Protective Effects of N-Acetyl Cysteine on Undescended Testis after Orchiopexy: A Rat-model Study
By Eray Kemahli1, Ugur Uyeturk1, Ayhan Cetinkaya2, Sevilay Erimsah3, Ummugul Uyeturk4, Adnan Gucuk5Affiliations
doi: 10.29271/jcpsp.2023.03.319ABSTRACT
Objective: To assess the effectiveness of utilising N-acetyl cysteine (NAC) to treat tissue damage brought on by undescended testis (UT) in rats after orchiopexy.
Study Design: Experimental study.
Place and Duration of the Study: Bolu Abant İzzet Baysal University, Bolu, Turkey, from January 2018 to June 2020.
Methodology: The UT model was created by administering flutamide to pregnant rats. Four groups of animals were created as the control group (offsprings of pregnant rats without flutamide), group II (UT), group III (UT + orchiopexy), and group IV (UT + orchiopexy + NAC); each containing eight animals.
Results: Group IV had a higher level of glutathione peroxidase than groups III and II (p=0.001 and p=0.002, respectively). Malondialdehyde was reduced in group IV compared with groups III and II (both p<0.001). There were differences in mean apoptotic cell counts (ACC) among the groups (p<0.001). ACC in group IV was lower than in group III (p<0.001). Sperm counts were higher in group IV than ın groups III and II, and in group III they were higher than group II (p<0.001 all) and similar between groups IV and control group (p=0.102).
Conclusion: Orchiopexy reduced UT-related testicular damage, additionally using NAC following orchiopexy may further reduce testicular damage through its antioxidant effects.
Key Words: Undescended testis, Testis damage, Orchiopexy, N-acetyl cysteine, Antioxidant.
INTRODUCTION
Cryptorchidism, which includes ectopic testicles, undescended testis (UT), and inadequate testicular descent, is one of the prevalent congenital anomalies of the urogenital system in children.1 An UT is defined as a testis in a suprascrotal position that cannot be pulled down into the scrotum.2 Early detection and treatment of these kids are crucial for the best results in reducing disease-related problems.3 However, even in developed countries, the average treatment age is 4.9 years; only 36.4% of patients receive treatment before age of two years.4 Although the surgical treatment is highly effective at reducing testicular damage, UT has negative effects on fertility, more frequently in those who receive treatment at older ages.5,6
The UT experiences a higher temperature than is experienced by a scrotal testis and this increases the UT oxidative stress and production of reactive oxygen species (ROS) while reducing superoxide dismutase and catalase activities. The elevated ROS increases DNA fragmentation, which has a negative effect on the UT tissues. Apoptosis also increases in a temperature-dependent manner, resulting in UT germ cell dysmaturation and reduced fertility.7 Therefore, suppressing ROS reactions by antioxidants could aid in maintaining fertility in the UT.
One of the body’s main antioxidants known for its ROS-scavenging effect is glutathione. Its precursor, N-acetyl cysteine (NAC), is widely used in medicine for the treatment of acetaminophen poisoning, contrast nephropathy, chronic obstructive pulmonary disease, chronic bronchitis, cardiotoxicity, and some neurological disorders.8 An experimental study has shown that NAC can reduce the damage in UT testicular tissue without orchiopexy and protect against infertility.9 The possibility of NAC to be used even in newborns with relevant indications also provides an opportunity for using the molecule in a broad age group.10
The literature search did not reveal any previous investigation of NAC on testis that had undergone orchiopexy due to UT. The purpose of this study was to determine if adding NAC to the medication affected reproductive parameters in rats with orchiopexy due to UT.
METHODOLOGY
This study was conducted at the Laboratory Animals Research and Implementation Center at University, after the local Ethics Committee approval. Wistar albino rats were housed at 20 ± 2°C, 12-h light/12-h dark, and 55–60% humidity and fed with the standard palette (210 kcal100 gday) and tap water.
The UT model was created by providing pregnant rats with 7.5 mg flutamide (FM) from day 14 to 20 of pregnancy.11 Their unilateral UT rat offspring were included in the study and underwent orchiopexy at 5 weeks of age, with the testis fixed to the scrotum as Mizuno et al. performed.12 The tunica vaginalis was briefly opened with the incision made from the inguinal area. The testis was transplanted into the scrotum after the distal gubernaculum was cut open. Testicles were fixed under the tunica albuginea. The inguinal skin was closed. Starting the day of surgery, NAC (1 mL) was administered intragastrically (i.g.) at 150 mg/kg/day for 8 weeks. The animals were separated into four groups of eight animals each.
Group I (Control) was without UT rats that were born from mothers for whom FM was not administered. They received i.g. saline. Group II (UT) was unilateral UT rats that did not undergo orchiopexy and were administered i.g. saline. Group III (UT +orchiopexy) was unilateral UT rats that underwent orchiopexy and were administered i.g. saline.
Group IV (UT + orchiopexy + NAC) was rats with unilateral UT that underwent orchiopexy and were administered NAC.
All of the animals were sacrificed after being given 90 mg/kg of ketamine and 10 mg/kg of xylazine intraperitoneally to induce anaesthesia. Testis tissues were collected for histopathologic analyses.
