Prognostic Markers after Hepatic Function Impairment in Patients with Pancreatic Adenocarcinoma
By Mustafa Buyukkor, Fatih Tay, Cengiz Karacin, Ayse Ocak DuranAffiliations
doi: 10.29271/jcpsp.2023.01.36ABSTRACT
Objective: To determine the factors affecting survival after hepatic failure in patients with pancreatic adenocarcinoma (PAC) who developed hepatic dysfunction accompanied by hyperbilirubinemia.
Study Design: Observational Study.
Place and Duration of Study: Department of Medical Oncology, Abdurrahman Yurtaslan Ankara Oncology Training and Research Hospital, Ankara, Turkey, from January 2017 to May 2022.
Methodology: The clinical characteristics of adult patients who developed hepatic dysfunction accompanied by hyperbilirubinemia with a diagnosis of PAC and died in their follow-up were recorded from the hospital's patient registry database. Patients without medical record were excluded. The effective parameters of overall survival after hepatic failure (aHFOS) were determined.
Results: The study included 57 patients with PAC (56.1% males) who developed hepatic dysfunction during their follow-up. According to the CA 19-9 value at the time of diagnosis, the tumour localisation was predicted to be located in the head and neck (Cutoff ≤1400, AUC 0.77, sensitivity 73.2%, specificity 75%; p=0.002). Values of international normalised ratio (INR, p=0.010), blood urea nitrogen (BUN, p=0.002) that were measured during hepatic dysfunction, and tumour location in the head and neck in the pancreas (p=0.028) were determined as independent variables affecting aHFOS in patients with PAC. In addition, percutaneous transhepatic biliary drainage (PTDB) application during liver failure and initiation of chemotherapy in appropriate patients also positively affected aHFOS (2.62 months vs. 0.92 months, p=0.016 and 3.45 months vs. 1.11 months, p=0.003; respectively).
Conclusion: Sufficient liver function reserve in malignant patients is highly effective in the curability and survival of patients. In this regard, it is crucial to improve prognosis by identifying the factors affecting aHFOS in patients with PAC who develop hepatic dysfunction due to liver metastasis or the primary tumour characteristics.
Key Words: Pancreatic adenocarcinoma, CA 19-9, Hepatic failure, Prognosis, Survival.
INTRODUCTION
Pancreatic cancer is one of the malignancies with high mortality and is a difficult disease to treat.1 Additionally, it is a ductal adenocarcinoma that spreads rapidly 85-90% of the time and can metastasize to surrounding tissues and distant organs.2 It is one of the leading malignancies with a high mortality rate, annually affecting more than 200,000 deaths worldwide.3,4 It ranks 14th among global common cancer types and 7th in cancer-related mortality. Thereabout 60%-70% of pancreatic cancers are located in the head of the pancreas, 15% in the body and 15% in the tail.5 It ranks 4th in cancer-related deaths in the USA, and its 5-year survival rate ranges from 5% to 15%.
The annual incidence is increasing between 0.5% and 1% each year and is expected to rise to the 2nd rank in cancer-related deaths by 2030. The only curative option is surgical resection, but only 20% of patients can be caught in the resectable period.6 Pancreatic cancer metastases are most common in the liver, and at the time of diagnosis, liver metastases account for 37-41.9% of patients, with a 5-year overall survival (OS) of 2% and a median life expectancy of less than one year.7,8 As in all malignancies, it is possible to see hyperbilirubinemia due to liver metastasis in pancreatic cancer. In addition, hyperbilirubinemia caused by malignant biliary obstruction is quite common in patients with advanced pancreatic disease and is challenging in oncological treatment.9,10
This study aimed to determine the approach to managing patients with liver dysfunction diagnosed with pancreatic adenocarcinoma (PAC), either secondary to liver metastasis or with hyperbilirubinemia due to the primary tumour characteristics.
METHODOLOGY
Patients aged 18 years and over who were diagnosed with pancreatic adenocarcinoma in the Medical Oncology Unit of Dr. Abdurrahman Yurtaslan Ankara Oncology Hospital, between January 2017 and May 2022, who were hospitalised, followed up, and treated due to the development of liver dysfunction with hyperbilirubinemia and died in later follow-ups were included. The study was prepared according to the Declaration of Helsinki. Patient information was recorded by retrospectively scanning the hospital database. In addition, patients with non-malignant hyperbilirubinemia and liver failure, with missing data and with synchronous second primary malignancy were also excluded. Approval for the study was obtained from the central ethics committee with the number 2022-05/1858 dated 08.06.2022.
