Prevalence, Clinical Characteristics, and Clinical Outcomes of New-onset Diabetes Mellitus among COVID-19 Patients in Developing and Developed Countries: A Systematic Review
By Khunsa Junaid1, Nauman Dawood2, Muhammad Daood1, Fawad Ahmad Randhawa3, Muhammad Kamran Yousaf2, Mian Sajjad Ahmad2Affiliations
doi: 10.29271/jcpsp.2023.06.691ABSTRACT
Diabetes mellitus (DM) is linked to poor clinical outcomes and high mortality in Coronavirus patients. The primary objective of this systematic review was to determine the prevalence, clinical features, glycemic parameters, and outcomes of newly diagnosed diabetes in individuals with COVID-19 in developing and developed countries. By searching PubMed, Medline, Scopus, Embase, Google Scholar, and PakMediNet databases, an online literature search was conducted from March 2020 to November 2021. Guidelines for reporting systematic reviews and meta-analyses (PRISMA) were used. There were 660 publications found, of which 27 were original studies involving 3241 COVID-19 patients were selected. In the COVID-19 patients with new-onset diabetes, mean age was 43.21±21.00 years. Fever, cough, polyuria, and polydipsia were the most frequently reported symptoms, followed by shortness of breath, arthralgia, and myalgia. The developed world reported (109/1119) new diabetes cases (9.74%), while the developing world reported (415/2122) (19.5%). COVID-19 new-onset diabetic mortality rate was 470/3241 (14.5%).
Key Words: COVID-19, New onset diabetes mellitus, SARS-CoV-2, Prevalence, Clinical outcomes, Developing countries, Developed countries.
INTRODUCTION
SARS coronavirus 2 (SARS-CoV-2) is a new enveloped RNA beta-coronavirus that causes severe acute respiratory syndrome. In early 2020, the WHO declared COVID-19 a global pandemic.1,2 The virus can be disseminated by asymptomatic patients or by infected individuals. COVID-19 individuals can have no symptoms, mild disease, or serious illness with multi-organ failure and death. COVID-19 mortality ranges from 0.7 to 10.8%.3,4 Survival decreases, and more problems emerge in advanced age groups and patients with underlying comorbidities. This has been a source of concern for people living with chronic conditions such as Type 1 diabetes mellitus (T1DM).5
Diabetes mellitus (DM) is a common chronic metabolic illness that causes significant morbidity and mortality globally. Diabetics are more prone to major consequences like SARS and multi-organ failure.6 Diabetic individuals have a more severe COVID-19 manifestation. The condition of diabetic ketoacidosis is prevalent. A few patients with normal HbA1c and no family history of diabetes presented with new-onset hyperglycemia. COVID-19 patients have developed new-onset diabetes globally.7 COVID-19-related DM raises management concerns. Eventually, they all get subcutaneous or intravenous insulin to maintain normoglycaemia while hospitalised. However, COVID-19 linked DM may acquire normoglycaemia with insulin faster than preexisting DM.8 A register of COVID-19-related DM is required from various COVID centres and hospitals globally. Additionally, it is critical to determine if these patients will develop diabetes or enter remission.9
Several biological processes have been related to SARS-CoV-2 infection in DM. To begin, DM decreases immunity. Chemotaxis, phagocytosis, and complement fixation are all affected by hyperglycemia. Second, DM can induce an inflammatory state characterised by higher levels of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumour necrosis factor (TNF), which has been related to multi-organ failure in SARS-CoV-2 patients.7,8 Third, in diabetic patients, the angiotensin-converting enzyme 2 (ACE2) receptor, which permits SARS-CoV-2 to enter human cells, is upregulated, increasing the likelihood of virus infection. Fourth, COVID-19 virus can infect endocrine pancreas cells expressing ACE2 receptors, limiting insulin production and aggravating or causing DM.8,9 DM may potentially be caused by insulin resistance caused by high interleukin-6 and tumour necrosis factor-alpha levels in COVID-19 patients. Hyperglycemia increases the susceptibility of lung cells to viral infection and replication.9,10
COVID-19 and DM are dependent on one another. Diabetes is associated with a poor prognosis for COVID-19. These patients had to develop new-onset DM and severe DM complications such as diabetic ketoacidosis (DKA) and hyperosmolarity.7,8 Diabetes prevalence has risen sharply in developing countries in recent decades. Despite this, many published works focused on the impact of pre-existing DM on COVID-19 clinical course and outcome.8,10 There are few data on the prevalence, clinical characteristics, types, and outcomes of new-onset DM in COVID-19 patients. To provide reliable data on newly diagnosed diabetes in COVID-19 patients, large-scale studies are now required. Therefore, the objective of this study was to assess the prevalence, clinical characteristics, types of newly diagnosed DM, glycemic parameters, and outcome of newly diagnosed DM in COVID-19 patients from developed and developing countries.