After specimens were fixed in Bouin solution and embedded in paraffin, testicular tissues underwent histopathological evaluation in all the groups. Light microscopy was used to analyse the results of the hematoxylin and eosin staining (Nikon Eclipse 80i light microscope and NIS-Elements D3.2 software). The germinal epithelium was analysed quantitatively by Johnsen, scoring in 50 seminiferous tubules at 10× magnification.13 Each seminiferous tubule was scored from 1 to 10 based on the presence of spermatogenic cells.
TdT-mediated dUTP nick end labelling (TUNEL) assays were used to measure the apoptosis of spermatogenic cells in the seminiferous tubules, and the number of apoptotic cells were counted in 50 positively stained seminiferous tubules under 40x magnification.
For 15 minutes, cauda epididymis tissues were immersed in 2 mL of pre-warmed phosphate-buffered saline (PBS, pH 7.2). The sperms were released into the PBS by clamping the epididymis and cutting it into small pieces, and the sperm numbers (×106 cells/ml) and motility (progressive and non-progressive; %) were determined by pipetting a drop of sperm into a Makler counting chamber (Sefi Medical Instruments LTD, Haifa, Israel). The sperm concentration per mL was expressed as the sperm number in 10 chamber frames, and the motility percentage was expressed as the ratio of the progressively and non-progressively moving sperms to the total sperm number.
Smears were dried for sperm morphology analysis, fixed in 3% for 30 minutes, and stained by the Papanicolaou method. For each smear, 200 sperms were counted at 100× magnification, and the percentages of sperms with abnormal morphologies were calculated. All testicular tissue cross-sections were analysed by a histologist who was unaware of the experimental groups.
100 mg of testicular tissues were washed once with PBS and stored in glass tubes at -80C. The samples were thawed, homogenized in PBS (1 mL), centrifuged at 2-8oC, 5,000 g for 5 minutes, and the supernatant was recovered for biochemical analysis.
Malondialdehyde (MDA) and glutathione peroxidase (GPx) were quantitatively analysed using “Sandwich-ELISA” kits (GPx: ElabScience E-EL-H5410 96T; MDA: ElabScience E-EL-0060 96T).
SPSS software v22 (Windows; SPSS Inc. Chicago, Ill. USA) was used to analyse the data. In order to determine variance homogeneity, the Levene test was used. The Shapiro-Wilk test was performed to determine whether the continuous variable distributions were normal.
The appropriate means, medians, or standard deviations are used to present the data. One-way ANOVA or Welch ANOVA was used to compare the mean differences between groups. The post hoc Tukey HSD or Tamhane T2 test for parametric data were used to evaluate the significance of differences between groups. Statistical significance was defined as a p-value <0.05. Multiple comparisons were conducted with the Bonferroni adjustment (p = 0.008) to reduce type I errors.
RESULTS
Mean MDA (nmol/minute/mL) levels in the control group, group II, group III, and group IV were 0.75±0.05, 1.52±0.42, 1.24±0.21, and 1.04±0.10, respectively (Table I). In comparison to the control group, MDA levels were significantly higher in groups II, III, and IV (p = 0.001). The MDA level was significantly lower in group IV compared to group III (p = 0.001) and slightly lower in group III compared to group II (p = 0.953, Table II).
Mean GPx (nmol/minute/mL) levels in the control group, group II, group III, and group IV were 2.14±0.47, 0.39±0.22, 0.39±0.23, and 1.06±0.35, respectively (Table I). Groups II, III, and IV had significantly reduced GPx levels when compared to the control group (p = 0.001). Group III had a lower GPx level than group IV (p=0.032), while group II had a slightly lower level than group III (p=0.131, Table II).
Table I: Descriptive analysis of the malondialdehyde, glutathione peroxidase levels, and apopitotic cell count, Johnsen score, sperm count, sperm motility percentage, and abnormal sperm morphology percentage in groups.
