluated. It showed that in patients with an SIII value <80.75, the mortality rate increased 2.549 times and 3.732 times in patients with a PLR value <37.86 (Table III).
Table I: Mortality analysis of influencing factors and distribution of the cases according to the years.|
|
Survival (n:68) |
Non-survivors (n:72) |
p-value |
|||
|
Median (min-max)/n |
Median (min-max)/% |
Median (min-max)/n |
Median (min-max)/% |
|||
|
Year |
2012 |
9 |
(47.37) |
10 |
(52.63) |
0.425 |
|
2013 |
8 |
(66.67) |
4 |
(33.33) |
||
|
2014 |
5 |
(50.00) |
5 |
(50.00) |
||
|
2015 |
7 |
(43.75) |
9 |
(56.25) |
||
|
2016 |
1 |
(20.00) |
4 |
(80.00) |
||
|
2017 |
1 |
(25.00) |
3 |
(75.00) |
||
|
2018 |
4 |
(36.36) |
7 |
(63.64) |
||
|
2019 |
8 |
(36.36) |
14 |
(63.64) |
||
|
2020 |
15 |
(65.22) |
8 |
(34.78) |
||
|
2021 |
10 |
(55.56) |
8 |
(44.44) |
||
|
Age (year) |
45.44±18.90 |
43 (29.5-62) |
53.69±19.08 |
60 (34.5-69.5) |
0.009² |
|
|
Gender |
Female |
21 |
(30.9) |
28 |
(38.9) |
0.321 |
|
Male |
47 |
(69.1) |
44 |
(61.1) |
||
|
Length of stay (day) |
7.76±3.35 |
7 (5-9) |
3.82±2.96 |
3 (2-4.5) |
<0.001² |
|
|
Comorbidity |
Absent |
49 |
(72.1) |
37 |
(51.4) |
0.012 |
|
Present |
19 |
(27.9) |
35 |
(48.6) |
||
|
Neutrophil |
3.07±2.57 |
2.07 (1.27-4.18) |
4.38±4.38 |
3.05 (1.19-6.01) |
0.229² |
|
|
Lymphocyte |
0.68±0.42 |
0.53 (0.4-0.82) |
1.51±1.13 |
1.26 (0.75-1.85) |
<0.001² |
|
|
Platelet |
42.1±26.12 |
36 (26-49) |
20.51±14.00 |
16 (10-27) |
<0.001² |
|
|
NLR |
4.67±2.92 |
3.97 (2.59-5.74) |
3.04±2.49 |
2.34 (1.49-3.7) |
<0.001² |
|
|
SIII |
200.09±246.28 |
138.58 (82.07-207.01) |
50.6±32.03 |
44.44 (24.83-73.1) |
<0.001² |
|
|
PLR |
83.43±71.29 |
68.58 (32.45-105.05) |
22.82±24.30 |
12.1 (7.17-30.85) |
<0.001² |
|
|
CRP (mg/L) |
69.58±80.00 |
47.1 (23.7-83.5) |
54.46±43.15 |
42.8 (19.05-76.65) |
0.554² |
|
|
|
Survival (n:68) |
Non-survivors (n:72) |
|
|||
|
Median (min-max)/n |
Median(min-max)/% |
Median (min-max)/n |
Median(min-max)/% |
|||
|
Year |
2012 |
9 |
(47.37) |
10 |
(52.63) |
0.425 |
|
2013 |
8 |
(66.67) |
4 |
(33.33) |
||
|
2014 |
5 |
(50.00) |
5 |
(50.00) |
||
|
2015 |
7 |
(43.75) |
9 |
(56.25) |
||
|
2016 |
1 |
(20.00) |
4 |
(80.00) |
||
|
2017 |
1 |
(25.00) |
3 |
(75.00) |
||
|
2018 |
4 |
(36.36) |
7 |
(63.64) |
||
|
2019 |
8 |
(36.36) |
