Quality of Life Index in Patients with Vitiligo
By Saadia Tabassum1, Atiya Rahman2, Rabia Ghafoor3, Qamaruddin Khan4, Najia Ahmed5, Tazein Amber1, Nadia Iftikhar7, Bazil Musharraf1, Nadia Ali Azfar7, Uzma Bashir8, Zartash Arshad9, Safia Awan1Affiliations
doi: 10.29271/jcpsp.2023.05.521ABSTRACT
Objective: To ascertain the quality of life (QoL) impairment among the Pakistani population with vitiligo and to determine the relationship between sociodemographic and clinical characteristics.
Study Design: Cross-sectional study.
Place and Duration of the Study: Dermatology Outpatients at the Aga Khan University in collaboration with outpatients of seven tertiary care hospitals of Sindh, Punjab, KPK, Balochistan, and AJK to collect data from March 2015 to April 2019.
Methodology: All clinically diagnosed patients of vitiligo, who signed consent and assent forms, were included in the study. A validated 25-item, QoL scale for vitiligo was used. Socio-economic status of the patients, clinical assessment of the disease and patients’ engagement in social and domestic lives was noted.
Results: Five hundred and seventy-three patients were enrolled in the study, having mean age 29.8 ± 16.2 years. In 306 (53.4%) males and 267 (46.65%) females; 21.8% were below 18 years. Mean vitiligo QoL index was 38.4 ± 11.8. Patients of vitiligo with disease duration 5-10 years, those affected on exposed parts, more than five body sites, rapidly progressing disease and of female gender had a higher impairment of quality of life. These scores were found significantly higher as compared to other levels of these parameters (p<0.05).
Conclusion: Patients with vitiligo experience low self-esteem. The disease adversely affects their quality of life. The authors recommend the use of disease-specific instruments to assess the quality of life which enables the treating physician to devise best possible management plan individually.
Key Words: Vitiligo, Quality of life, Pakistan, Vitiligo life quality index.
INTRODUCTION
Vitiligo is a disorder characterised by milky white macules and patches which runs a chronic unpredictable course. The worldwide incidence is 0.5-2% with average age of onset being 20 years.1 The aetiology remains unclear; however, the autoimmune hypothesis is the most widely accepted one. There is no ethnic or racial predilection and affects both genders equally.1
It is one of the most aesthetically disfiguring conditions in people of colour with serious implications on quality of life (QoL). In South Asian countries, including Pakistan, the depigmented patches are often in stark contrast to normal skin, making the patients suffer psychologically to the extent of social isolation. There is also a significant role of media in promoting the ethos of physical beauty.2 This demands special consideration while treating this emotionally morbid condition and to assess the QoL in these individuals.1,3 The relationship between skin diseases and QoL is taken as a guideline to tailor individual treatment. Various scoring tools are available e.g. vitiligo life quality index (VLQI), Vitiligo impact scale (VIS), VITIQol, and VIS-22.3,4
Few studies to assess QoL in vitiligo patients have already been conducted in this region. However, they are single centred studies employing non-specific tools like Dermatology Life Quality Index (DLQI).5 Keeping in view the diverse cultural variations across the country, this study covered most regions and used a validated disease-specific tool.4 The aim of this study was to ascertain the QoL impairment among the Pakistani population with vitiligo and to determine the relationship between sociodemographic and clinical characteristics with QoL.
METHODOLOGY
This cross-sectional study involved collaboration of The Aga Khan University Hospital with seven secondary and tertiary care hospitals situated in four provinces of Pakistan namely Combined Military Hospital Lahore, Jinnah Postgraduate Medical Center Karachi, Combined Military Hospital Peshawar, Sheikh Khalifa Bin Zaid Hospital Azad Jammu and Kashmir, Military Hospital Rawalpindi, Ganga Ram Hospital Lahore, and Combined Military Hospital Quetta. The data collection started after approval from the Ethical Review Committee of Aga Khan University. The duration of data collection was from April 2015 to April 2019. A validated 25-item, QoL scale developed by Senol et al., named as Vitiligo Life Quality Index (VLQI) was used.4 This scale consists of 25 questions comprising of demographic and clinical parameters of the patients. It is a reliable tool with high internal consistency, with test-retest for reproducibility.4 The study participants were consecutively enrolled using convenient sampling technique as they presented to the clinic and fulfilled the inclusion criteria. Patients of all age groups and genders, clinically diagnosed with vitiligo, who consented to participate in the study were included. In case of children <7 years, assent forms were filled by the guardian/parent. Patients with other skin diseases affecting pigmentation, those with underlying neuropsychiatric illnesses, functional disability or deformity and pregnancy, were excluded as these conditions may already affect the QoL. Clinical assessment, socioeconomic status, and engagement in social and domestic lives were recorded in a separate room maintaining confidentiality. The performas were kept under lock and key and were only accessible to research team members.