METHODOLOGY
This study used PRISMA (Principles for Reporting Systematic Reviews and Meta-Analyses) guidelines.11 Because this was a systematic review, no IRB approval was required. Eligibility criterion for this systematic review was determined using the PICOS criteria.
PubMed / Medline / Scopus, Embase, Google Scholar, and PakMediNet were searched for all peer-reviewed articles published from March 2020 to November, 2021. Synonyms, Boolean operators, and truncation strategies were used to include as many articles as possible. 'New-onset diabetes', 'newly diagnosed diabetes', 'transient hyperglycaemia', and 'secondary hyperglycaemia' are all search terms that can be used in conjunction with 'COVID-19'. The search was restricted to the English language. Additionally, the reference lists of relevant articles were sorted through in order to identify additional studies that met the criteria.
This review included only observational studies that provided data on the number or percentage of newly diagnosed diabetes among COVID-19 patients. COVID-19 (lab-confirmed or clinically diagnosed) and new-onset DM were considered regardless of age, gender, or nationality. Non-peer-reviewed papers, COVID-19 patients with pre-existing diabetes and hyperglycemia, letters to editors, editorials, commentaries, review articles; animal studies, and publications in languages other than English were excluded from this review.
Newly diagnosed diabetes was defined as new-onset diabetes when an individual has been diagnosed with diabetes for the first time, they have no prior history of the disease and have fasting plasma glucose (FPG) levels less than or equal to 7.0 millimol/L or random blood glucose levels less than or equal to 11.1 millimol/L (RBG) levels or less than or equal to 6.5 percent (HbA1c).
After retrieving titles and abstracts, two authors checked for inclusion criteria. The remaining full texts were then screened for insufficient data and duplicates. At this stage, studies and cases in case series were double-checked. Three reviewers used standard data extraction formats to extract data from included studies. The extracted data included the type of study, the first author, the year of publication, the country of publication, the number of patients, their age, gender, symptoms, and glycemic parameters findings in COVID-19 patients admitted to the hospital. The review also included data on the number of newly diagnosed diabetes cases, the type of newly diagnosed diabetes mellitus, the time of diagnosis, the definition of newly diagnosed diabetes, and clinical outcomes. Author’s disagreements were resolved by consensus.
The Newcastle–Ottawa Scale was used to assess study quality (NOS). This scale assesses both cross-sectional and cohort studies. Selection, comparability, and exposure/outcome are each given a maximum of four, two, or three points. On this scale, high-quality studies get a 7, while moderate-quality studies get a 5–7. Using the Joanna Briggs Institute (JBI) tool, the authors assessed the risk of bias in case reports and case series. This tool asked nine questions about the target population, sample size, condition being measured, and statistical analysis. For the quality assessment, the questions in the checklists must be answered with yes, no, unclear, or not applicable. The risk of bias classification is high for 1–3 yes responses, moderate for 4–6 yes responses, and low for 7–8 or more yes responses.12,13 The authors undertook a world population review data using the Human Development Index (HDI) to classify countries in developing and developed countries list. HDI ranges from 0 to 1. Countries with a score above 0.80 are considered developed, while those with a score below 0.80 are considered developing.14,15
All statistical analyses were performed using SPSS 24.0 (SPSS Inc. Chicago, IL, USA, 2020). The results are presented descriptively. Quantitative variables are summarised using mean and standard deviation, while qualitative variables are presented as frequencies (n) and percentages (%). Each case report/series contained individual-level data. The authors present all available data and the percentage of variables with missing data. Valid denominators were used to calculate percentages (i.e. denominator signifies the number of patients who have data for the characteristic of interest). Hozo et al. method was to used estimate the median, average, and standard deviation of variables found in many cohort studies.16 Among the studies that included glycemic parameters measurements, the authors chose those that included laboratory measurements during hospital admission.