Liver dysfunction was defined as hyperbilirubinemia (total bilirubin≥1.5 times of normal values) accompanied by high-level alanine aminotransferase (ALT) or aspartate aminotransferase (AST) values in their follow-up. The effect of various clinical and laboratory variables on survival after the development of hepatic dysfunction was calculated.
Statistical analyses of the study were evaluated by SPSS version 24.0. Categorical data were expressed as counts and percentages. Receiver operating characteristic (ROC) curve was used for determining the role of CA 19-9 to predict the location of primary pancreas tumour. In survival analysis, the Kaplan-Meier curve was used and compared with the log-rank test, and significant variables in univariate analyses were evaluated in multivariate cox regression analysis. A p-value of <0.05 was considered significant in all statistical tests.
RESULTS
Of the 57 patients included, 25 (43.9%) were females and 32 (56.1%) were males. Thirty (52.6%) of patients were over 55 years old. The primary location of the tumour was in the head and neck of pancreas in 41 (71.9%) cases, and in the body and tail in 16 (28.1%) cases. When evaluated according to liver metastasis status, 44 (77.2%) patients were de novo metastatic or had metastasis in their follow-up, while liver metastasis was not detected in 13 (22.8%) patients.
While percutaneous transhepatic biliary drainage (PTDB) was applied to 33 (57.9%) of the patients, 24 (42.1%) were not suitable for PTDB. Twenty-five (43.9%) patients could receive chemotherapy during hepatic dysfunction, and 32 (56.1%) patients could not receive chemotherapy at all. The demographic and clinical characteristics of the patients are summarised in Table I.
ROC analysis predicted that if the CA 19-9 value at the time of diagnosis was 1400 U/ml and below, the primary tumour was localised in the head and neck of the pancreas (AUC: 0.770, sensitivity 73.2%, specificity 75%; p=0.002, Figure 1).
In the multivariate cox regression analysis performed between the Univariate Cox regression analysis and the factors affecting overall survival after hepatic failure (aHFOS), it was seen that the increase in BUN and INR values as independent risk factors negatively affected survival (HR: 1.049 95% CI: 1.017-1.081, p=0.002; and HR 2.168 95% CI 1.205-3.902 p=0.010, respectively, Table II). In addition, when the head and neck region, where pancreatic tumours are most common, was taken as reference, tumours involving the body and tail region were also more mortal as an independent risk factor during liver failure (HR: 1.984 95% CI: 1.077-3.654, p=0.028, Figure 2).
The duration of aHFOS was approximately three times longer in patients with PTDB than in those who did not receive PTBD (2.62 months vs. 0.92 months p=0.016, Figure 2a). Additionally, the duration of aHFOS was approximately three times longer in patients who received chemotherapy after hepatic failure than in those who did not (3.45 months vs. 1.11 months, p=0.003, Figure 2b).
Table I: Clinical and demographical features of patients.