The sample size was calculated using online sample size calculator of proportion available at www.openepi.com, after inserting 2% worldwide incident rate of vitiligo at 1.4% margin of error and 95% confidence interval, the authors have required at least n=571 samples for this study.
Data were stored and analysed using IBM-SPSS version 23.0, Count with percentages were reported for qualitative characteristics of vitiligo patients, information on diseases history, its duration, progression, diet habits and lifestyles. Mean with standard deviation reported for quality of life scores obtained using vitiligo questionnaire, the mean scores were compared using independent sample-test and one-way ANOVA across studied factors. Post hoc analysis was done using Tukey’s HSD test. Correlation analysis of quality of life scores with emotion, anxiety, personal relations, leisure activities, school/work, and clinical findings domains was also done using Pearson correlation, p-values less than 0.05 were considered statistically significant. Pie chart, bar diagram, and scatter Plots were also done to give a useful presentation of data.
RESULTS
The study comprised of 573 patients; 53.4% (306) were males and 46.6% (267) were females. Majority of the patients belonged to age group between 26-45 years, as shown in Table I. Representation from various provinces was ensured, with patients from Sindh making up the greater part i.e. 44% of the total study population. The majority (71.4%) of the patients had vitiligo for five or fewer years and majority (74.8%) had two or more body sites involvement. Sixty-four (11.2%) patients thought their disease was slowly spreading.
The mean score of quality of life assessed by VLQI of all study patients was 38.4±11.8. The mean scores of quality of life was 45.6±12.5 in AJK samples, 44.3±13 among samples with age group 26-45 years, 41.7±12.3 among females, 45.3±12.2 among Sindhi ethnicity samples, 42.3 ±11.9 among samples with 5-10 years of vitiligo history, 42.3±13 among samples with more than five affected areas, 40.9±12.5 among samples with symmetrical vitiligo pattern, and 45.5±14 among rapidly progressing ones. These scores were found significantly higher as compared to other levels of these parameters (p<0.01).
When QoL was compared with treatment and its outcome, as shown in Table I, patients who had previously sought medical advice for their skin disease were 178 (59.9%). However, those who had received treatment had higher VLQI score (40.3 ±12.1) as compared to those who were not on treatment (38.4±12.1). About half of the patients 183 (43.2%) had been on treatment for more than a year. Large proportion of the patients had received topical creams and ointments (40.5%), followed by systemic steroids (25.3%) and phototherapy (11.5%) as part of their treatment, as depicted in Figure 1. Only 5 patients (0.9%) noticed complete re-pigmentation of the lesions after completion of treatment. No significant association was established between VLQI scores and treatment duration (p=0.24) or response to treatment (p=0.58).
Family history of vitiligo was present in 146 (25.7%) of the patients, whereas family history of other autoimmune disorders was noted in 174 (30.4%). Patients with family history of vitiligo had mean VLQI of 41.9±13.2. Those having history of other autoimmune disorders had mean VLQI of 45.5±12.9. Both these scores were significantly higher as compared to other comparable levels (p<0.05).
Four hundred and twenty-six (74.3%) patients did not wear sunblock before going out in the sun. Noteworthy, 20.2% (116) had a sunburn reaction in their life and had statistically higher VLQI scores (43.5, p=<0.01). Ninety-nine (17.3%) of the patients were active smokers, and had a disturbed VLQI (41.4) as compared to non-smokers. Two hundred and sixty-five (46.2%) of patients were physically active in terms of exercise or playing sports. Majority of them preferred walking (n=165, 51.7%). Higher VLQI scores were noted in those who did not practice any physical activity (42.03) with a statistically significant p-value of <0.01.