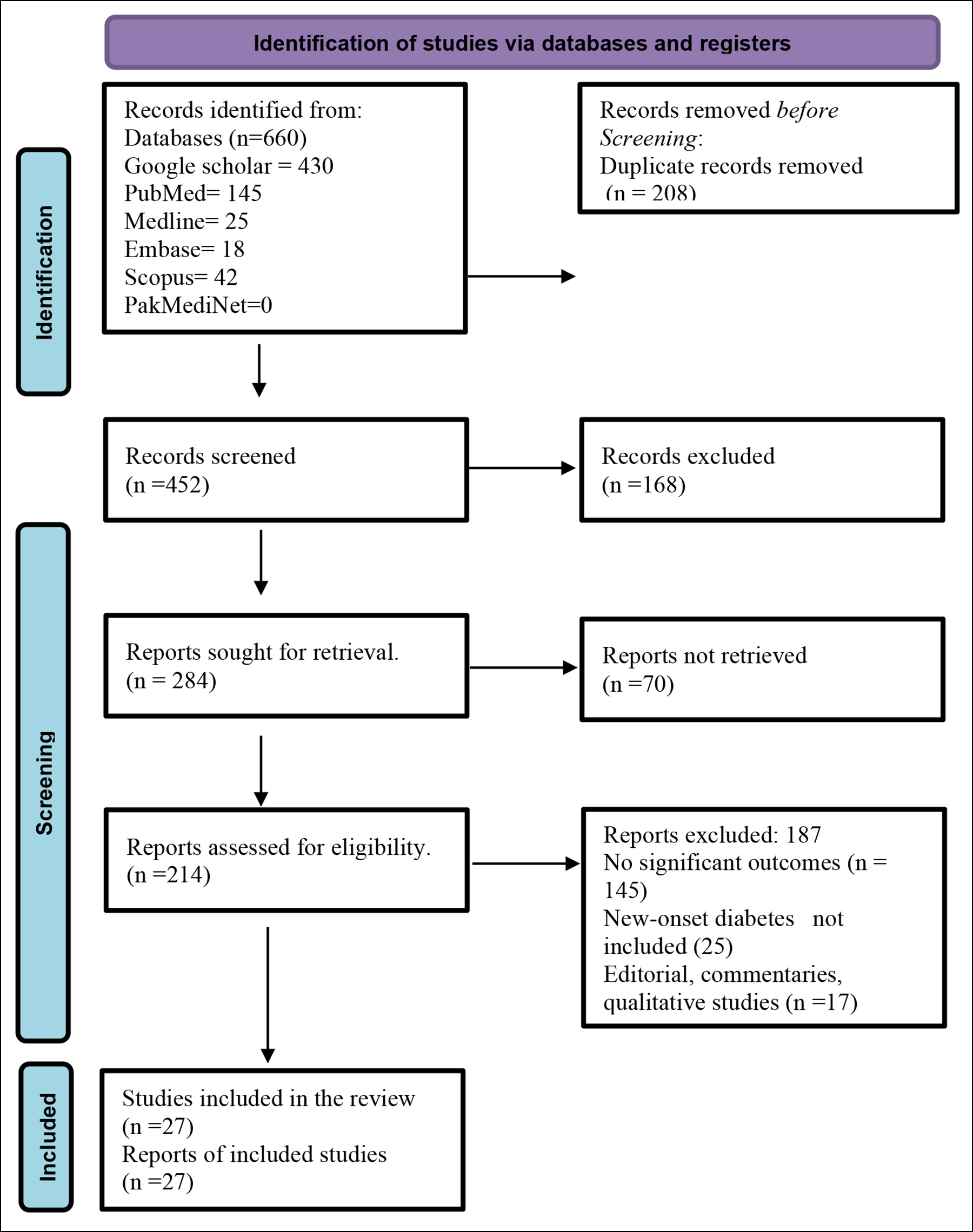 Figure 1: PRISMA flow chart of the study selection procedure.
Figure 1: PRISMA flow chart of the study selection procedure.