|
|
Total N: 57 (%) |
|
|
Gender |
Male |
32 (56.1) |
|
Female |
25 (43.9) |
|
|
Age |
<55 |
27 (47.4) |
|
>55 |
30 (52.6) |
|
|
ECOG performance score |
1 |
8 (14) |
|
2 |
37 (64.9) |
|
|
3 |
11 (19.3) |
|
|
4 |
1 (1.8) |
|
|
Diabetes at the time of diagnosis |
Present |
32 (56.1) |
|
Absent |
25 (43.9) |
|
|
Adjuvant chemotherapy |
Received |
8 (14) |
|
Did not receive |
49 (86) |
|
|
Localisation |
Head+Neck |
41(71.9) |
|
Body+Tail |
16 (28.1) |
|
|
Hepatic metastasis |
Absent |
13 (22.8) |
|
Present (de novo + follow-up) |
44 (77.2) |
|
|
Oxaliplatin (adjuvant or metastatic step) |
Did not receive |
29 (50.9) |
|
Received |
28 (49.1) |
|
|
Irinotecan (adjuvant or metastatic step) |
Did not receive |
31 (54.4) |
|
Received |
26 (45.6) |
|
|
Taxane (adjuvant or metastatic step) |
Did not receive |
37 (64.9) |
|
Received |
20 (35.1) |
|
|
BMI (during hepatic failure) |
Cachectic |
9 (15.8) |
|
Normal weight |
34 (59.6) |
|
|
Overweight |
14 (24.6) |
|
|
PTDB |
Absent |
24 (42.1) |
|
Present |
33 (57.9) |
|
|
Chemotherapy during liver failure |
Received |
25 (43.9) |
|
Did not receive |
32 (56.1) |
|
|
BMI: Body mass index; PTDB: Percutaneous transhepatic biliary drainage; ECOG: Eastern cooperative oncology group. |
||
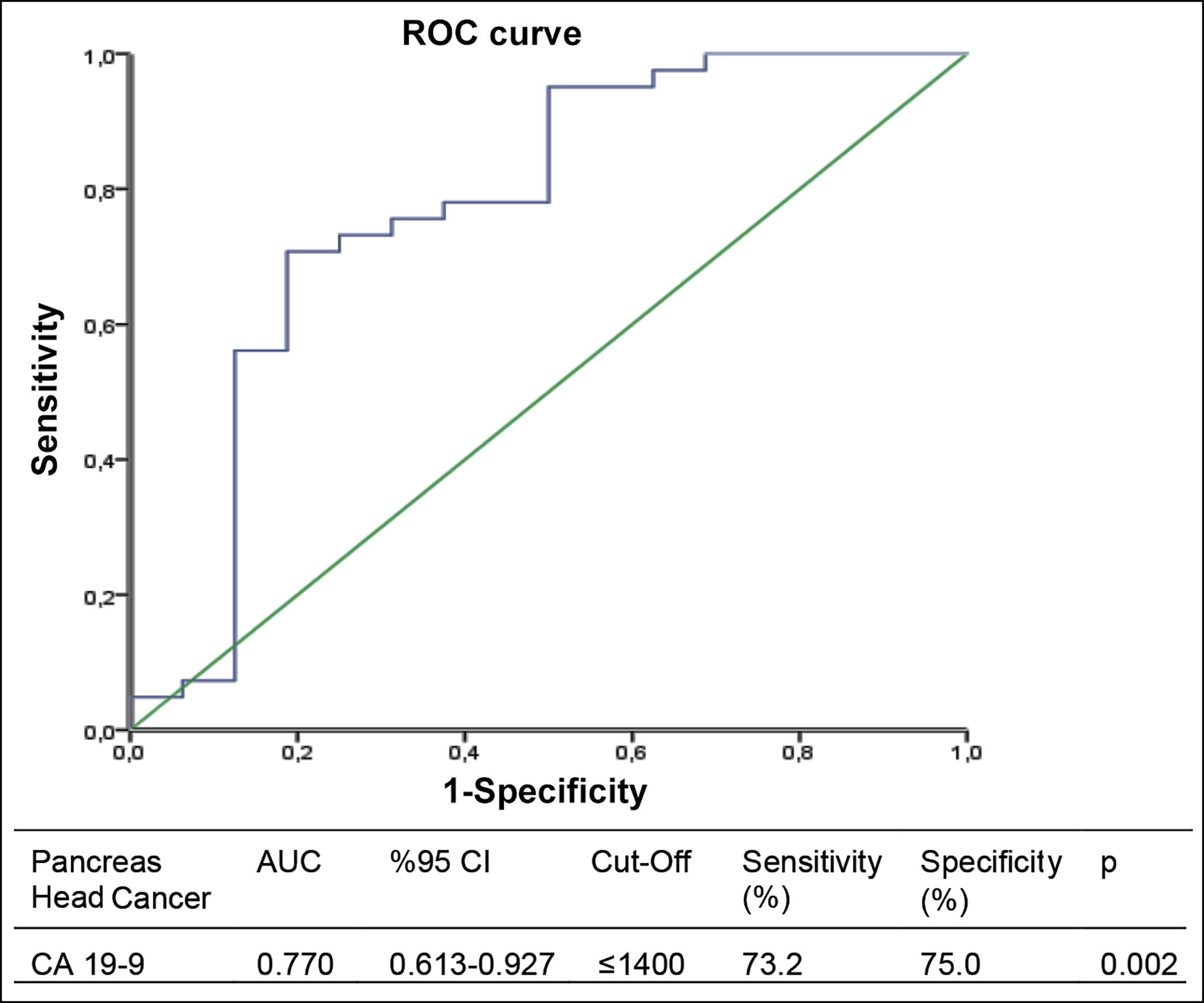 Figure 1: Evaluation of the CA 19-9 variable at the time of diagnosis with the ROC curve of tumour location affecting the pancreatic head and neck.