14 |
(63.64) |
||
|
2020 |
15 |
(65.22) |
8 |
(34.78) |
||
|
2021 |
10 |
(55.56) |
8 |
(44.44) |
||
|
Age (year) |
45.44±18.90 |
43 (29.5-62) |
53.69±19.08 |
60 (34.5-69.5) |
0.009² |
|
|
Gender |
Female |
21 |
(30.9) |
28 |
(38.9) |
0.321 |
|
Male |
47 |
(69.1) |
44 |
(61.1) |
||
|
Length of stay (day) |
7.76±3.35 |
7 (5-9) |
3.82±2.96 |
3 (2-4.5) |
<0.001² |
|
|
Comorbidity |
Absent |
49 |
(72.1) |
37 |
(51.4) |
0.012 |
|
Present |
19 |
(27.9) |
35 |
(48.6) |
||
|
Neutrophil |
3.07±2.57 |
2.07 (1.27-4.18) |
4.38±4.38 |
3.05 (1.19-6.01) |
0.229² |
|
|
Lymphocyte |
0.68±0.42 |
0.53 (0.4-0.82) |
1.51±1.13 |
1.26 (0.75-1.85) |
<0.001² |
|
|
Platelet |
42.1±26.12 |
36 (26-49) |
20.51±14.00 |
16 (10-27) |
<0.001² |
|
|
NLR |
4.67±2.92 |
3.97 (2.59-5.74) |
3.04±2.49 |
2.34 (1.49-3.7) |
<0.001² |
|
|
SIII |
200.09±246.28 |
138.58 (82.07-207.01) |
50.6±32.03 |
44.44 (24.83-73.1) |
<0.001² |
|
|
PLR |
83.43±71.29 |
68.58 (32.45-105.05) |
22.82±24.30 |
12.1 (7.17-30.85) |
<0.001² |
|
|
CRP (mg/L) |
69.58±80.00 |
47.1 (23.7-83.5) |
54.46±43.15 |
42.8 (19.05-76.65) |
0.554² |
|
|
¹Chi-square test, ²Mann whitney U test; NLR: Neutrophil/lymphocyte ratio; SIII: Systemic immune-inflammation index; PLR: Platelet/lymphocyte ratio; CRP: C-reactive protein; p<0.05: Statistically significant. |
||||||
Table II: Kaplan-Meier survival analysis.
|
|
|
Estimate |
Std. error |
95% confidence interval |
p-value |
|
|
Lower bound |
Upper bound |
|||||
|
|
Overall |
9.758 |
0.684 |
8.418 |
11.099 |
|
|
Gender |
Female |
9.049 |
1.164 |
6.768 |
11.330 |
0.416 |
|
Male |
9.710 |
0.779 |
8.183 |
11.236 |
||
|
Comorbidity |
Absent |
11.391 |
0.852 |
9.721 |
13.060 |
0.029 |
|
Present |
7.696 |
0.875 |
5.981 |
9.411 |
||
|
NLR |
>1.85 |
10.906 |
0.741 |
9.454 |
12.358 |
<0.001 |
|
<1.85 |
5.188 |
0.832 |
3.557 |
6.818 |
||
|
SIII |
>80.75 |
14.238 |
0.757 |
12.754 |
15.722 |
<0.001 |
|
<80.75 |
6.519 |
0.704 |
5.138 |
7.900 |
||
|
PLR |
>37.86 |
14.047 |
0.822 |
12.435 |
15.659 |
<0.001 |
|
<37.86 |
6.291 |
0.720 |
4.879 |
7.703 |
||
|
Kaplan-Meier analysis; NLR: Neutrophil/lymphocyte ratio; SIII: Systemic immune-inflammation index; PLR: Platelet/lymphocyte ratio; p<0.05: Statistically significant. |
||||||
Table III: The effect of NLR, PLR and SIII on mortality.