RESULTS
A comprehensive literature review found 660 studies in databases such as Google Scholar, PubMed, Medline, Embase, Scopus, and PakMediNet (Figure 1). Two hundred and eight studies were removed due to duplication, and 168 were removed after the title and abstract were screened. The eligibility of 214 reports was determined, and 27 were selected for the final systematic review (Figure 1).17-43
To examine the onset of diabetes in COVID-19 patients, 27 observational studies were identified, including ten case reports, eight case series, eight cohort studies, and one cross-sectional study. The participants comprised 3241 COVID-19 patients with a non-significant prior history of diabetes mellitus. The clinical characteristics and symptoms of COVID-19 patients are summarized in the table below (Table I). Patients included in the study ranged in age from infants to adults and the elderly. The mean age of included COVID-19 patients was 43.21 ± 21.00 years, with a greater proportion of men 1636 (50.4%). Age was reported as the median (IQR) in six case series and seven cohort studies. In one cross-sectional study and one cohort study, the average age of all participants was mentioned (Table I). As a result of the distribution being approximately normally distributed in many studies, the median age was substituted for the mean and the interquartile range (IQR) for the standard deviation. Fever, cough, polyuria, and polydipsia were the most frequently reported symptoms, followed by shortness of breath, arthralgia, and myalgia. These were all hospital-based studies (Table I). Fourteen studies were conducted in developed countries, 13 in developing countries. The majority of studies were conducted in China (5 studies; 18.5%), India (5 studies; 18.5%) and Saudi Arabia (3 studies; 11.11%, Table I.
The mean random blood glucose level was 25.54 ±15.02. The mean ± SD of HbA1c was 91.85± 31.49 mmol/mol (Table II). Out of 3241 COVID-19 patients, (524/3241, 16.1%) had newly diagnosed diabetes from 27 studies. The developed countries reported (109/1119, 9.74%) new diabetes cases, while the developing world reported (415/2122, 19.5%). Eleven studies report Type 1 diabetes, while only three report Type 2 diabetes. The majority of studies 21(77.7%) detected newly diagnosed diabetes on the first day of hospitalisation. The mortality rate in new-onset diabetic patients with COVID-19 was (470/3241, 14.50%). The three studies did not report patient outcomes (Table III).
With respect to methodological quality of Newcastle–Ottawa Scale (NOS), cross-sectional study had moderate quality and low risk of bias and all cohort studies had moderate study quality and moderate risk of bias. The Joanna Briggs Institute (JBI) tool was used to assess the methodological quality of case reports and case series. Four studies had a moderate risk of bias, and fourteen studies had a low risk of bias, according to the results.
DISCUSSION
SARS-CoV-2 has been identified as the new virus causing this global pandemic. With severe insulin resistance and insulin insufficiency, COVID-19-associated diabetes is difficult to manage.8,9 Although numerous studies have linked COVID-19 to DM, this is the first comprehensive review to synthesise clinical characteristics, prevalence of new-onset DM in COVID-19 patients, types, and outcomes in developed and developing countries. In this review, the most common COVID-19 patient’s symptoms were fever, cough, polyuria, and polydipsia, as well as shortness of breath, arthralgia, and myalgia. This finding is in alignment with previous studies.44,45 Diabetes mellitus is becoming more common all over the world, is a big public health problem, and is a big problem in the medical field. Diabetes mellitus long-term illness that is very serious can reduce a person's life expectancy, contribute to making their quality of life worse, and cost more to cure. The disease characteristics, prevalence, mortality rates and symptoms, differ broadly among different regions of the world.5,7 This finding suggests that, in addition to fever, cough, and dyspnea, diabetes screening is critical in COVID-19 patients, and it seems that COVID-19 patients with these signs and symptoms need extra attention.
The overall mean age of the participants in this review was 43 years. The median age of hospitalized COVID-19 patients was 47 years in a previous study.44 In developing countries, the majority of diabetics are between the ages of 45 and 65, whereas in developed countries, the majority over the age of 64. By 2030, diabetes will affect over 82 million people aged 64 and older in developing countries and over 48 million in developed countries, according to demographic trends.
Table I: Baseline characteristics of included COVID-19 patients.
