Radiological Recovery of Osteoid Osteoma after CT Guided Percutaneous Radiofrequency Ablation
By Cennet Sahin1, Aylin Hasanefendioglu Bayrak2, Mujdat Bankaoglu1, Mehmet Ali Talmac3, Ozgur Genc2Affiliations
doi: 10.29271/jcpsp.2022.08.1056ABSTRACT
Objective: To observe the necessity and usefulness of follow-up Magnetic Resonance Imaging (MRI) and Computed Tomography Imaging (CTI) after RFA of osteoid osteoma.
Study Design: A descriptive study.
Place and Duration of Study: Department of Radiology, Sisli Etfal Training and Research Hospital, Istanbul, Turkey, between May 2015 and January 2020.
Methodology: Patients, who underwent CT-guided RFA for osteoid osteoma treatment, were followed-up both clinically and radiologically. MRI was recommended between the third and sixth months and CTI at 12th month or later for follow-up. All the pre and post-treatment radiological images were evaluated retrospectively. Radiological recovery was noted in three categories as complete/almost-complete, partial, and minimal-no recovery according to the healing of pre-treatment radiological findings.
Results: One-hundred and thirty-one patients with at least one follow-up CT or MRI were included. All had technically and clinically successful RFA treatments. Of 131 patients, 64.1% had CTI and 82.4% had MRI follow-up. In follow-up images, complete/almost-complete-recovery was observed in 70.2%, partial recovery in 26.7%, and minimal recovery in 3.1% of the cases. Re-ablation therapies were applied in 2 cases in this study due to pain recurrence after three months of successful treatments.
Conclusion: Radiological follow-up is beneficial for the evaluation of outcome after RFA of osteoid-osteoma. At least one follow-up MRI may be helpful for the assessment of healing or recurrence. Follow-up CTI may not be needed unless planning a re-ablation.
Key Words: Osteoma osteoid, Radiofrequency ablation, Tomography, Magnetic resonance imaging.
INTRODUCTION
Osteoid osteoma (OO) is a benign bone tumor presented with pain symptom.1-8 Radiofrequency-Ablation (RFA) has been preferred as the first choice of treatment. After RFA, noticeable pain relief occurs mainly within 24 hours.1-8 Previous studies have established the details about the radiological findings of OO, RFA procedure, and clinical follow-up findings after treatment.1-10 However, radiological success and expected radiological findings have been uncertain and there are few reports.11-14
Although clinical recovery is satisfactory, an objective indicator as radiological demonstration of treatment response is needed. Being familiar with regular postablative radiological changes and being aware of the expected and unexpected radiological findings after RFA may provide a better evaluation of the patients with pain recurrence. The present follow-up radiological findings of both clinically successful and unsuccessful treatments may be helpful to understand what is expected and what is not.
This study aimed to observe the usefulness and necessity of radiological follow-up after RFA. To the authors knowledge, this is one of the very few reports demonstrating radiological recovery of OO with demonstrative Magnetic Resonance Imaging (MRI) and Computed Tomography Imaging (CTI) findings.
METHODOLOGY
The study was approved by the Institution’s Ethical Committee (Number: 1879; Date: 23.01.2018). Informed patients’ consent were obtained from all the patients for RFAs and follow-up radiological examinations. Patients, who had CT-guided RFA between May 2015 and Jan 2020 at the Department of Radiology, Sisli Etfal Training and Research Hospital, Turkey, were included. Two interventional radiologists performed all the interventions with 13 and 6 years of interventional radiology experience. All the patients already had diagnostic pretreatment MRIs and CTIs when they were admitted to the interventional radiology clinic. After the interventions, for radiological follow-up, MRI (without contrast medium administration) was recommended between the third and sixth months and CTI after the 12th month. Follow-up MRI was recommended for all the patients (if possible and could be done without sedation in pediatrics). Follow-up CTI was not routinely recommended for the patients younger than a 10-year-old to protect them from irradiation except for suspicious findings on follow-up radiographic images or clinical requirements. All the CT examinations were performed under the supervision of the radiologist who performed the interventions to obtain a targeted low-dose CT examination. Shields were used to protect the gonads during CT examinations.