|
|
B |
SE |
p |
Exp(B) |
95.0% CI for Exp (B) |
|
|
Lower |
Upper |
|||||
|
Comorbidity |
0.323 |
0.242 |
0.183 |
1.381 |
0.859 |
2.222 |
|
NLR (<1.85) |
0.352 |
0.256 |
0.169 |
1.422 |
0.861 |
2.348 |
|
SIII (<80.75) |
0.936 |
0.372 |
0.012 |
2.549 |
1.229 |
5.286 |
|
PLR (<37.86) |
1.317 |
0.360 |
<0.001 |
3.732 |
1.843 |
7.557 |
|
COX Regresyon analizi; NLR: Neutrophil/lymphocyte ratio; SIII: Systemic immune-inflammation index; PLR: Platelet/lymphocyte ratio; B: Regression coefficient; SE: Standard error of the coefficient; Exp(B): Hazard ratio; CI: Confidence interval; p<0.05: Statistically significant. |
||||||
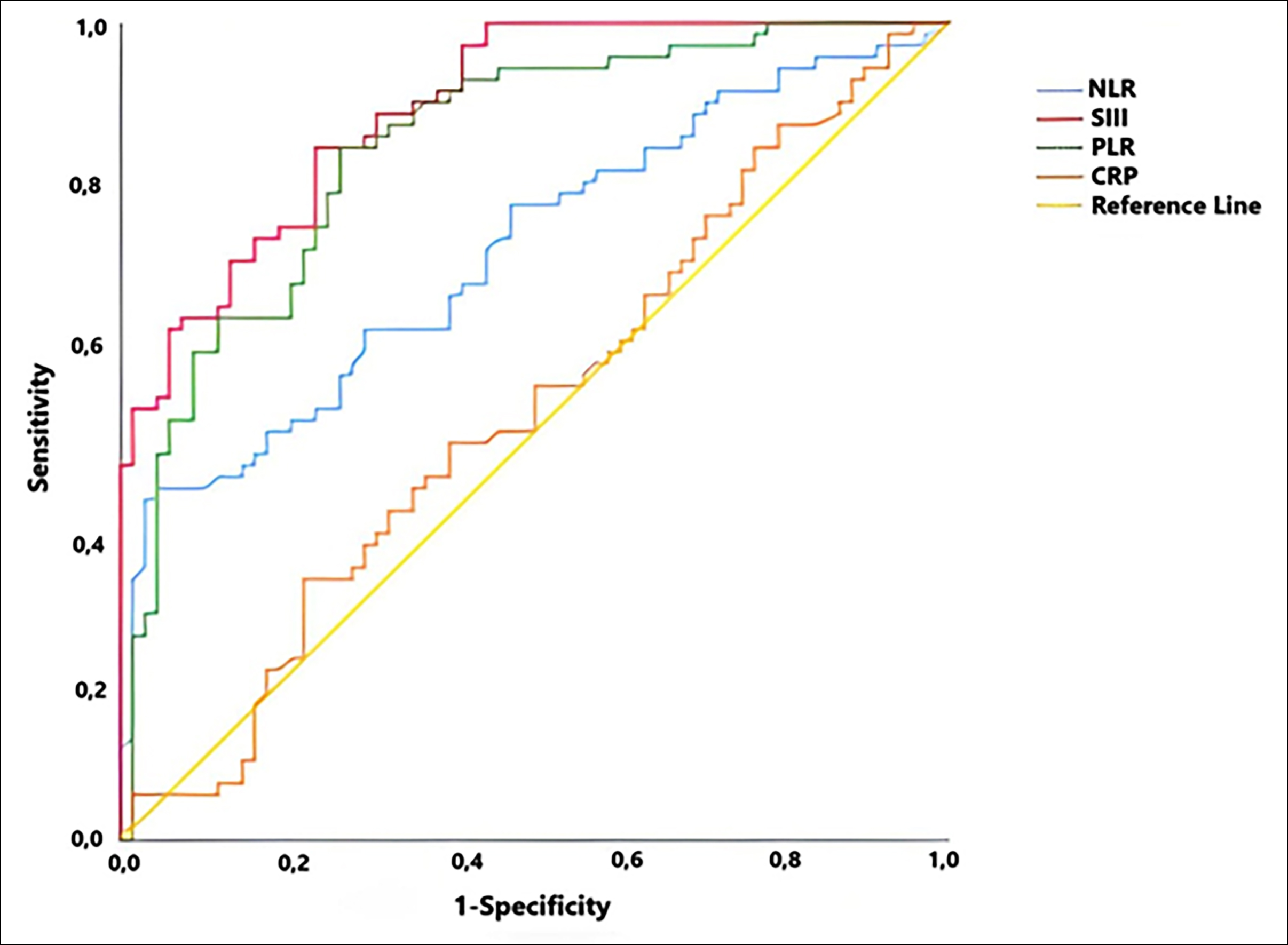 Figure 1: ROC analysis of NLR, SIII, PLR and CRP to predict mortality.