Table I: Correlation between characteristics of vitiligo patients with quality of life (using one way ANOVA and independent sample t-test).
|
Parameters |
n (%) |
QoL mean (SD) |
p-value |
|
|
Region |
Sind |
252 (44) |
42 (12.8) |
<0.01* |
|
Punjab |
121 (21.2) |
39.5 (11.6) |
||
|
KPK |
115 (20.1) |
33.5 (8.9) |
||
|
Baluchistan |
33 (5.8) |
36.3 (7.8) |
||
|
AJK |
52 (9.1) |
45.6 (12.5) |
||
|
Age group |
<18 yrs |
125 (21.8) |
36.3 (9.1) |
<0.01* |
|
18-25 |
138 (24.1) |
37.3 (12) |
||
|
26-45 |
215 (37.5) |
44.3 (13) |
||
|
>45 yrs |
95 (16.6) |
37.6 (10.9) |
||
|
Gender |
Male |
306 (53.4) |
38.1 (11.8) |
<0.01* |
|
Female |
267 (46.6) |
41.7 (12.3) |
||
|
Vitiligo duration |
Less than one years |
198 (34.6) |
38.1 (11.3) |
<0.01* |
|
1-5 years |
211 (36.8) |
41.1 (13.2) |
||
|
5-10 years |
84 (14.7) |
42.3 (11.9) |
||
|
>10 Years |
80 (14) |
37.5 (11) |
||
|
Progression of vitiligo |
Not spreading |
169 (29.5) |
36.7 (11) |
<0.01*
- |
|
Slowly spreading |
340 (59.3) |
40.2 (11.9) |
||
|
Rapidly spreading |
64 (11.2) |
45.5 (14) |
||
|
Have you had treatment for your vitiligo? |
Yes |
178(59.9) |
40.3(12.1) |
0.08 |
|
No |
119(40.1) |
38.4(12.1) |
||
|
Treatment Duration |
≤1 years |
180(42.5) |
40.2(12.1) |
0.24 |
|
2 -10 years |
183 (43.2) |
42.4(13.1) |
||
|
>10 years |
57 (13.4) |
39(11.5) |
||
|
After this treatment, what was the status of your vitiligo? |
No improvement |
244 (42.6) |