|
|
|
Group I |
Group II |
Group III |
Group IV |
|
GPx |
Mean ±SD |
2.14 ±0.47 |
0.39 ±0.22 |
0.39 ±0.23 |
1.06 ±0.35 |
|
Median (IQR) |
2.14 (1.84-2.27) |
0.39 (0.19-0.55) |
0.39 (0.24-0.39) |
1.06 (0.80-1.10) |
|
|
MDA |
Mean ±SD |
0.75 ±0.05 |
1.52 ±0.42 |
1.24 ±0.21 |
1.04 ±0.10 |
|
Median (IQR) |
0.75 (0.74-0.78) |
1.40 (1.10-1.97) |
1.24 (1.09-1.24) |
1.04 (0.95-1.04) |
|
|
ACC |
Mean ±SD |
26.75 ±4.46 |
193.0 ±26.28 |
74.50 ±10.33 |
47.25 ±11.48 |
|
Median (IQR) |
27.0 (23.0-30.0) |
193.0 (175.75-205.75) |
74.50 (66.50-82.0) |
47.0 (38.0-51.0) |
|
|
JS |
Mean ±SD |
9.76 ±0.13 |
4.96 ±0.38 |
7.83 ±0.31 |
9.01 ±0.22 |
|
Median (IQR) |
9.80 (9.70-9.87) |
4.95 (4.67-5.35) |
7.80 (7.70-7.90) |
9.05 (9.0-9.17) |
|
|
SC |
Mean ±SD |
46.50 ±9.25 |
0.24 ±0.28 |
25.25 ±8.31 |
38.62 ±8.73 |
|
Median (IQR) |
46.50 (39.25-52.50) |
0.15 (0.0-0.54) |
25.0 (20.25-32.0) |
38.0 (31.0-45.75) |
|
|
SM% |
Mean ±SD |
71.75 ±7.16 |
2.0 ±1.69 |
32.0 ±7.07 |
48.50 ±9.39 |
|
Median (IQR) |
71.0 (66.50-72.75) |
2.0 (0.25-3.0) |
32.0 (26.50-34.25) |
48.0 (42.0-58.0) |
|
|
ASM% |
Mean ±SD |
9.56 ±2.30 |
80.0 ±6.41 |
55.37 ±5.45 |
44.56 ±8.23 |
|
Median (IQR) |
9.51 (8.12-11.53) |
79.68 (72.25-89.58) |
53.90 (46.75-59.65) |
45.12 (35.22-50.05) |
|
|
MDA: Malondialdehyde, GPx: Glutathione peroxidase, ACC: Apopitotic cell count, SC: Sperm count, SM %: Sperm motility percentage, ASM %: Abnormal sperm morphology percentage. |
|||||
Table II: Multiple comparison of the malondialdehyde, glutathione peroxidase levels and apopitotic cell count, Johnsen score, sperm count, sperm motility percentage, and abnormal sperm morphology percentage among the groups and associated p-values (The post hoc analyses of one-way Anova).
|
|
Group I vs. Group II |
Group I vs. Group III |
Group I vs. Group IV |
Grup II vs. Group III |
Group II vs. Group IV |
Group III vs. Group IV |
|
MDA |
p<0.001 |
p<0.001 |
p<0.001 |
p=0.953* |
p<0.001 |
p<0.001 |
|
GPx |
p<0.001 |
p<0.001 |
p<0.001 |
p=0.131* |
p=0.008 |
p=0.032* |
|
ACC |
p<0.001 |
p<0.001 |
p<0.001 |
p<0.001 |
p<0.001 |
p<0.001 |
|
Johnsen score |
p<0.001 |
p<0.001 |
p<0.001 |
p<0.001 |
p<0.001 |
p<0.001 |
|
SC |
p<0.001 |
p<0.001 |
p=0.102* |
p<0.001 |
p<0.001 |
p<0.007 |
|
SM % |
p<0.001 |
p<0.001 |
p<0.001 |
p<0.001 |
p<0.001 |
p<0.001 |
|
ASM % |
p<0.001 |
p<0.001 |
p<0.001 |
p<0.001 |
p<0.001 |
p=0.008 |
|
MDA: Malondialdehyde, GPx: Glutathione peroxidase, ACC: Apopitotic cell count, SC: Sperm count, SM %: Sperm motility percentage, ASM %: Abnormal sperm morphology percentage. |
||||||
Mean ACC were 26.75±4.46, 193±26.2, 74.5±10.337, and 47.25±11.48 in the control group, group II, group III, and group IV, respectively (Table I). The mean apoptotic cell count (ACC) varied significantly between groups (p 0.001). ACC in group IV was lower than in group III (p = 0.001, Table II).
Mean Johnsen scores were 9.76±0.13, 4.96±0.38, 7.83±0.31, and 9.01±0.22 in the control group, group II, group III, and group IV respectively (Table I). There were significant differences in mean Johnsen score among the groups, (p<0.001). The Johnsen score in group IV was higher in comparison with group III (p<0.001, Table II).
Mean sperm count (SC) were 46.5±9.25, 0.22±0.28, 25.25±8.31, and 38.6±8.73 in the control group, group II, group III, and group IV, respectively (Table I). There was no statistical difference when group I and group IV were compared (p=0.102). SC was higher in group IV than in group III (p<0.007, Figure 1, Table II).
Mean sperm motility (SM) percentages were 71.75±7.16, 2±1.69, 32±7.07, and 48.5±9.39 in the control group, group II, group III, and group IV respectively (Table 1). There were significant differences in mean sperm motility among the groups (p<0.001). SM was higher in group IV in comparison with group III (p<0.001, Table II).
Mean abnormal sperm morphology (ASM) percentages were 9.56±2.30, 80±6.41, 55.37±5.45, and 44.56±8.23 in the control group, group II, group III, and group IV respectively (Table I). ASM percentage was statistically lower in group IV than in group III (p<0.08, Table II).