Figure 1: ROC analysis of NLR, SIII, PLR and CRP to predict mortality.
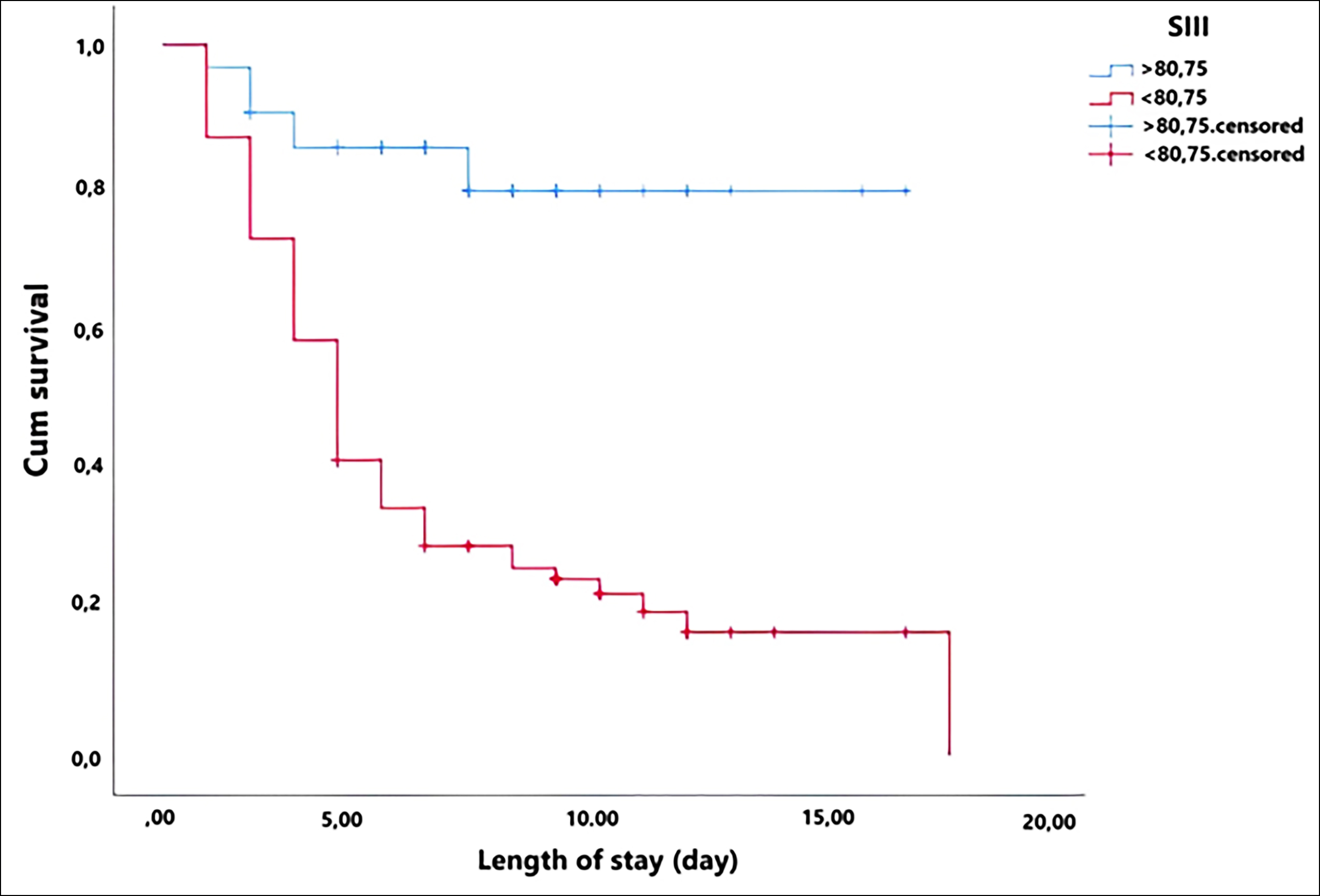 Figure 2: Kaplan-Meier survival analysis for SIII.