41.8(12.8) |
0.58 |
|
Slightly improvement |
247 (43.1) |
40.5(12.3) |
||
|
Significantly improvement |
77 (13.4) |
39.4(11.8) |
||
|
Complete regimentation of the lesions |
5 (0.9) |
41.3(10.3) |
||
|
Is there a history of vitiligo in your family? |
Yes |
146(25.7) |
41.9(13.2) |
0.014* |
|
No |
421(74.3) |
39(11.7) |
||
|
Do you use sun block |
Whenever i go out in sun |
48(8.4) |
44.06(13.15) |
<0.01* |
|
|
Often but not always |
41(7.2) |
42.24(11.89) |
|
|
|
Occasionally |
58(10.1) |
45.5(11.77) |
|
|
|
Never |
426(74.3) |
38.15(11.69) |
|
|
Have you ever had sunburn? |
Yes |
116(20.2) |
43.53(11.87) |
<0.01* |
|
|
No |
457(79.8) |
38.71(11.99) |
|
|
*p-value <0.05 was considered statistically significant. |
||||
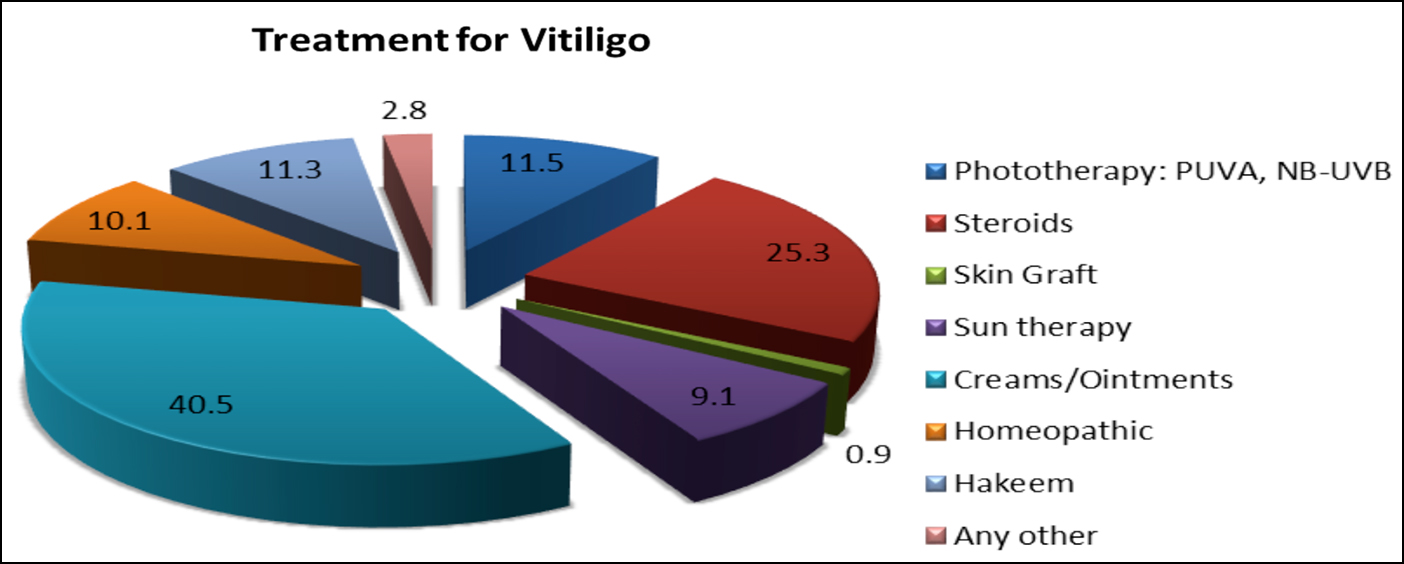 Figure 1: Different medications used by study patients.
Figure 1: Different medications used by study patients.
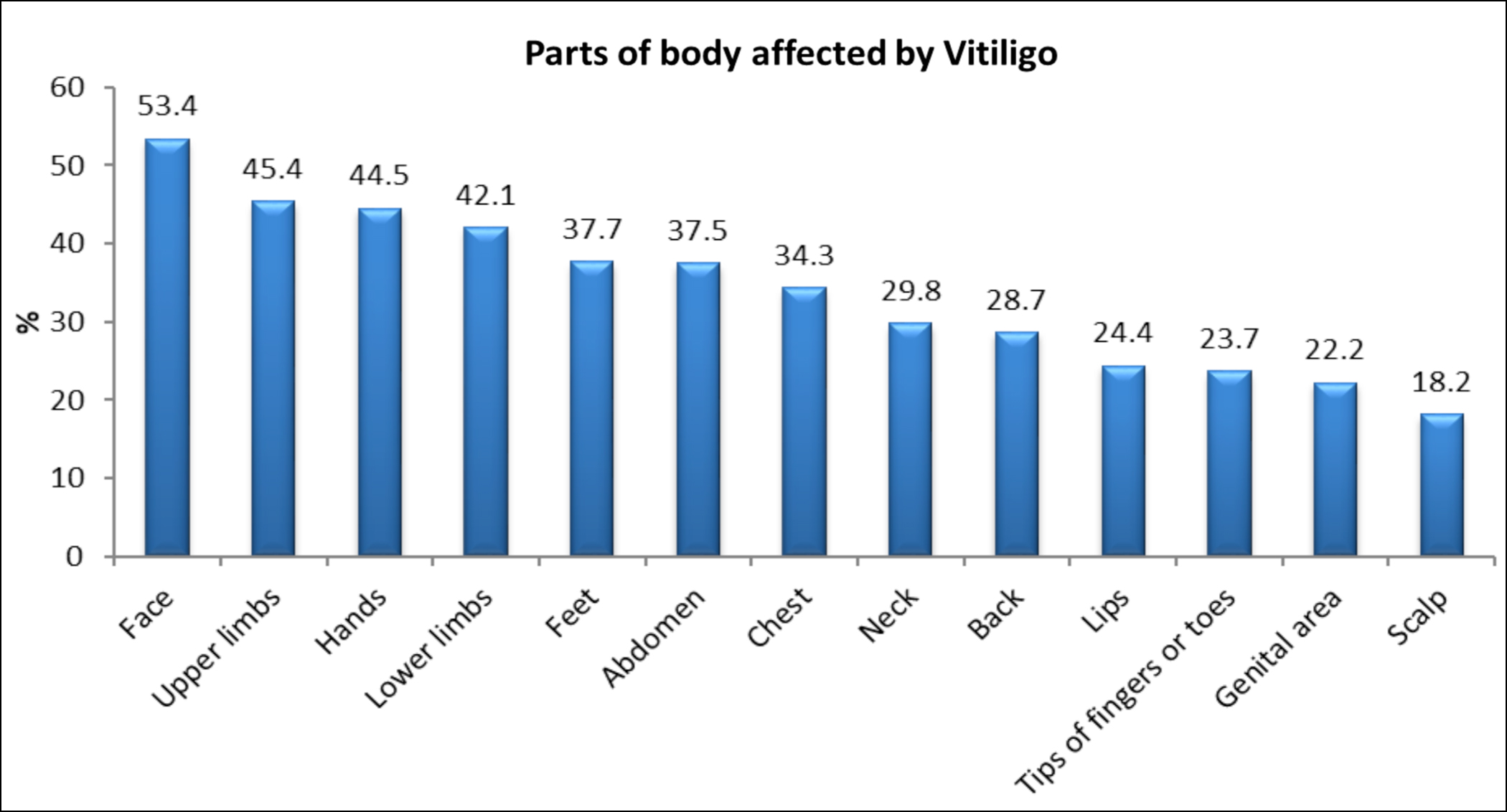 Figure 2: Different body sites involved by vitiligo in the study participants.
Figure 2: Different body sites involved by vitiligo in the study participants.
Table II: Body sites involvement compared to quality of life (using independent sample t-test).
|
Site |
Yes |
No |
p-value |
|
Face |
38.3 ± 12.3 |
34.6 ± 11.2 |
<0.001 |
|
Scalp |
40.2 ± 13.3 |
35.7 ± 11.5 |
0.001 |
|
Neck |
40.0 ± 13.2 |
35.0 ± 11.1 |
<0.001 |
|
Upper limbs |
39.5 ± 12.7 |
34.1 ± 10.7 |
<0.001 |
|
Hands |
39.7 ± 12.7 |