|
Study type |
First author (Ref) |
Country |
Sample size (n) |
Age, Mean, median (IQR) (years) |
Male N (%) |
Female N (%) |
Symptoms N (%) |
|
Case report |
Al-Naami 202017 |
Saudi Arabia |
1 |
46 |
1 |
0 |
Fever, cough, shortness of breath, irritability, increased thirst and urination for one week |
|
Case report |
Soliman 202018 |
Qatar |
1 |
0.7 |
N/R |
N/R |
Two days’ history of fever, vomiting, 10% dehydration and rapid breathing |
|
Case report |
Rabizadeh 202019 |
Iran |
1 |
16 |
1 |
0 |
Seven-day history of fatigue, weakness weight loss, nausea, polyuria, polydipsia, and abdominal pain. |
|
Case report |
Daniel 202020 |
India |
1 |
15 |
0 |
1 |
Abdominal pain and vomiting |
|
Case report |
Marchand 202021 |
France |
1 |
29 |
0 |
1 |
Severe asthenia, fever, stiffness and dyspnea. Polyuria, polydipsia syndrome |
|
Case report |
Ali 202122 |
Qatar |
1 |
53 |
1 |
0 |
History of fever, cough, and shortness of breath for 2 days, associated with vomiting once. |
|
Case report |
Alfishawy 202123 |
Egypt |
1 |
17 |
1 |
0 |
Fever, palpitation, and cough of four-week duration |
|
Case report |
Albuali & AlGhamd202124 |
Saudi Arabia |
1 |
7 |
0 |
1 |
Polyuria, polydipsia, weight loss, fatigue and vomiting |
|
Case report |
Ordooei 202125 |
Iran |
1 |
10 |
1 |
0 |
History of polyuria, vomiting and polydipsia from ten days |
|
Case report |
Ghosh 202126 |
India |
1 |
60 |
1 |
0 |
Dull aching pain in his abdomen for the last 3 days associated with fever, cough, throat ache, malaise, increased thirst, excessive frequency of urination and generalised weakness |
|
Case series |
Kuchay 202027 |
India |
3 |
30,60,34 |
3 |
0 |
Fever or chills, Cough, Shortness of breath or difficulty breathing, Fatigue, Muscle or body aches, Headache |
|
Case series |
Yang 202028 |
China |
n = 69 among 120 evaluated |
61 years (IQR, 52 to 67) |
34 (49.3) |
35 (50.72) |
Fever :62 (89.9), Cough: 45 (65.2), Sputum: 12 (17.4), Dyspnea: 30 (43.5), Fatigue: 26 (37.7), Diarrhea: 12 (17.4) |
|
Case series |
Alsadhan 202029 |
Saudi Arabia |
5 |
47 (42–62.5) |
3 (0.6) |
2 (0.4) |
4 days’ history of shortness of breath, cough, and confusion |
|
Case series |
Zavaleta 202030 |
Peru |
14 |
64 (42.5–71.2) |
2(0.14) |
12(85.71) |
N/R |
|
Case series |
Reddy 202031 |
India |
2 |
45 (30–45) |
2(2.0) |
0(0.0) |
General weakness, fever, loss of taste and mild dyspnea |
|
Case series |
Plasencia-Dueñas 202132 |
Peru |
13 |
64 (42.5–71.2) |
3(0.23) |
10(76.92) |
N/R |
|
Case series |
Shankar 202133 |
India |
10 |
13 (11-15) |
4(0.4) |
6(0.6) |
General weakness, fever |
|
Case series |
Suwanwongse & Shabarek 202134 |
United States |
3 |
18,51,64 |
2(66.66) |
1(33.33) |
Fever, cough, Anorexia, vomiting, nausea:1 (33.33) Fatigue:2(66.66),polydipsia:3(100), polyuria:3(100.0) |
|
Cohort
|
Li 202035 |
China |
453 |
61.0 (49-68) |
59(13.0) |
394(86.9) |
Fever: 77 (81.9), Cough: 61(64.9), Dyspnea: 35 (37.2), Nausea or vomiting: 9 (9.6), Fatigue: 47 (50.0), Diarrhoea: 12 (12.8), Poor appetite: 14 (14.9), Palpitation: 5 (5.3), Chest distress: 30 (31.9) |
|
Cohort
|
Zhou 202036 |
China |
80 |
47 (35-56) |
48 (60) |
32(40) |