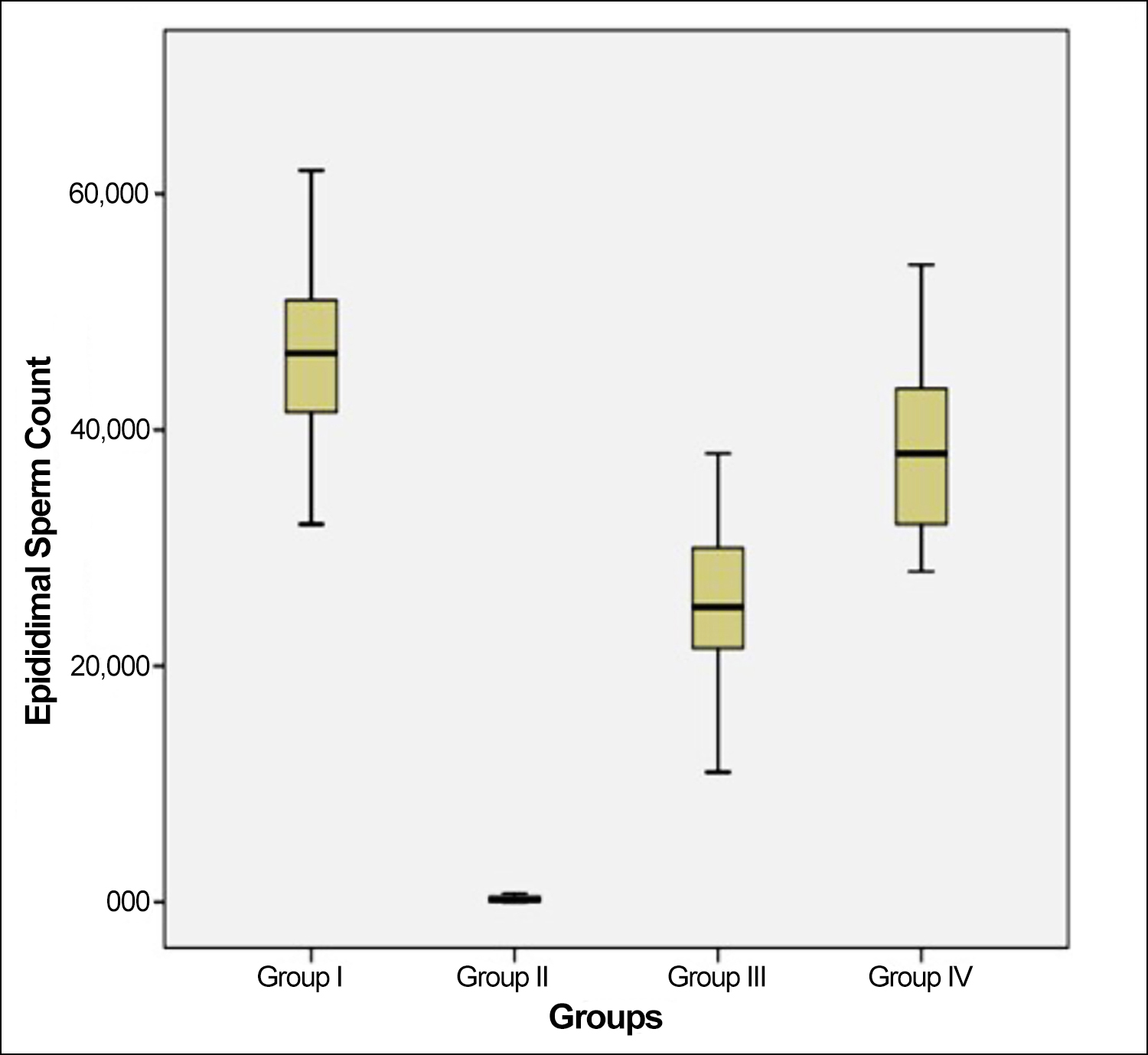 Figure 1: Epidimal sperm counts of rat groups.
Figure 1: Epidimal sperm counts of rat groups.
The histological analyses revealed normal seminiferous tubules and complete spermatogenesis in the control group, whereas group II showed atrophy of the seminiferous tubules, a significant reduction in germinal epithelium, and fibrosis in the interstitial area. Group III displayed an increase in germinal cell counts and an increase in tubule length. The seminiferous tubular epithelium was more organised in group IV than in group III and had a more noticeable increase in the spermatogenic cells (Figure 2). The TUNEL assays confirmed a higher degree of apoptosis in group II than in group III, whereas the degree of apoptosis was lower in group IV (Figure 3).