34.0 ± 10.7 |
<0.001 |
|
Lips |
39.2 ± 12.9 |
35.7 ± 11.6 |
0.002 |
|
Trunk |
37.5 ± 11.1 |
39.9 ± 12.2 |
0.09 |
|
Genitalia |
39.5 ± 12.7 |
39.7 ± 1..9 |
0.85 |
The correlation analysis of quality of life showed a significant positive association with scores of emotions (87.3%), anxiety (86.5%), personal relations (79.7%), leisure activities (74.1%), school/ work and 56.6% with clinical findings. All these correlations were statistically significant with p-value <0.01.
Involvement of different body parts with vitiligo is shown in Figure 2. QoL was impaired significantly when the head and neck region and upper limbs were involved. However, QoL was unaffected by genitalia or trunk involvement (p >0.05).
DISCUSSION
Vitiligo has a significant negative impact on the lives of affected individuals. Anxiety, depression, and significant psychosocial consequences are incumbent in these patients.6 The studies have shown that tools specifically designed for cutaneous disorders are better in gauging the impairment in QoL e.g. Psoriasis Quality of Life Scale (PSORIQoL) and Acne Quality of Life Scale (AQOLS) etc.4, 7-10; in contrast to generic tools like DLQI. It has been argued that DLQI might not be a suitable tool for assessing QoL impairment in Indian vitiligo patients.7 The authors have used the VLQI scoring method,4 which is a validated, reliable, and feasible tool with established internal consistency.