Patients with at least one follow-up MRI and/or CTI were included in the study. Patients who were lost for follow-up or did not accept the invitation for follow-up radiological imaging were excluded. All the follow-up MRIs and CTIs were evaluated retrospectively. Radiological findings such as healing of the nidus, reactive sclerosis, periosteal reaction, regression of reactive synovitis, edema (in bone-marrow and adjacent soft tissue), and recovery of muscle atrophy were evaluated by comparing the pre and post-treatment images. Healing of these findings was considered as recovery while worsening was considered as relapse. The radiological response was assessed according to the last follow-up radiological findings of either MRI or CTI. Response to the treatment was noted in three categories in accordance to recovery rate of radiological findings as complete/almost-complete, partial, and minimal-no recovery. If regression of the pretreatment radiological findings (such as healing of edema in bone-marrow and adjacent soft-tissue), reduction of joint synovitis, recovery of reactive sclerosis, regression of periosteal thickening, healing of muscle atrophy, mineralization of nidus) was clearly prominent compared to the posttreatment findings, it was defined as complete/almost-complete radiological recovery. If edema/ synovitis prominently regressed while periosteal thickening/ sclerosis or muscle atrophy slightly regressed, it was defined as partial radiological recovery. If there was no prominent change in any of the pretreatment findings, it was defined as minimal-no recovery accordingly.
For statistical analysis, Statistical Package for the Social Sciences (SPSS) for Windows (Version 15.0, Chicago, SPSS Inc.) program was used. Descriptive statistics were given as numbers and percentages for categorical variables and mean, standard deviation, minimum, maximum, and median for numerical variables.
RESULTS
A total of 131 patients [90 (68.70%) males and 41(31.30%) females; mean age 16.0±7.58 years] with at least one follow-up CTI or MRI were included in this study. Considering the localization of the OOs, 84 (64.12%) of them were localised in the femur head, neck, and diaphysis. The mean follow-up period was 38 (8-59) months and 123 (94%) of the patients had more than 1-year of follow-up.
All the patients had pretreatment CTIs as a part of the routine diagnostic workup. Also, 124 (95%) of the patients had pre-treatment MRIs. After RFAs, 84 (64.1%) had CTI and 108 (82.4%) had MRI follow-up. Forty-two (32.1%) of the patients had more than one follow-up MRI while none had more than one follow-up CTI.
Evaluating the technical and clinical success, all the patients had successful RFA treatments that all were pain-free in 24 hours. The Re-ablation therapies were applied in 2 cases due to known OO pain recurrence on the 12th and 29th months. Nine minor complications were seen related to the RFAs and all were treated successfully. No major complications occurred.
Evaluating the radiological success with follow-up CTIs and MRIs, complete/ almost complete-recovery (reduced size of the niduses, its surrounding sclerosis, amount of edema in bone-marrow or adjacent soft-tissue, and posture-deformity) was observed in 92 (70.2%), partial-recovery in 35 (26.7%), and minimal-recovery in 4 (3.1%) of all cases.