Figure 1: Evaluation of the CA 19-9 variable at the time of diagnosis with the ROC curve of tumour location affecting the pancreatic head and neck.
|
Overall survival after hepatic failure (aHFOS) |
||||
|
|
Univariate Cox Regression |
Multivariate Cox Regression |
||
|
|
HR (CI 95%) |
p-value |
HR (CI 95%) |
p-value |
|
BUN |
1.043 (1.014-1.073) |
0.003 |
1.049 (1.017-1.081) |
0.002 |
|
Albumin |
0.607 (0.422-0.873) |
0.007 |
0.710 (0.482-1.046) |
0.083 |
|
INR |
2.476 (1.436-4.271) |
0.001 |
2.168 (1.205-3.902) |
0.010 |
|
Gender |
0.573 (0.328-1.002) |
0.051 |
0.539 (0.283-1.029) |
0.061 |
|
Tumour localisation (1=Head+Neck, 2=Body+Tail) |
1.995 (1.093-3.640) |
0.024 |
1.984 (1.077-3.654) |
0.028 |
|
*Cox regression analysis |
||||
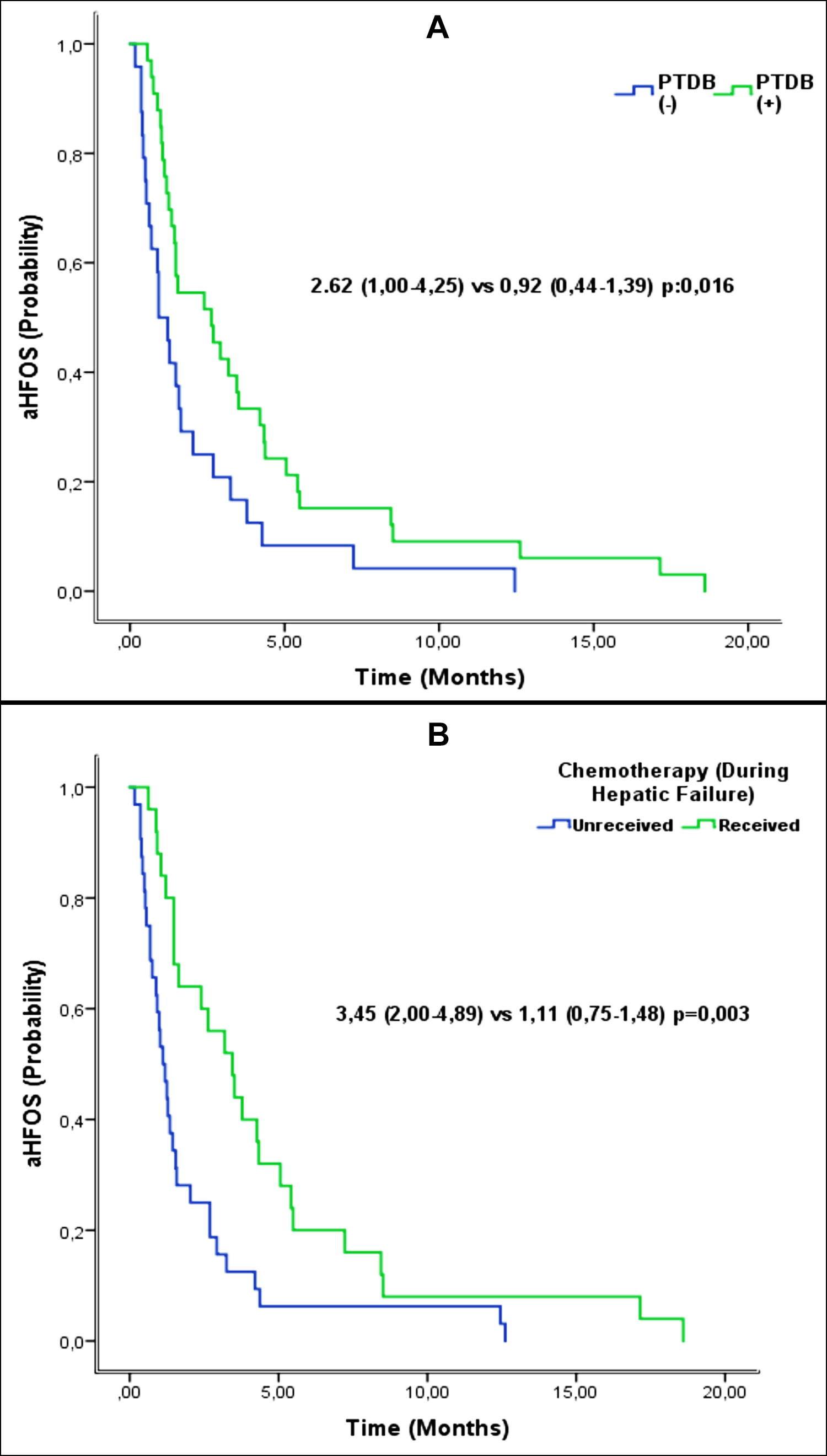 Figure 2: p-values of aHFOS Kaplan-Meier curves were derived from log-rank tests (A) Effect of PTDB administration on aHFOS during hepatic failure (B) Effect of chemotherapy administration during hepatic failure on αHFOS; PTDB: percutaneous transhepatic biliary drainage; aHFOS: overall survival after liver failure.
Figure 2: p-values of aHFOS Kaplan-Meier curves were derived from log-rank tests (A) Effect of PTDB administration on aHFOS during hepatic failure (B) Effect of chemotherapy administration during hepatic failure on αHFOS; PTDB: percutaneous transhepatic biliary drainage; aHFOS: overall survival after liver failure.
DISCUSSION
Pancreatic cancer is primarily seen in men, occurs more frequently in advanced ages (40-85 years), and is a malignancy with high mortality.11 The prognosis is poor despite advances in treatment options. Even if patients receive adjuvant chemotherapy after complete surgery, approximately 80% die within 5 years.12 In patients who have metastasised, the survival rate is further reduced, and the liver is the most common site of pancreatic cancer metastasis.7,8 Liver dysfunction can be seen secondary to liver metastasis in most cancer patients. However, in pancreatic cancer, liver