Impaired QoL varies with gender, culture, race and geographical region. Significant impairment has been documented in dark-skinned individuals; especially in South Asia where rejection and intolerance prevail regardless of socioeconomic status.7,11 They suffer pain in terms of social spurn, embarrassment, and humiliation. This study found participants from KPK province having the least impairment in their QoL i.e.33.5±8.9 which was statistically significant from the rest of the provinces (p<0.01). This ascribes to the fairer complexion of Pathans generally as compared to the rest of the population as well as socio-cultural norms of that area. A recent study has shown moderate to extremely large impairment of QoL in 65.3% of vitiligo patients employing DLQI scale; whereas the same study population using VIS-22 scale indicated 32.7% to have moderate to extremely large effect on their QoL. No or minimal effect on QoL was almost equal on both scales, respectively [DLQI (14.7%) and VIS-22 (13.3%)].12 Senol et al. found mean VLQI score of 44.0±12,4 which is higher than this study’s overall VLQI score of 38. These results are corroborated by earlier findings documented by Amer and Gao who found Turkish, Arabs, and Indians to have significantly higher impairment of QoL than Europeans.13
Total body surface area involved by vitiligo correlates with the QoL impairment.4,12,13 Body surface area involvement of >5% having statistically significant impairment of QoL as compared to those with less than 5% involvement (p = 0.015).4 This study took into account number of body sites involved and found vitiligo affecting single body part had mean QoL of 35.1±9.5, whereas score rose to 42.3±13 in those afflicted by vitiligo on more than five body sites.
Disease evolution influences the QoL, patients with regressive vitiligo had statistically significantly less mean scores on DLQI and VIS-22 as compared to patients with stable or progressive vitiligo (p-values ≤0.014 and 0.006, respectively).12 Similar results were seen in this study, where patients with rapidly spreading vitiligo had the worst QoL (p <0.01).
A noteworthy finding in this study was that there was no significant relationship between treatment history and QoL (p = 0.08). Literature review indicated similar trend.4 Similarly, the relationship between the length of disease and QoL is variable.13 This studys’ results showed patients with longer disease duration (more than 10 years) had lower scores than those with disease duration 1-10 years. Perhaps the patients overcome their feelings of discomfort and isolation as they accept with time the chronic nature of the disease.
There is lack of consensus regarding the influence of gender on QoL. Certain studies have shown no effect of gender,12,14,15 while others have demonstrated females having more impaired QoL as compared to males.16-18 Some studies have shown single women to have worse QoL and others married women.13,14 It indicates that the psychological effects of vitiligo are complex and multi-factorial and generalisations should be avoided in this condition. This study found females having worse QoL as compared to males (41.7±12.3 vs. 38.1±11.8). Women are more conscious of their physical appearance and societal expectation from them to have perfect skin might be responsible for their having lower QoL as compared to their male counterparts. Similarly, lesions on exposed parts e.g. face, neck, and hands had statistically significant impairment in QoL as supported by earlier studies.4,14 In a Mexican study conducted by Morales-Sanchez et al. QoL was found to be worst in patients with genital lesions as compared to lesions elsewhere on the body (p<0.001).19
Similar to this study, young adults are found to have the highest impairment of QoL.12,15,20 Kota et al., found age group 18-30 years more adversely affected by vitiligo.12 Bae et al. found the worst QoL during third decade of life.14 Yang et al. concluded that 20-59 year olds and people of higher socio-economic strata have more impaired QoL.21 This implies that the QoL of people who are generally in their prime productive years of life and contributing significantly towards social and economic progression are worst affected. Other studies of children have shown impaired QoL as they grow and 90% of the adolescent population aged 15-17 years was bothered by vitiligo 18,22
A large multi-ethnic study conducted in UK,23 divided patients into 3 groups: Caucasians, Pakistani- Indians, and Afro-Caribbeans. They found that the Pakistani-Indian subgroup scored significantly higher on the consequences scale as compared to the other two groups. The disease would be more prominent in them than the Caucasians. Interestingly the consequences scale was not statistically different amongst the Caucasian and Afro-Caribbean groups. This highlights the significance of the cultural norms or beliefs associated with the disease. Thought provokingly, significant number of study participants believed themselves or their friends/family members that vitiligo is an infectious disease. This indicates gross misconceptions about the disease, leading to stigmatisation and isolation.
QoL with vitiligo has been found to be equal to or even worse than people having chronic systemic disorders like arthritis, cancer, congestive heart failure, myocardial infarction, and diabetes etc.21 Baidya et al. studied 80 vitiligo patients and employed DLQI and Hamilton rating scale of depression and found depression significantly impairing their QoL.24 Similarly, Nazar et al., in a single centre study from Pakistan, found significant impairment in psychosocial predictors of QoL; such as body image, self-esteem, and perceived stigmatisation.25
In this study nearly 20% of the patients gave history of sunburn and had significantly higher VLQI (43.53 vs. 38.71) than those who did not experience sunburn. Vitiliginous skin, devoid of melanin, sunburns readily and QoL is negatively impacted. Dermatologists need to emphasize judicious use of sunscreens. Although medical literature generally supports that vitiligo does not increase the chances of skin cancer but few case reports and studies of non-melanoma skin cancers arising in vitiliginous skin have been published.