DISCUSSION
In OO patients, the most crucial determinant factor in evaluating the response to treatment is pain-relief.1-8 However, it is a matter of curiosity whether the radiological response accompanied the clinical response. Current studies in the literature have shown that, although radiological response supports clinical response, it does not always reflect the success of the procedure.11-14
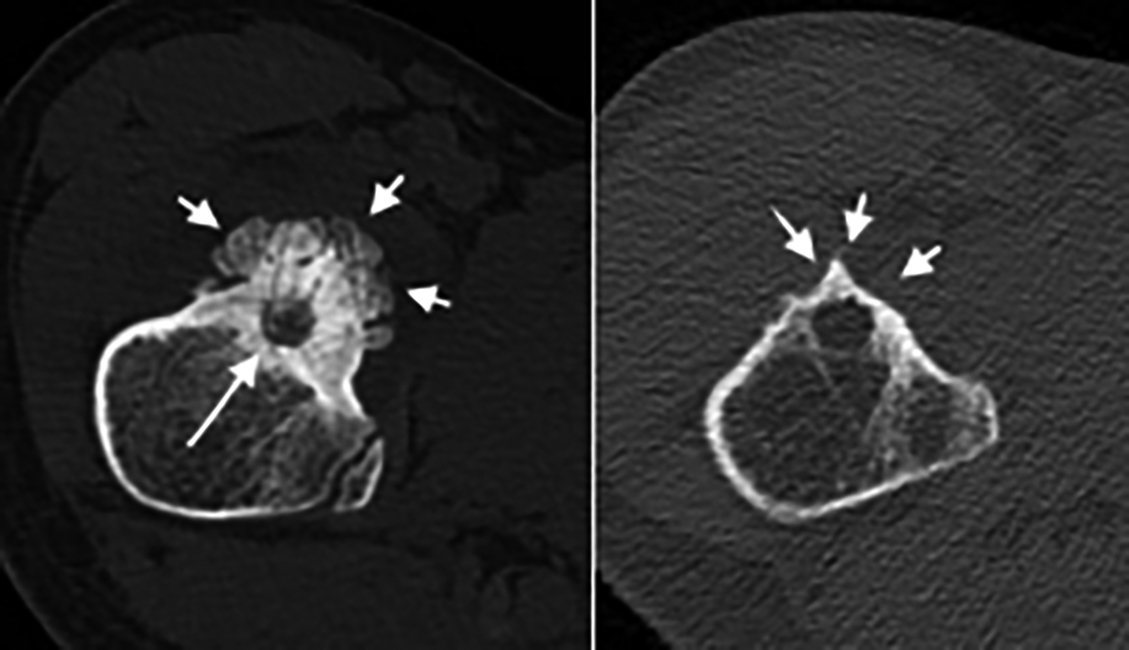 Figure 1 (a,b): A 13-year-old boy with an osteoid osteoma in the right femoral neck. The radiolucent nidus (arrow) and prominent reactive periosteal reaction (arrowheads) are well presented in pretreatment CTI (a). Although the nidus was visible, significant regression of periosteal reaction and cortical sclerosis was demonstrated at follow-up CTI at 18th month (b). This patient was categorised as complete/almost complete recovery group.
Figure 1 (a,b): A 13-year-old boy with an osteoid osteoma in the right femoral neck. The radiolucent nidus (arrow) and prominent reactive periosteal reaction (arrowheads) are well presented in pretreatment CTI (a). Although the nidus was visible, significant regression of periosteal reaction and cortical sclerosis was demonstrated at follow-up CTI at 18th month (b). This patient was categorised as complete/almost complete recovery group.
In this study, considering 83% of the patients presented with a complete (/almost complete) recovery, the radiological success supports the clinical success substantially (Figure 1). On the other hand, although 17% of the patients had a partial or minimal recovery, none had pain relapse. This result supports the fact that radiological recovery may be partial, although the clinical recovery is total.
While the clinical response may be present within a short period, such as 24 hours, seeing the radiological response may take longer. Since there is no specific algorithm or recommendation for optimal follow-up times, different follow-up protocols were established in the upcoming literature.15-20 As there was insufficient experience in this subject, follow-up imaging times were planned according to the authors’ observation. Although nidus of OO, reactive sclerosis and periosteal-reaction were best visualised in CTI, it was not as good as MRI to reveal healing of edema in bone marrow and soft tissue. Edema signs showed a prominent decrease in the MRIs performed in the third month or later. If MRIs were performed earlier than three months, post-RFA edema and reactive synovitis could be confusing or accidentally interpreted as pseudo-progression of reactive synovitis or muscle atrophy, especially for intra-articular OOs. Presentation of radiological response in CTI almost in all the patients took more than one year. Thus, MRI, were performed in the third month or later and the cases were not invited for follow-up CT earlier than one year of follow-up time in this study.