Figure 2: Kaplan-Meier survival analysis for SIII.
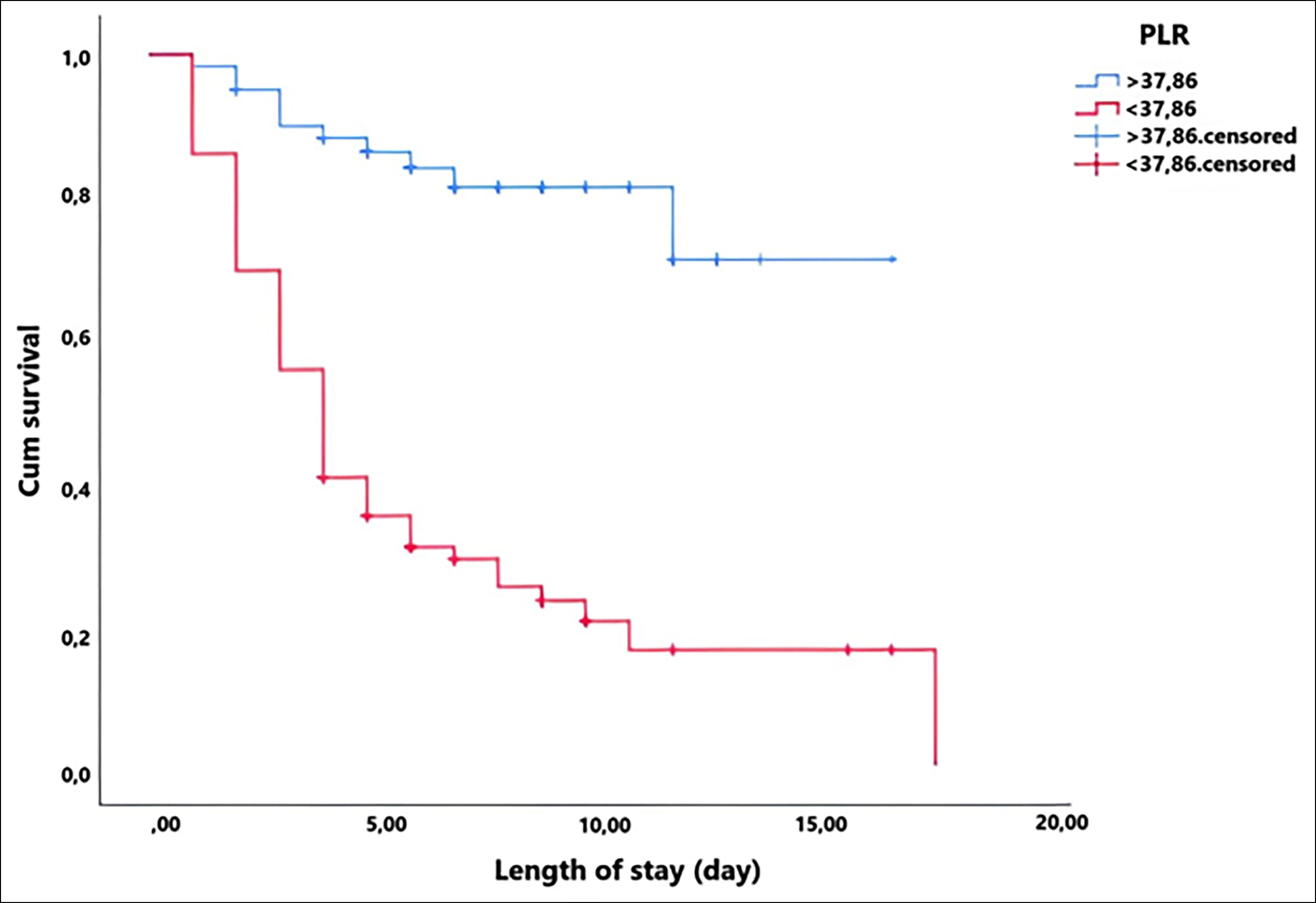 Figure 3: Kaplan-Meier survival analysis for PLR.