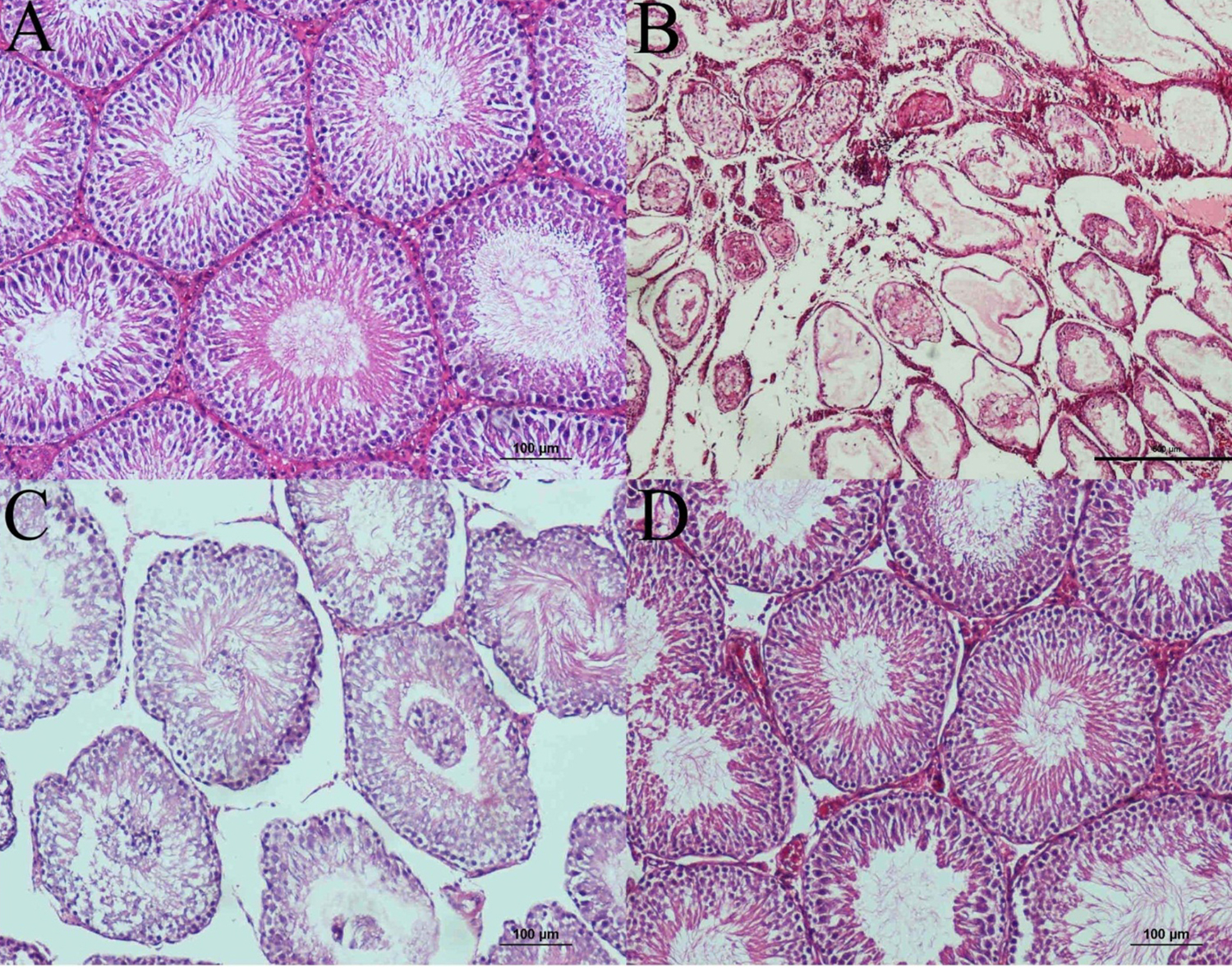 Figure 2: (A) Normal seminiferous tubule structure and spermatogenesis in the control group. (B) Reduction in the seminiferous tubule diameter and spermatogenic cell counts, as well as edema and fibrosis in the inter tubular areas, in UT without treatment group (H&E, scale bar 500 μm). (C) Increase in post-orchiopexy in tubule diameters and epithelial germ cell counts and improvement in spermatogenesis in UT plus NAC group. (D) More organised seminiferous tubule epithelium and spermatozoa in the lumen in UT plus NAC plus orchiopexy group (H&E, scale bar 100 μm).
Figure 2: (A) Normal seminiferous tubule structure and spermatogenesis in the control group. (B) Reduction in the seminiferous tubule diameter and spermatogenic cell counts, as well as edema and fibrosis in the inter tubular areas, in UT without treatment group (H&E, scale bar 500 μm). (C) Increase in post-orchiopexy in tubule diameters and epithelial germ cell counts and improvement in spermatogenesis in UT plus NAC group. (D) More organised seminiferous tubule epithelium and spermatozoa in the lumen in UT plus NAC plus orchiopexy group (H&E, scale bar 100 μm).