dysfunction accompanied by hyperbilirubinemia can also be observed in cases of compression of the bile ducts by localising the head and neck of the primary tumour. There is no clear consensus on follow-up and treatment approaches in malignant patients with hepatic failure. The goal of this study was to determine the parameters that will affect the overall survival in patients with liver failure accompanied by hyperbilirubinemia and to contribute to the literature on the medical approach in these patients.
Thereabout 60-70% of pancreatic cancers are localised to the head of the pancreas,9 and in 70-80% of these patients, central bile duct obstruction causes hyperbilirubinemia.13 Liver functions, including the coagulation pathway, protein synthesis, and immunological mechanisms, are impaired either by liver metastasis or by a mechanism due to malignant obstruction.14,15 Other causes of hyperbilirubinemia were excluded in the patients included in This study, and the primary tumour location of all patients without liver metastasis was pancreatic head and neck. Again, in accordance with the literature, 41 patients (71.9%) were localised in the pancreatic head and neck, while most of the patients (52.6%) were over 55 years old and 32 (56.1%) were males. All patients died within 5 years from the date of diagnosis.
In the study by Artinyan et al., tumour localisation in pancreatic cancers significantly affected overall survival. Patients with body and tail localisation had less median survival (4 months vs. 6 months, p<0.001), increased incidence of metastasis (67% vs. 36%, p<0.001), and less surgical feasibility (16% vs. 30%, p<0.001) compared to patients with head localisation.16 The authors observed that primary tumour localisation in aHFOS had a positive effect in favour of head + neck as an independent variable in patients with pancreatic adenocarcinoma who developed liver failure (HR: 1.984, p=0.028). This finding can be explained by the earlier symptoms of head + neck tumours.