Radiological follow-up is especially beneficial to diagnose a recurrence. For example, in the first follow-up MRI of a patient after RFA, reduction of bone marrow and soft tissue edema supports healing of an OO and benefit of a treatment. If relapse of the pain develops in the same patient, re-occurrence of the edema in the second follow-up MRI strongly supports the relapse of the OO. Then a second RFA can be planned undoubtedly. In this study, re-ablation therapy was applied in 2 cases due to the pain recurrence (11 and 13-year boys who had OOs in femur-diaphysis). Recurrence of edema in bone marrow and adjacent soft tissue were the signs of relapse in both of the patients. Follow-up MRIs were supportive and beneficial for planning re-ablation therapies (Figure 2).
Follow-up imaging is not only valuable in terms of monitoring the OO nidus and reactive sclerosis but also in monitoring the secondary radiological findings. In two patients (a 10-year-girl and a 12-year-boy with OOs in the femur neck), who had intra-articular OO, edema, and periarticular synovial-fluid persisted for 18 and 28 months after RFA, respectively. An increase was observed in periarticular synovial-fluid amount which could be assumed as pseudo progression (Figure 3). Since children had no pain relapse, re-ablation was not planned. The boy was referred to physical-rehabilitation-therapy due to postural-distortion and secondary muscle atrophy occurred as a long-term outcome of OO. The girl recovered faster and did not need physical therapy. Given these two patients, regression of extra-synovial fluid may be prolonged in especially intra-articular OOs. In two other patients, post treatment muscle atrophy occurred due to tendon injury in needle-track and they have recovered after receiving physical therapy. It should be kept in mind that post treatment MRI is also necessary to evaluate procedure-related secondary radiological findings.
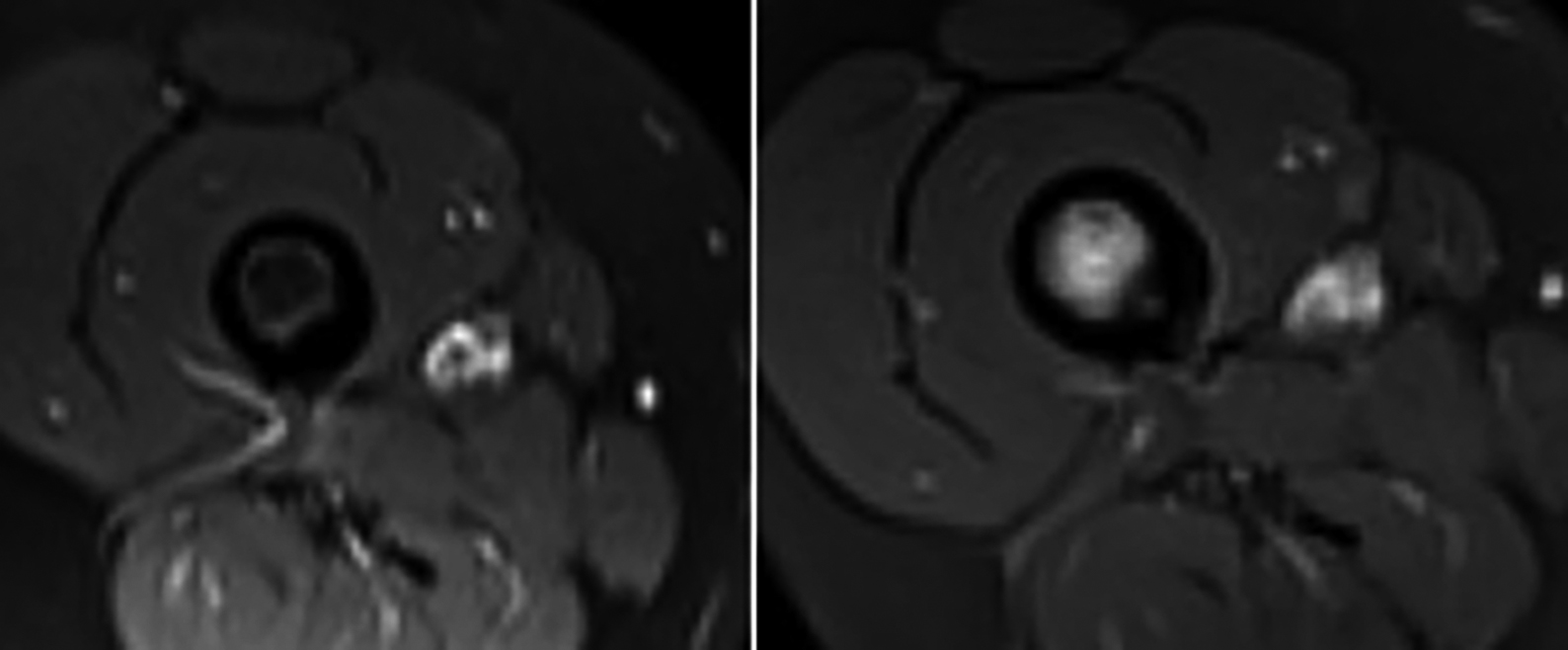 Figure 2 (a,b): An 11-year-old boy with osteoid osteoma in the diaphysis of the right femur. After RFA, follow-up MRI at 6th month (a) revealed treatment success. The boy had a pain relapse in the 12th month. In the 12th months follow-up MRI (b), bone marrow and soft tissue edema were seen which supported the relapse. Thus, a second successful RFA was performed.