Figure 3: Kaplan-Meier survival analysis for PLR.
DISCUSSION
According to the findings in this study, in CCHF patients, the level of thrombocytopenia at admission to the ICU helped to predict mortality much more than the severity of inflammation. The SIII and PLR values have a comparable power in the prediction of mortality. In this disease, although severe inflammation is the cause of thrombocytopenia, the severity of thrombocytopenia was useful in the determination of the prognosis, even though inflammation was suppressed as a result of the antiviral treatment at admission to the ICU.
Recent studies showed that SIII might be used for the predictions of the prognosis and mortality in patients with cancer, coronary artery disease, hip fractures and Covid-19 patients.8-13 The common feature of these conditions is inflammation but there is no study published in the literature focused on SIII in CCHF which is also an inflammatory disease. This study is unique in this respect. As CCHF is restricted to a certain geographical area, studies focused on the diagnosis and prognosis of the disease are critical for the increase of awareness about the disease worldwide.
The factors which determined the mortality in CCHF included: age, haemorrhage, hepatomegaly, organ failure, aspartate transaminase, alanine transaminase, lactate dehydrogenase, leukocyte count, prolonged prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalised ratio (INR), and platelet count. A scoring system named Severity Grading Score (SGS) was developed with the help of these parameters and the sensitivity, specificity, PPV, and NPV were 96%, 100%, 97%, and 44% respectively in CCHF.14 In a study conducted by Bakır et al., SGS was used for the determination of the CCHF mortality, and data obtained at first application to the hospital were analysed.14 This study differs with respect to the patient population. In this study, data from the blood sample was obtained at admission to the ICU from the patients, who were transferred to the ICU, while they were under treatment in the hospital with the diagnosis of CCHF. Instead of the interpretation of SGS, which consists of 12 factors, introducing a tool like SIII with few factors and practical mortality prediction properties to the literature, will be more useful in routine practice. However, it is also a fact that it is not surprising that the variables in SGS, which bring organ failure to the forefront have higher predictive power compared to the hemogram parameters in predicting mortality.
In this study, the reason SIII was preferred to predict CCHF mortality was that thrombocytopenia has a central place in the pathogenesis of the disease. The most common laboratory finding is thrombocytopenia at the first application to the hospital.15 The most important reason for the progress to the hemorrhagic period is not the prolongation of PT, aPTT, and INR, but thrombocytopenia, which is determined before these parameters deteriorate. Therefore, the authors investigated PLR in one of this prior studies.1 The difference between SIII from PLR is that SIII shows the severity of inflammation with the patient's immune response taking the neutrophil count into the calculation. In this study, which was conducted with 34 ICU patients in 2020, the sensitivity and specificity of PLR were 81.3% and 100% respectively. In this study, which was conducted with 140 patients, the sensitivity and specificity of PLR were 84.72% and 73.53% respectively. With the increase in the sample size, it was observed that the mortality rate was comparable between these two studies. The decrease in PLR level from 100% to 73.53% can be explained by the smaller sample size of the previous study. Nevertheless, despite the increase in sample size, the high sensitivity of the PLR in predicting mortality was preserved.