This study is the first largest multicentre study on vitiligo from Pakistan. However, there were study limitations like sample collection from dermatology clinics, which may not be the actual representation of vitiligo in the community. Nevertheless, the present authors have included major hospitals from the provinces of Pakistan to address this sampling bias. Another limitation was that the authors excluded patients with underlying depression or psychiatric diseases only based on past medical history and a screening tool which cannot be replaced by psychiatric evaluation.
The results of this study and comparison with previous published research indicate that there are diverse factors affecting the QoL of vitiligo patients. Routine use of a tool to assess QoL amongst vitiligo patients shall enable the treating physician to devise the best possible management plan tailored according to the needs of individual vitiligo patients.
CONCLUSION
Patients with vitiligo experience low self-esteem and the disease adversely affects QoL in Pakistani population. The vulnerable groups identified in this study were patients with disease duration 5-10 years, those with exposed parts or more than five body sites involvement, those with rapidly progressing disease, female gender, and age bracket 26-45 years.
DISCLOSURE:
The preliminary analysis-based results of this study were presented as an e-poster at the World Congress of Dermatology 2019, in Milan.
ETHICAL APPROVAL:
Approval from the Ethical Review Committee of Aga Khan University was obtained prior to initiation of the research work.
PATIENTS’ CONSENT:
Written informed consent from each patient was obtained that this data shall be published with anonymity.
COMPETING INTEREST:
The authors declare no competing interest.
AUTHORS’ CONTRIBUTION:
ST, AR: Conceptualisation, literature search, study design, data collection, data analysis plan, data interpretation, manuscript writing and proofreading.
RG, QK, NO, NI, NAA, UB, ZA: Study design and data collection.
TA, BM: Data entry, analysis, and manuscript writing.
SA: Data analysis and interpretation.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Bergqvist C, Ezzedine K. Vitiligo: A review. Dermatol 2020; 236(6):571-92. doi: 10.1159/000506103.
- Ivie EJ, Pettitt A, Moses LJ, Allen NB. A meta-analysis of the association between adolescent social media use and depressive symptoms. J Affect Disord 2020; 275:165-74. doi: 10.1016/j.jad.2020.06.014.
- Lilly E, Lu PD, Borovicka JH, Victorson D, Kwasny MJ, West DP, et al. Development and validation of a vitiligo-specific quality-of-life instrument (VitiQoL). J Am Acad Dermatol 2013; 69(1):e11-8. doi: 10.1016/j.jaad.2012.01.038.
- Şenol A, Yücelten AD, Ay P. Development of a quality of life scale for vitiligo. Dermatol 2013; 226(2):185-90. doi: 10.1159/000348466.
- Noor SM, Khurshid K, Mahmood T, Haroon TS. Quality of life in vitiligo patients. J Pak Assoc Dermatol 2004;14(2):55-8. jpad.com.pk/index.php/jpad/article/view/782.
- Topal IO, Duman H, Goncu OE, Durmuscan M, Gungor S, Ulkumen PK. Knowledge, beliefs, and perceptions of Turkish vitiligo patients regarding their condition. An Bras Dermatol 2016; 91(6):770-5. doi: 10.1590/abd1806-4841.20165060.
- Narahari SR, Prasanna KS, Aggithaya MG, Bose KS, Praseeda TR. Dermatology life quality index does not reflect quality of life status of indian vitiligo patients. Indian J Dermatol 2016; 61(1):99-100. doi: 10.4103/0019-5154. 174048.
- McKenna SP, Cook SA, Whalley D, Doward LC, Richards HL, Griffiths CE, et al. Development of the PSORIQoL, a psoriasis-specific measure of quality of life designed for use in clinical practice and trials. Br J Dermatol 2003; 149(2):323-31. doi: 10.1046/j.1365-2133.2003.05492.x.
- Gupta MA, Johnson AM, Gupta AK. The development of an acne quality of life scale: Reliability, validity, and relation to subjective acne severity in mild to moderate acne vulgaris. Acta Derm Venereol 1998; 78(6):451-6. doi: 10.1080/00 0155598442773.