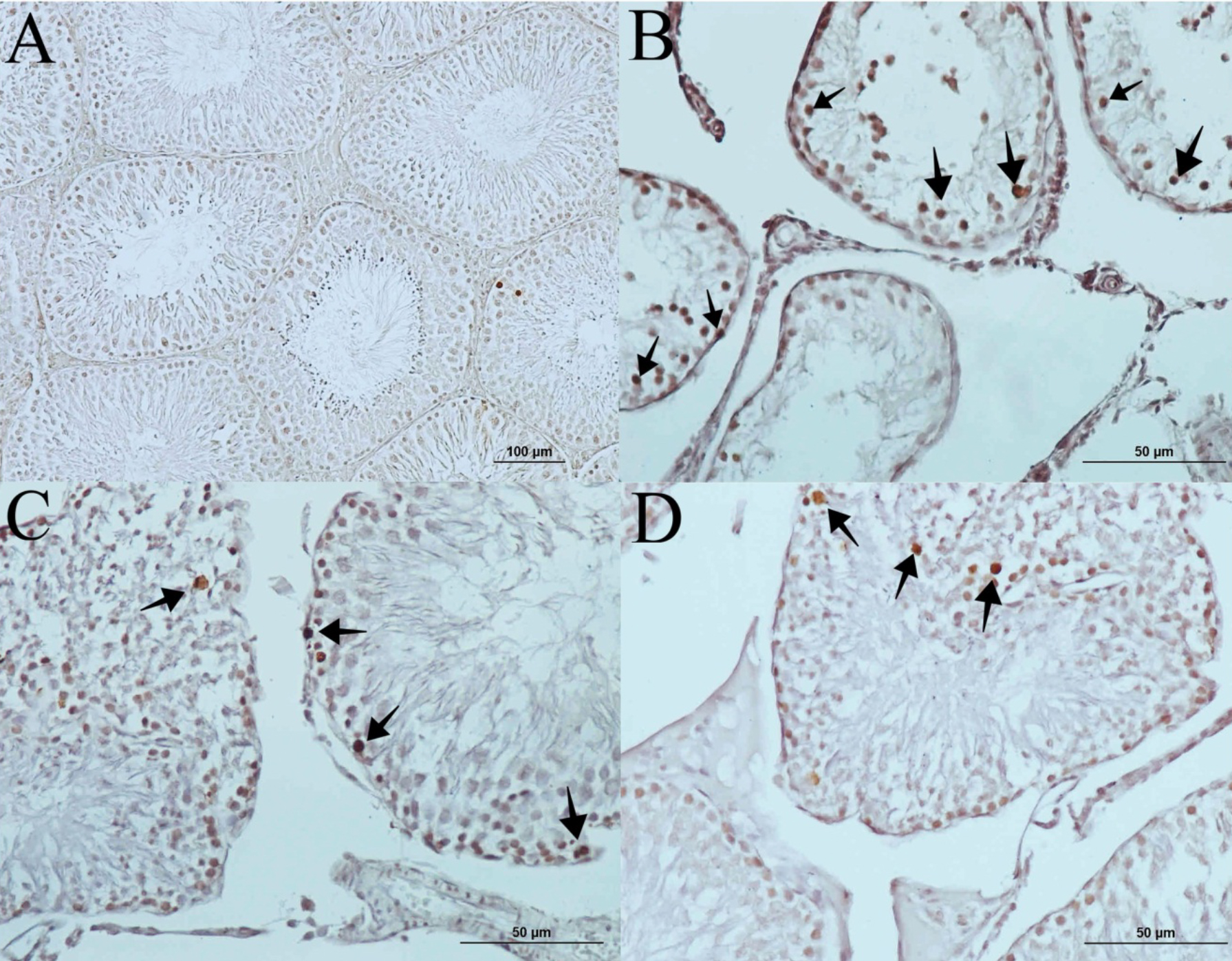 Figure 3: (A) Control group (TUNEL, scale bar 100 μm). (B) Several apoptotic germ cells in UT group (→). (C) Reduced apoptotic germ cell counts in the orchiopexy group (→). (D) Noticeable reduction in the apoptotic cell counts in the group that received both orchiopexy and NAC (→) (TUNEL stain, scale bars indicate 50 μm).
Figure 3: (A) Control group (TUNEL, scale bar 100 μm). (B) Several apoptotic germ cells in UT group (→). (C) Reduced apoptotic germ cell counts in the orchiopexy group (→). (D) Noticeable reduction in the apoptotic cell counts in the group that received both orchiopexy and NAC (→) (TUNEL stain, scale bars indicate 50 μm).
DISCUSSION
In some UT patients, the testis spontaneously drops into the scrotum at an earlier age, but UT prevalence is as high as 2% in children older than 3 months.14 UT can affect fertility negatively even in patients who have been treated for UT, especially if the onset of treatment is delayed.15
Various experimental studies have been conducted to prevent and treat problems caused by this disorder, and UT modelling is the usual basis for these studies. One of the options is to surgically transfer the testis that has already entered the scrotum back into the abdomen.16 However, this procedure may cause undesired side effects, including inflammatory tissue changes. Another model involves the administration of FM, a selective protein that binds to androgen receptors, in pregnant rats. The offspring born to those mothers are used, and this reduces the testis damage that may occur due to the surgery used to establish the model.12 In the current study, the researchers used a cryptorchidism model induced by FM, as this further reduces both the potential for testicular damage while forming the UT model and the stress on the rats undergoing surgical testis descent. NAC was also administered by an oral method, as it was felt that this administration would be the simplest for long-term clinical treatments aimed at minimising oxidative stress.
Oxidative stress causes noticeable changes in the lipids of damaged tissues. The authors examined MDA, as it is one among the most significant byproducts of polyunsaturated fatty acid peroxidation. It is also a good indicator of tissue damage, as its levels increase with elevated oxidative stress, while exogenous administration of antioxidants reduces its levels. A reduction in tissue MDA levels by antioxidant administration is also viewed as an indicator of a reduction in oxidative.17,18 The authors also used GPx activity as another measure of oxidative damage. The GPx enzyme uses glutathione as a cofactor to reduce H2O2 and organic hydroperoxides. Several enzymatic and non-enzymatic defensive mechanisms, including as ascorbic acid, α-tocopherol, superoxide dismutase, and GPx, can prevent oxidative damage in tissues. The GPx levels are generally higher in tissues where oxidative damage is low than in tissues where oxidative damage is high.17,19 The highest GPx value was observed in the control group. The group receiving NAC had the highest GPx level among the UT-induced groups, whereas the GPx levels in the groups receiving just orchiopexy did not alter appreciably. In comparison to the groups that did not receive NAC, the MDA level was lower in the NAC-administered group. The MDA level was also lower in the group that underwent orchiopexy than in the group that did not, but the difference was not statistically significant. The significant increase in GPx activity and the reduction in MDA levels in response to NAC was interpreted as a potential indicator of the antioxidant activity of NAC. NAC administration could be effective in reducing the oxidative stress that develops in relation to UT.