The mortality analysis done in this study showed that mortality increased by 2.54 and 3.73 times respectively, when SIII and PLR values were under the cut-off values, while NLR did not reveal a significant correlation with the increase in the mortality rate. Therefore, following the COX regression analysis with the high specificity and low sensitivity found for NLR<1.85, it was observed that high NLR levels did not significantly elevate the mortality rate. As it can be understood from this result, PLR and SIII, which are thrombocytopenia-based parameters, are more powerful tests to display whether the disease will lead to death, rather than NLR, which reflects the severity of inflammation in the hemorrhagic period of CCHF. Bleeding, which is a clinical finding closely related to thrombocytopenia and emerges during the progress of the disease, is the cause of organ failure and death, Therefore, the analysis of these parameters is consistent with the clinical course of the disease. It should be kept in mind that the cut-off values determined for NLR, PLR, and SIII were obtained during the hemorrhagic period of the disease. Different comments can be proposed for the NLR values determined during the incubation and prehemorrhagic periods. To demonstrate the effectiveness of the severity of inflammation in predicting the progress of the disease, an investigation of the inflammation parameters during the incubation and prehemorrhagic periods might provide more valuable findings.
CRP, which is another inflammation marker, did not provide a significant cut-off level for mortality in this study. There are studies published in the literature, which reported that like CRP, also NLR indicated inflammation.16-19 In this study, the reason why NLR displayed more significant results than CRP in indicating inflammation is that this study was designed for a viral disease. As lymphopenia is in the foreground in acute viral infections, NLR provided more significant results in CCHF compared to CRP. CRP increases in many inflammatory events and thus it is not specific to any inflammatory factor. Although high-sensitivity CRP (hs-CRP) levels provide significant results for mortality in CCHF patients, which may progress with liver damage, the investigation with SGS did not change the sensitivity of SGS.20
There are some limitations of this study. The retrospective design may cause some biases. The use of hs-CRP, which became popular in CCHF recently, instead of CRP might reveal more significant results. The investigation of these parameters in patients, who had received ribavirin treatment, was another limitation of this study. The included patients were patients, who were transferred to ICU despite they were under ribavirin treatment in the clinic. Therefore, the authors did not have treatment-naive patients. The lack of information about the viral load in patients was another limitation of the study.
CONCLUSION
Similar sensitivity and specificity levels were found for SIII and PLR regarding the mortality prediction power and impact on mortality. Both tests can be used for the prediction of mortality during the hemorrhagic period in patients with severe CCHF.
ETHICAL APPROVAL:
The study was approved by the local Ethics Committee of the institute (Institutional Review Board (IRB) No. 2022-01/04 IRB date: 13.01.2022).
PATIENTS’ CONSENT:
Consent for publication was obtained from the patients whose data are included in this manuscript.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
OG: Conception and designing of the study, selected patients, created the study plan, and wrote the manuscript.
OA: Selected patients analysed the data, and critically reviewed for important intellectual content.
Both authors have agreed on the final version of the manuscript for publication.
REFERENCES
- Avcı O, Gündoğdu O. The Relationship between platelet/lymphocyte and neutrophil/lymphocyte ratios and mortality in intensive care patients with crimean-congo hemorrhagic fever. Erciyes Med J 2020; 42(4):425-30. doi: 10.14744/etd.2020.32966.
- Kouhpayeh H. An overview of complications and mortality of crimean-congo hemorrhagic fever. Int J Infect 2019; 6(2): e91707. doi: 10.5812/iji.91707.
- Lombe BP, Saito T, Miyamoto H, Mori-Kajihara A, Kajihara M, Saijo M, et al. Mapping of antibody epitopes on the crimean-congo hemorrhagic fever virus nucleoprotein. Viruses 2022; 14(3):544. doi: 10.3390/v14030544.
- Tumturk A. Crimean-congo haemorrhagic fever in a middle anatolian city: Five years of experience. Trop Doct 2019; 1-3. doi: 10.1177/0049475519891337.
- Ergonul O. Crimean-congo haemorrhagic fever. Lancet Infect Dis 2006; 6:203-14. doi: 10.1016/S1473-3099(06) 70435-2.
- Shen Y, Huang X, Zhang W. Platelet-to-lymphocyte ratio as a prognostic predictor of mortality for sepsis: Interaction effect with disease severity- a retrospective study. BMJ Open 2019; 9:e022896. doi: 10.1136/bmjopen-2018- 022896.