- Whalley D, McKenna SP, Dewar A Erdman RA, Kohlmann T, Niero M, et al. A new instrument for assessing quality of life in atopic dermatitis: International development of the Quality of Life Index for Atopic Dermatitis (QoLIAD). Br J Dermatol 2004; 150(2):274-83. doi: 10.1111/j.1365-2133. 2004.05783.x.
- Thompson AR, Clarke SA, Newell RJ, Gawkrodger DJ. Vitiligo linked to stigmatization in British South Asian women: Aqualitative study of the experiences of living with vitiligo. Br J Dermatol 2010; 163(3):481-6. doi: 10.1111/j.1365-2133. 2010.09828.x.
- Kota RS, Vora RV, Varma JR Kota SK, Patel TM, Ganjiwale J. Study on assessment of quality of life and depression in patients of vitiligo. Indian Dermatol Online J 2019; 10(2):153-7. doi: 10.4103/idoj.IDOJ_14_18.
- Amer AA, Gao XH. Quality of life in patients with vitiligo: An analysis of the dermatology life quality index outcome over the past two decades. Int J Dermatol 2016; 55(6):608-14. doi: 10.1111/ijd.13198.
- Bae JM, Lee SC, Kim TH, Yeom SD, Shin JH, Lee WJ, et al. Factors affecting quality of life in patients with vitiligo: A nationwide study. Br J Dermatol 2018; 178(1):238-44. doi: 10.1111/bjd.15560.
- Aradhya S, Manjunath K, Kasturi, Somaiah S. Quality-of-life and psychosocial impact of vitiligo in Indian patients. Pigment Int 2015; 2(1):28-33. doi: 10.4103/2349-5847. 159392.
- Ingordo V, Cazzaniga S, Medri M, Raone B, Digiuseppe MD, Musumeci ML, et al. To what extent is quality of life impaired in vitiligo? A multicenter study on Italian patients using the dermatology life quality index. Dermatol 2014; 229(3):240-7. doi: 10.1159/000363407.
- Sangma LN, Nath J, Bhagabati D. Quality of life and psychological morbidity in vitiligo patients: A study in a teaching hospital from north-East India. Indian J Dermatol 2015; 60(2):142-6. doi: 10.4103/0019-5154.152508.
- Catucci Boza J, Giongo N, Machado P, Horn R, Fabbrin A, Cestari T. Quality of life impairment in children and adults with vitiligo: A cross-sectional study based on dermatology-specific and disease-specific quality of life instruments. Dermatol 2016; 232(5):619-25. doi: 10. 1159/000448656.
- Morales-Sánchez MA, Vargas-Salinas M, Peralta-Pedrero ML, Olguín-García MG, Jurado-Santa Cruz F. Impact of vitiligo on quality of life. Actas Dermosifiliogr 2017; 108(7):637-42. doi: 10.1016/j.ad.2017.03.007.
- Wang KY, Wang KH, Zhang ZP. Health-related quality of life and marital quality of vitiligo patients in China. J Eur Acad Dermatol Venereol 2011; 25(4):429-35. doi: 10. 1111/j.1468-3083.2010.03808.x.
- Yang Y, Zapata L, Rodgers C, Hernandez K, Iyer M, Jia G, et al. Quality of life in patients with vitiligo using the Short Form-36. Br J Dermatol 2017; 177(6):1764-6. doi: 10. 1111/bjd.15936.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol 2014; 31(3):309-18. doi: 10.1111/pde.12226.
- Papadopoulos L, Bor R, Walker C, Flaxman P, Legg C. Different shades of meaning: Illness beliefs among vitiligo sufferers. Psychol Health Med 2002; 7(4):425-33. doi.org/ 10.1080/1354850021000015249.
- Baidya S, Dey P, Mohanty R. Assessment of quality of life in vitiligo patients attending a tertiary care hospital- A cross sectional study. Ind Psychiatry J 2021; 30:62-6. doi: 10.4103/ipj.ipj_16_20.
- Nazar I, Kamran F and Masood A. Psychosocial Predictors of quality of life in patients with vitiligo. Pak J Psychol Res 2021; 36(1): 19-36. doi:10.33824/PJPR.2021.36.1.02.