Apoptosis is a form of cell death that is characterised by a number of morphological and structural changes that are seen in old and damaged cells. Testicular damage increases the ACC in the testicular tissue, and an increase in ACC and a parallel loss of germinal cells are frequently encountered in UT cases.20,21 This situation is accepted as an important cause of the reduced fertility potential observed in UT.22 The authors observed an increased ACC in the UT when compared to the normal testis, while orchiopexy significantly decreased the ACC. The administration of NAC further reduced the ACC following surgery, indicating a positive effect of NAC on the testicular tissue.
The Johnsen score is a technique that quantifies testis histology on a scale of 1 to 10, with higher scores indicating better spermatogenesis.23 Here, the Johnsen score was used as one of the analysis criteria for the testis. The UT scored very low, but the score improved in testes that underwent orchiopexy, indicating that surgery is an effective method for UT treatment. However, the finding that adding NAC noticeably increases the score in group IV, compared to group III, led the authors to consider that adding NAC treatment to surgery may have a positive effect on spermatogenesis.
The SC and motility values are frequently examined in spermiograms conducted to assess fertility potential.24 Sperm morphology is another parameter that has been used for years to assess fertility potential, as an increase in ASM is believed to reduce the fertility potential of the individual and negatively affect the fertility process up to the time of conception.25
The assessment of SC, motility, and morphology revealed lower SC, motility, and higher ASM rates in the UT group without treatment than in the other groups. Orchiopexy provided a significant improvement in these values. By comparison, the sperm morphology, counts, and motility were further improved when NAC was added to the orchiopexy treatment. The SC in the group that received NAC in addition to orchiopexy noticeably increased, even to the level that they were not statistically different from those of the control group.
The previous experimental studies on NAC, which has antioxidant characteristics, have revealed a protective effect on UT tissue that has not been subjected to orchiopexy.8 However, descending the testis to its normal place by medical or surgical methods continues to be the only treatment option in clinical practice today. In the current study, a noticeable improvement was observed in all histological parameters by surgically descending the testis to its correct place. Administering NAC in addition to surgical treatment led to significant improvements in the GPx and MDA values. A noticeable improvement was also observed by administering NAC in addition to orchiopexy in terms of the ACC, Johnsen score, sperm morphology, SC, and SM values. The SC, in particular, reached similar values to those of the control group.
Since this research was carried out under certain conditions in a small group of animals, it may not always show the facts in their entirety. It may need to be repeated with more variables and parameters and so the long-term and comparative animal studies, as well as clinical research are still needed.
CONCLUSION
In the experimental rat model, orchiopexy reduced UT-related testicular damage, but it did not eliminate this damage completely. Adding NAC to surgical treatment may further and significantly reduce testicular damage, probably facilitating this improvement by reducing the oxidative stress in the tissue.
ETHICAL APPROVAL
This study was conducted at the Laboratory Animals Research and Implementation Center at University, after the local Ethics Committee approval.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
EK, UU: Conception and design of the study and manuscript writing.
AC: Intellectual content of the study and technical procedures.
SE: Histopathological examinations.
UU: Statistical analysis and technical procedures.
AG: Conception and design of the study.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Rodprasert W, Virtanen HE, Makela JA, Toppari J. Hypogonadism and Cryptorchidism. Front Endocrinol (Lausanne) 2020; 10:906. doi:10.3389/fendo.2019.00906.
- Cortes D, Kjellberg EM, Breddam M, Thorup J. The true incidence of cryptorchidism in Denmark. J Urol 2008; 179(1):314-8. doi: 10.1016/j.juro.2007.08.158.
- Ahmed R, Akhtar J, Taqvi SMRH, Zamir N, Bibi S. Clinical presentation, diagnostic approach, laparoscopic evaluation and treatment of ımpalpable testis. J Coll Physicians Surg Pak 2022; 32(4):478-82. doi: 10.29271/jcpsp.2022.04.478.
- Bergbrant S, Omling E, Bjork J, Hagander L. Cryptorchidism in Sweden: A nationwide study of prevalence, operative management, and complications. J Pediatr 2018; 194: 197-203 e6. doi: 10.1016/j.jpeds.2017.09.062.
- Hadziselimovic F. On the descent of the epididymo-testicular unit, cryptorchidism, and prevention of infertility. Basic Clin Androl 2017; 27:21. doi: 10.1186/s12610-017-0065-8.
- Li LW, Yang B, Liu D, Liu C, Guo SL. Laparoscopic versus conventional open surgery approach of tunica vaginalis for palpable cryptorchidism. J Coll Physicians Surg Pak 2022; 32(9):1122-6. doi: 10.29271/jcpsp.2022.09.1122.
- Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Adv Exp Med Biol 2008; 636:154-71. doi: 10.1007/978-0-387-09597-4_9.