Carbohydrate antigen 19-9 (CA 19-9), also known as Sialyl Lewis a (sLea), is a biomarker that is used routinely and has 82% sensitivity and 90% specificity for pancreatic cancer, approved by the FDA to support the diagnosis of pancreatic adenocarcinoma and used in the follow-up of the disease.17 Luo et al. showed that patients with CA 19-9 levels in the normal range have better survival rates than those with high CA 19-9 levels, independent from the pancreatic cancer stage. They also showed that advanced-stage pancreatic cancer patients with normal CA 19-9 levels had a better five-year survival rate (15.4%) compared to the early stage with high CA 19-9 levels (13.8%).18,19 The low levels of CA 19-9 associated with a better prognosis may be explained by the hypothesis that CA 19-9 promotes any cancer cell metastasis by binding to E-selectin, an adhesion receptor found on the surface of endothelial cells. Another hypothesis is that CA 19-9 promotes metastasis of pancreatic cancer cells.18,19 Therefore, this study predicted that the primary tumour location is in the head and neck of the pancreas (AUC: 0.770, sensitivity 73.2%, specificity 75%; p=0.002) if the CA 19-9 value at the time of diagnosis, determined by ROC analysis, is 1400 U/ml and below. In addition, it can be hypothesised that the low CA 19-9 value at the diagnosis positively affects pancreatic cancer survival and predicts that the primary tumour localisation is in the head and neck. However, larger studies are needed to confirm this hypothesis.
There is no standard method for defining and classifying hepatic failure, especially in cancer patients. The main variables used to define the severity of hepatic failure are albumin, blood coagulation parameters (prothrombin time and INR), hepatic encephalopathy, and decompensation. However, hypoalbuminemia can also be considered a suboptimal parameter related to malnutrition and weight loss.20 This study predicted that the increase in INR measured during liver failure is an independent variable negatively affecting aHFOS (HR: 2.168, p=0.010). It was also predicted that gender and albumin are dependent variables affecting aHFOS, and the increase in the BUN value measured during liver failure, as an indicator of ascites formation possibly due to decreased effective circulatory volume in the vessel, is an independent poor prognostic indicator of aHFOS (HR: 1.049, p=0.002).
Hyperbilirubinemia is associated with short survival in patients with pancreatic cancer,21 and biliary compression contributes positively to morbidity and mortality in patients with obstructive causes. Percutaneous transhepatic biliary drainage (PTBD) is one of the methods used in the treatment of biliary obstruction and endoscopic biliary drainage (EBD) is the other one. Obstruction of the bile ducts by a malignant tumour raises serum bilirubin levels and disrupts almost all homeostasis mechanisms in the body with systemic effects. It was observed that our patients who underwent PTDB had a significant positive effect on aHFOS compared to those who did not (2.62 months vs. 0.92 months, p=0.016).
Many phase 1, 2, and 3 studies exclude patients with abnormal liver function tests, including elevated bilirubin; therefore, a remarkable patient population in this condition that could benefit from treatments cannot be identified.22 For this reason, there is not any clear consensus about the initiation of chemotherapy in patients with pancreatic cancer with hyperbilirubinemia. In the study by Alvarez et al., bilirubin levels are > 3 mg/dl, chemotherapy can be considered in appropriate patients with close follow-up. This recommendation is based on the practical recovery of liver functionality and the survival benefit associated with the early initiation of chemotherapy treatment.20 In the present study, regardless of whether PTDB was applied or not, it has been shown that in patients with clinical and laboratory-appropriate chemotherapy toxicities, the treatment decision is individualised, and starting chemotherapy with appropriate agents and doses in liver dysfunction accompanied by hyperbilirubinemia has a positive effect on aHFOS (3.45 months vs. 1.11 months, p=0.003). The heterogeneity of the patient groups included in the study, different treatment modalities applied to patients and the retrospective design are the main limitations of this study.
CONCLUSION
The follow-up and treatment of pancreatic adenocarcinoma patients who develop liver dysfunction with hyperbilirubinemia are complex and challenging for oncologists. Although there is no clear data on this subject, this study is important in determining the parameters that predict the prognosis in these patients and how to approach the follow-up and treatment. Further large-scale studies are needed to use this information in clinical practice.
ETHICAL APPROVAL:
Ethics Committee approval was received from the Ethics Committee of Dr. Abdurrahman Yurtaslan Ankara Training and Research Hospital (Number 2022-05/1858, dated June 8, 2022).