Figure 2 (a,b): An 11-year-old boy with osteoid osteoma in the diaphysis of the right femur. After RFA, follow-up MRI at 6th month (a) revealed treatment success. The boy had a pain relapse in the 12th month. In the 12th months follow-up MRI (b), bone marrow and soft tissue edema were seen which supported the relapse. Thus, a second successful RFA was performed.
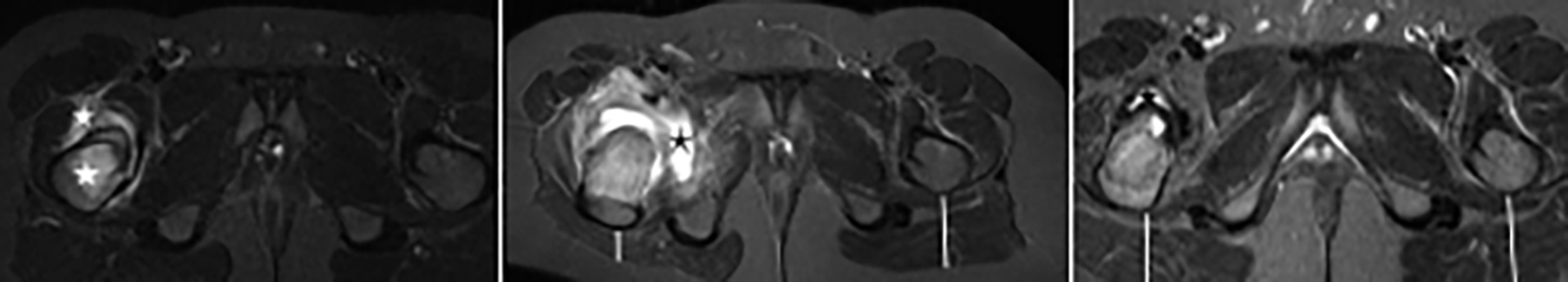 Figure 3a-c: A 12-year-old boy with an osteoid-osteoma in the right femoral-neck. In pre-treatment MRI (a), nidus, reactive cortical-sclerosis, periosteal-reaction, bone marrow edema, and synovitis (asterisks) in the right hip joint are seen clearly. On follow-up MRI at the 6th (b) month, an increase in joint effusion and atrophy of the right gluteal muscles were noticeable (line) which could be misdiagnosed as pseudoprogression. Finally, at 28th month, follow-up MRI (c) revealed recovery of the gluteal muscles (lines) and regression in the hip joint fluid (asterisk).
Figure 3a-c: A 12-year-old boy with an osteoid-osteoma in the right femoral-neck. In pre-treatment MRI (a), nidus, reactive cortical-sclerosis, periosteal-reaction, bone marrow edema, and synovitis (asterisks) in the right hip joint are seen clearly. On follow-up MRI at the 6th (b) month, an increase in joint effusion and atrophy of the right gluteal muscles were noticeable (line) which could be misdiagnosed as pseudoprogression. Finally, at 28th month, follow-up MRI (c) revealed recovery of the gluteal muscles (lines) and regression in the hip joint fluid (asterisk).