- Pei Y, Liu H, Yang Y, Yang Y, Jiao Y, Tay FR, et al. Biological activities and potential oral applications of N-acetylcysteine: Progress and prospects. Oxid Med Cell Longev 2018; 2018:2835787. doi: 10.1155/2018/2835787.
- Uyeturk U, Cetinkaya A, Ozyalvacli G, Tekce BK, Ozyalvacli ME, Kemahli E, et al. Protective effects of N-acetylcysteine on experimentally undescended testis. J Urol 2014; 191(4):1168-73. doi: 10.1016/j.juro.2013.08.053.
- Aiyagari R, Gelehrter S, Bove EL, Ohye RG, Devaney EJ, Hirsch JC, et al. Effects of N-acetylcysteine on renal dysfunction in neonates undergoing the arterial switch operation. J Thorac Cardiovasc Surg 2010; 139(4):956-61. doi: 10.1016/j.jtcvs..2009.09.025.
- Mizuno K, Hayashi Y, Kojima Y, Kurokawa S, Sasaki S, Kohri K. Influence for testicular development and histological peculiarity in the testes of flutamide-induced cryptorchid rat model. Int J Urol 2007; 14(1):67-72. doi: 10.1111/j.1442- 2042.2006.01654.x.
- Mizuno K, Hayashi Y, Kojima Y, Kurokawa S, Sasaki S, Kohri K. Early orchiopexy improves subsequent testicular development and spermatogenesis in the experimental cryptorchid rat model. J Urol 2008; 179(3):1195-9. doi: 10.1016/j.juro. 2007.10.029.
- Johnsen SG, Agger P. Quantitative evaluation of testicular biopsies before and after operation for varicocele. Fertil Steril 1978; 29(1):58-63. doi: 10.1016/s0015-0282(16) 43038-4.
- Boisen KA, Kaleva M, Main KM, Virtanen HE, Haavisto AM, Schmidt IM, et al. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet 2004; 363(9417):1264-9. doi: 10.1016/S0140- 6736 (04)15998-9.
- Allin BSR, Dumann E, Fawkner-Corbett D, Kwok C, Skerritt C. Paediatric surgery trainees research N. Systematic review and meta-analysis comparing outcomes following orchidopexy for cryptorchidism before or after 1 year of age. BJS Open 2018; 2(1):1-12. doi: 10.1002/bjs5.36.
- Kheradmand A, Dezfoulian O, Alirezaei M, Hadian B. Ghrelin is a suppressor of testicular damage following experimentally induced cryptorchidism in the rat. J Pediatr Surg 2014; 49(4):593-8. doi: 10.1016/j.jpedsurg.2013.10.003.
- Kuzay D, Ozer C, Sirav B, Canseven AG, Seyhan N. Oxidative effects of extremely low frequency magnetic field and radio frequency radiation on testes tissues of diabetic and healthy rats. Bratisl Lek Listy 2017; 118(5):278-282. doi: 10.4149/BLL_2017_055.
- Malmir M, Soleimani Mehranjani M, Naderi Noreini S, Faraji T. Protective antioxidant effects of N-acetylcysteine against impairment of spermatogenesis caused by paranonyl-phenol. Andrologia 2018; 50(10):e13114. doi: 10.1111/ and.13114.
- Robaczewska J, Kedziora-Kornatowska K, Kozakiewicz M, Zary-Sikorska E, Pawluk H, Pawliszak W, et al. Role of glutathione metabolism and glutathione-related antioxidant defense systems in hypertension. J Physiol Pharmacol 2016; 67(3):331-7.
- Aksu EH, Kandemir FM, Kucukler S, Mahamadu A. Improvement in colistin-induced reproductive damage, apoptosis, and autophagy in testes via reducing oxidative stress by chrysin. J Biochem Mol Toxicol 2018; 32(11):e22201. doi: 10.1002/jbt.22201.
- Mestrovic J, Drmic-Hofman I, Pogorelic Z, Vilovic K, Supe-Domic D, Seselja-Perisin A, et al. Beneficial effect of nifedipine on testicular torsion-detorsion injury in rats. Urology 2014; 84(5):1194-8. doi: 10.1016/j.urology.2014. 07.022.
- Ohta Y, Nishikawa A, Fukazawa Y, Urushitani H, Matsuzawa A, Nishina Y, et al. Apoptosis in adult mouse testis induced by experimental cryptorchidism. Acta Anat (Basel) 1996; 157(3):195-204. doi: 10.1159/000147881.
- Tang WH, Zhou SJ, Song SD, He HY, Wu H, Zhang Z, et al. A clinical trial on the consistency of bilateral testicular tissue histopathology and Johnsen score: Single side or bilateral side biopsy? Oncotarget 2018; 9(35):23848-59. doi: 10.18632/oncotarget.24748.
- Lavranos G, Balla M, Tzortzopoulou A, Syriou V, Angelopoulou R. Investigating ROS sources in male infertility: A common end for numerous pathways. Reprod Toxicol 2012; 34(3):298-307. doi: 10.1016/j.reprotox. 2012.06.007.
- Khatun A, Rahman MS, Pang MG. Clinical assessment of the male fertility. Obstet Gynecol Sci 2018; 61(2):179-91.