PATIENTS’ CONSENT:
Because this study was retrospective, patients' consent was waived.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS' CONTRIBUTION:
MB, FT, CK, AOD: Carried out the conception and design of the research, drafted the manuscript, and carried out the analysis and interpretation of data.
MB, FT: Performed the statistical analysis and participated in data acquisition.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet 2020; 395(10242):2008-20. doi.10.1016/S0140- 6736(20)30974-0.
- Modi B, Shires GT. Pancreatic cancer, cystic pancreatic neoplasms, and other nonendocrine pancreatic tumor. Sleisenger Fordtran’s Gastrointest Liver Dis 2021; 947-65.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin Internet 2019; 69(1):7-34. doi 10.3322/caac. 21551.
- Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet 2016; 388(10039):73-85. doi. 10.1016/S0140- 6736(16)00141-0.
- McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2018; 24(43):4846–61. doi:10.3748/wjg. v24.i43.4846.
- Puckett Y, Garfield K. Pancreatic cancer. In: StatPearls. StatPearls Publishing; 2021.
- Hand F, Conlon KC. Pancreatic cancer. Surg 2019; 37(6):319-26. doi: 10.1016/j.mpsur.2019.03.005.
- Sohal DPS, Mangu PB, Khorana AA, Shah MA, Philip PA, O’Reilly EM, et al. Metastatic pancreatic cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol 2016; 34(23):2784-96. doi: 10.1200/JCO.2016.67. 1412.
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014; 371(11):1039-49. doi: 10.1056/NEJMra1404198.
- Jansen H, Pape UF, Utku N. A review of systemic therapy in biliary tract carcinoma. J Gastrointest Oncol 2020; 11(4): 770-89. doi: 10.21037/jgo-20-203.
- Organization WH. International agency for research on cancer. 2019;
- Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant Chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer. JAMA 2013; 310(14):1473. doi: 10.1001/jama.2013.279201.
- Seufferlein T, Bachet JB, Van Cutsem E, Rougier P. Pancreatic adenocarcinoma: ESMO–ESDO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23:vii33–40. doi: 10.1093/annonc/mds224.
- Nehéz L, Andersson R. Compromise of immune function in obstructive jaundice. Eur J Surg 2002; 168(6):315-28.
- Papadopoulos V, Filippou D, Manolis E, Mimidis K. Haemostasis impairment in patients with obstructive jaundice. J Gastrointest Liver Dis 2007; 16(2):177.
- Artinyan A, Soriano PA, Prendergast C, Low T, Ellenhorn JDI, Kim J. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB 2008; 10(5):371-6. doi: 10.1080/13651820802291233.
- Llop E, Guerrero PE, Duran A, Barrabés S, Massaguer A, Ferri MJ, et al. Glycoprotein biomarkers for the detection of pancreatic ductal adenocarcinoma. World J Gastroenterol 2018; 24(24):2537-54. doi: 10.3748/wjg.v24.i24.2537.
- Luo G, Jin K, Guo M, Cheng H, Liu Z, Xiao Z, et al. Patients with normal-range CA19-9 levels represent a distinct subgroup of pancreatic cancer patients. Oncol Lett 2017; 13(2):881–6. doi: 10.3892/ol.2016.5501.
- Luo G, Jin K, Deng S, Cheng H, Fan Z, Gong Y, et al. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim Biophys Acta - Rev Cancer 2021; 1875(2):188409. doi: 10.1016/j.bbcan.2020.188409.
- Álvarez R, Carrato A, Adeva J, Alés I, Prados S, Valladares M, et al. Management of hyperbilirubinaemia in pancreatic cancer patients. Eur J Cancer 2018; 94:26-36. doi: 10. 1016/j.ejca.2018.01.078.
- Strasberg SM, Gao F, Sanford D, Linehan DC, Hawkins WG, Fields R, et al. An important, poorly recognised risk factor for diminished survival in patients with adenocarcinoma of the head of the pancreas. HPB 2014; 16(2):150-6. doi: 10.1111/hpb.12094.
- Field KM, Michael M. Part II: Liver function in oncology: Towards safer chemotherapy use. Lancet Oncol 2008; 9(12):1181-90. doi: 10.1016/S1470-2045(08)70307-3.