There are a few limitations of this study. First, the severity of pre treatment radiological findings was not the same per patient due to the symptomatic time spent until the diagnosis, age of the patients, localisation, the severity of the secondary radiological findings. Thus, the speed and recovery rate of the posttreatment radiological findings were not the same either. Second, the best follow-up interval period has not been established yet. Thus, a standard follow-up protocol could not be applied and a cut-off time regarding the recovery ratio in radiological findings could not be achieved. Third, radiological follow-up examinations were offered to the patients only as a suggestion and patients were not obliged. So, follow-up images were not performed in a standard way for every patient at the 3rd, 6th, and 12th-month. Nevertheless, although almost one-third of the patients were lost in the course of follow-up, the number of patients adapting to radiological follow-up was considerable when comparing the studies in the literature.
CONCLUSION
Follow-up CTI and MRI findings have demonstrated that RFA is effective and safe in OO treatment. Although the radiological response is a strong indicator of success, a late or weak response does not indicate that the treatment has failed. Radiological recovery of OO after RFA may occur much later than clinical recovery. Follow-up MRI is beneficial, primarily if performed later than the 3rd month, and mineralisation of the nidus is better seen after the 12th month in CTI. After RFA, at least one follow-up MRI may suggest evaluating the treatment outcome and monitoring the healing or worsening of the radiological findings. A routine follow-up CTI may not be needed unless planning a re-ablation due to pain relapse.
ETHICAL APPROVAL:
The study was approved by the Ethical Committee of Istanbul Sisli Hamidiye Etfal Training and Research Hospital (No. 1879; Date: 23.01.2018) and was conducted in accordance with the 1964 Helsinki declaration.
PATIENTS’ CONSENT:
Informed patients’ consents were obtained from all the patients for RFAs and follow-up radiological examinations.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
CS, AHB: Planned and performed the interventions and supervised radiological follow-up examinations.
MAT: Performed orthopaedic physical examinations.
MB, OG: Contributed to the data collection.
All the authors contributed to the study design and interpretation of the data. All authors contributed to the preparation of the manuscript and gave final approval of the current version for submission.
REFERENCES
- Lassalle L, Campagna R, Corcos G, Babinet A, Larousserie F, Stephanazzi J, et al. Therapeutic outcome of CT-guided radiofrequency ablation in patients with osteoid osteoma. Skeletal Radiol 2017; 46(7):949-56. doi: 10.1007/s00256-017-2658-x.
- Cioni R, Armillotta N, Bargellini I, Zampa V, Cappelli C, Vagli P, et al. CT-guided radiofrequency ablation of osteoid osteoma: Long-term results. Eur Radiol 2004; 14(7):1203-8. doi: 10.1007/s00330-004-2276-6.
- Rosenthal DI, Springfield DS, Gebhardt MC, Rosenberg AE, Mankin HJ. Osteoid osteoma: Percutaneous radio-frequency ablation. Radiol 1995; 197(2): 451-4. doi: 10. 1148/radiology.197.2.7480692.
- Donkol RH, Al-Nammi A, Moghazi K. Efficacy of percutan-eous radiofrequency ablation of osteoid osteoma in children. Pediatr Radiol 2008; 38(2):180-5. doi: 10. 1007/s00247-007-0690-z.
- Weber MA, Sprengel SD, Omlor GW, Lehner B, Wiedenhofer B, Kauczor HU, et al. Clinical long-term outcome, technical success, and cost analysis of radiofrequency ablation for the treatment of osteoblastomas and spinal ostoeoid osteomas in comparison to open surgical resection. Skletal Radiol 2015; 44(7):981-93. doi: 10.1007/s00256-015-2139-z.
- Pinto CH, Taminiau AH, Vanderschueren GM, Hogendoorn PC, Bloem JL, Obermann WR. Technical considerations in CT-guided radiofrequency thermal ablation of osteoid osteoma: Tricks of the trade. AJR Am J Roentgenol 2002; 179(6):1633-42. doi: 10.2214/ajr.179.6.1791633.