- Turcato G, Sanchis-Gomar F, Cervellin G, Zorzi E, Sivero V, Salvagno G, et al. Evaluation of neutrophil-lymphocyte and platelet-lymphocyte ratios as predictors of 30-day mortality in patients hospitalised for an episode of acute decompansated heart failure. J Med Biochem 2019; 38: 452-460. doi: 10.2478/jomb-2018-0044.
- Van Berckelaer C, Van Geyt M, Linders S, Rypens C, Trinh XB, Tjalma WAA, et al. A high neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are associated with a worse outcome in inflammatory breast cancer. Breast 2020; 53: 212-20. doi: 10.1016/j.breast.2020.08.006.
- Yang YL, Wu CH, Hsu PF, Chen SC, Huang SS, Chan WL, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest 2020; 50:e13230. doi: 10.1111/eci.13230.
- Taha SI, Samaan SF, Baioumy SA, Shata AK, Youssef MK. Role of hemogram-derived ratios and systemic-immune inflammation index in prediction of COVID-19 progression in egyptian patients. Microbes Infect Dis 2021; 2:613-22. doi: 10.21608/MID.2021.84684.1168.
- Wang ZC, Jiang W, Chen X, Yang L, Wang H, Liu YH. Systemic immune-inflammation index independently predicts poor survival of older adults with hip fracture: A prospective cohort study. BMC Geriatr 2021; 21(1):155. doi: 10.1186/s12877-021-02102-3.
- Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol 2017; 23(34):6261-72. doi: 10.3748/wjg.v23.i34.6261.
- Wang K, Diao F, Ye Z, Zhang X, Zhai E, Ren H, et al. Prognostic value of systemic immune‑inflammation index in patients with gastric cancer. Chin J Cancer 2017; 36(1):75. doi: 10.1186/s40880-017-0243-2.
- Bakir M, Gozel MG, Koksal I, Asik Z, Gunal O, Yilmaz H, et al. Validation of a severity grading score (SGS) system for predicting the course of disease and mortality in patients with Crimean-Congo hemorrhagic fever (CCHF). Eur J Clin Microbiol Infect Dis 2015; 34(2):325-30. doi: 10.1007/ s10096-014-2238-0.
- Hamidi AA, Kescioğlu S. Diagnostic value of non-specific clinical and laboratory findings in patients suspected of crimean-congo hemorrhagic fever in an endemic region. Infect Dis Clin Microbiol 2021; 3:145-151. doi: 10.36519/idcm.2021.91.
- Yilmaz H, Yalcin KS, Namuslu M, Celik HT, Sozen M, Inan O, et al. Neutrophil-lymphocyte ratio (NLR) could be better predictor than c-reactive protein (CRP) for liver fibrosis in non-alcoholic steatohepatitis (NASH). Ann Clin Lab Sci 2015; 45(3):278-86. doi: 10.1097/MEG.0000000000001393.
- Duman D, Aksoy E, Agca MC, Kocak ND, Ozmen I, Akturk UA, et al. The utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophilia. Int J Chronic Obstructive Pulmonary Disease 2015; 10:2469-78. doi: 10.2147/COPD.S90330.
- Chandrashekara S, Mukhtar Ahmad M, Renuka P, Anupama KR, Renuka K. Characteriation of neutrophil-to-lymphocyte ratio as a measure of inflammation in rheumatoid arthritis. Int J Rheum Dis 2017; 20(10):1457-67. doi: 10.1111/ 1756-185X.13157.
- Liang P, Yu F. Value of CRP, PCT, and NLR in prediction of severity and prognosis of patients with bloodstream infections and sepsis. Front Surg 2022; 9:857218. doi: 10.3389/fsurg.2022.857218.
- Bozkurt I, Esen S. Association between severity grading score and acute phase reactants in patients with crimean congo hemorrhagic fever. Pathogens Global Health 2021; 115(7-8):496-8. doi: 10.1080/20477724.2021.1878450.