- Lindner NJ, Ozaki T, Roedl R, Gosheger G, Winkelmann W, Wortler K. Percutaneous radiofrequency ablation in osteoid osteoma. Percutaneous radiofrequency ablation in osteoid osteoma. J Bone Joint Surg Br 2001; 83(3):391-6. doi: 10.1302/0301-620x.83b3.11679.
- Martel J, Bueno A, Ortiz E. Percutaneous radiofrequency treatment of osteoid osteoma using cool-tip electrodes. Eur J Radiol 2005; 56(3):403-8. doi: 10.1016/j.ejrad. 2005.05.014.
- Greenspan A. Benign bone-forming lesions: Osteoma, osteoid osteoma, and osteoblastoma. Clinical, imaging, pathologic, and differential considerations. Skeletal Radiol 1993; 22(7):485-500. doi: 10.1007/BF00209095.
- Carneiro BC, Da Cruz IAN, Ormond Filho AG, Silva IP, Guimarães JB, Silva FD, et al. Osteoid osteoma: The great mimicker. Insig Imag 2021; 12(1):32. doi: 10.1186/ s13244-021-00978-8.
- Barei DP, Moreau G, Scarborough MT, Neel MD. Percutaneous radiofrequency ablation of osteoid osteoma. Clin Orthop Relat Res 2000; 373:115-24. doi: 10.1097/00003086-200004000-00014.
- Yuce G, Aytekin N, Eren S, Genç B, Ateş OF, Canyigit M. Is radiofrequency ablation safe and effective in treating osteoid osteomas? A prospective single-centre study with atypical cases. J Orthop Surg (Hong Kong) 2020; 28(3): 2309499020960555. doi: 10.1177/2309499020960555.
- De Filippo M, Russo U, Papapietro VR, Ceccarelli F, Pogliacomi F, Vaienti E, et al. Radiofrequency ablation of osteoid osteoma. Acta Biomed 2018; 89(1-S):175-85. doi: 10.23750/abm.v89i1-S.7021.
- Bhavin J, Nishigandha B. Percutaneous radiofrequency ablation for osteoid osteoma: How we do it. Indian J Radiol Imag 2009; 19(1):36-42. doi: 10.4103/0971- 3026.44523.
- Vanderschueren GM, Taminiau AH, Obermann WR, van den Berg-Huysmans AA, Bloem JL, van Erkel AR. The healing pattern of osteoid osteomas on computed tomography and magnetic resonance imaging after thermocoagulation. Skeletal Radiol 2007; 36(9):813-21. doi: 10.1007/s00256-007-0319-1.
- Rehnitz C, Sprengel SD, Lehner B, Ludwig K, Omlor G, Merle C, et al. CT-guided radiofrequency ablation of osteoid osteoma: Correlation of clinical outcome and imaging features. Diagn Interv Radiol 2013; 19(4):330-9. doi: 10.5152/dir.2013.096.
- Chahal A, Rajalakshmi P, Khan SA, Rastogi S, Srivastava DN, Gamanagatti S. CT-guided percutaneous radiofrequency ablation of osteoid osteoma: Our experience in 87 patients. Indian J Radiol Imag 2017; 27(2):207-15. doi: 10.4103/ijri.IJRI_260_16.
- Lorenc T, Kocoń H, Gołębiowski M. Computed tomography-guided percutaneous radiofrequency and laser ablation for the treatment of osteoid osteoma – long-term follow-up from 5 to 10 years. Polish J Radiol 2021; 86(1):19-30. doi: 10.5114/pjr.2021.102678.
- Chaudhry MBH, Salam B, Khandwala K, Sayani R, Muhammad A, Haq TU. Image-guided percutaneous radiofrequency ablation for osteoid osteoma: Experience from a Developing Nation. Cureus 2019; 11(9):e5633. doi: 10.7759/cureus.5633.
- Cantwell CP, Kerr J, O'Byrne J, Eustace S. MRI features after radiofrequency ablation of osteoid osteoma with cooled probes and impedance-control energy delivery. AJR Am J Roentgenol 2006; 186(5): 1220-7. doi: 10.2214/AJR.05.0149.