Fever: 68 (85.00), Fatigue: 30 (37.5), Cough: 45 (56.25), Chest tightness: 27 (33.75) Dyspnea: 8 (10.00), Diarrhea: 9 (11.39) |
|
Cohort
|
Wang 202037 |
China |
132 |
66 (56-(72) |
68(51.51) |
64(48.48) |
Fever: 34 (77.3), Cough: 31 (75.6), Fatigue: 11 (26.8), Dyspnea: 32 (78.0), Myalgia: 6 (14.6), Diarrhea 3 (7.3) |
|
Cohort
|
Zhang 202038 |
China |
166 |
62.7±14.2 |
85 (51.2) |
81(48.79) |
Fever: 16 (76.2), Cough: 15 (71.4), Dyspnea: 16 (76.4), Diarrhoea: 10 (47.6), Anorexia: 10 (47.6), Fatigue: 15 (71.4), Sore throat: 6 (28.6)
|
|
Cohort
|
Wang 202039 |
China |
605 |
59.0 (47.0, 68.0) |
322 (53.2) |
283 (46.8) |
Fever: 463(87.4), Cough: 404 (72.8), Muscular soreness: 129 (25.6), Fatigue: 300 (56.8), Diarrhoea: 91 (17.8) |
|
Cohort
|
Fadini 202040 |
Italy |
413 |
64.9±15.4 |
245(59.32) |
168(40.6) |
Fever: 272 (66.0%), Cough: 234 (63.9%), Dyspnea: 232 (61.9%), GI symptoms: 102 (28.5%) |
|
Cohort
|
Lampasona 202041 |
Italy |
509 |
64.0 (56.2-71.5) |
335(65.8) |
174(34.1) |
N/R |
|
Cohort
|
Smith 202142 |
USA |
184 |
64.4 (21-100) |
98(53.2) |
86(46.7) |
Hypoxia (83.7%) and fever 115 (62.5%) |
|
Cross-sectional |
Farag 202143 |
Egypt |
570 |
47.9 ± 10.9 |
317 (55.5) |
253(44.3) |
Fever: 70 (90.9%), Cough: 70 (90.9%), Dyspnea: 66 (85.7%), Diarrhoea: 11 (14.3%) |
|
Total=27 |
|
|
3241 |
43.21 ± (21.00) |
1636(50.4%) |
1604(49.4%) |
|
Table II: Glycemic parameters findings of COVID-19 patients at admission.
| Study type | First author (Ref) | Country | Random blood glucose (mmol/L) | HbA1c (mmol/mol) |
|
Case report |
Al-Naami 202017 |
Saudi Arabia |
36.5 |
87 |
|
Case report |
Soliman 202018 |
Qatar |
31.7 |
64 |
|
Case report |
Rabizadeh 202019 |
Iran |
28.41 |
108 |
|
Case report |
Daniel 202020 |
India |
22.97 |
119 |
|
Case report |
Marchand 202021 |
France |
20.5 |
105 |
|
Case report |
Ali 202122 |
Qatar |
16.4 |
53 |
|
Case report |
Alfishawy 202123 |
Egypt |
31.41 |
125 |
|
Case report |
Albuali & AlGhamd202124 |
Saudi Arabia |
30.80 |
86 |
|
Case report |
Ordooei 202125 |
Iran |
27.03 |
53 |
|
Case report |
Ghosh 202126 |
India |
29.97 |
N/R |
|
Case series |
Kuchay 202027 |
India |
30.80, 32.30, 52.17 |
86,108, 108 |
|
Case series |
Yang 202028 |
China |
6.5 |
N/R |
|
Case series |
Alsadhan 202029 |
Saudi Arabia |
23.3, 24 |
125,140 |
|
Case series |
Zavaleta 202030 |
Peru |
43.35,38.79, 67.60 |
108,133,143 |
|
Case series |
Reddy 202031 |
India |
N/R |
75,112 |
|
Case series |
Plasencia-Dueñas 202132 |
Peru |
N/R |
N/R |
|
Case series |
Shankar 202133 |
India |
N/R |
N/R |
|
Case series |
Suwanwongse & Shabarek 202134 |
United States |
27.53,44.12,19.59 |
90,112 |
|
Cohort |
Li 202035 |
China |
4.97 |
N/R |
|
Cohort |
Zhou 202036 |
China |
N/R |
N/R |
|
Cohort |
Wang 202037 |
China |
N/R |
53 |
|
Cohort |
Zhang 202038 |
China |
7.7 |
46 |
|
Cohort |
Wang 202039 |
China |
9.769 |
N/R |
|
Cohort |
Fadini 202040 |
Italy |
7.3 |
50.3 |
|
Cohort |
Lampasona 202041 |
USA |
9.93 |
57 |
|
Cohort |
Smith 202142 |
Italy |
5.5 |
N/R |
|
Cross-sectional |
Farag 202143 |
Egypt |
11.56 |
42 |
|
Total=27 |
|
|
25.54 ± 15.02 |
91.85± 31.49 |
Increases are anticipated in the Middle East crescent, Sub-Saharan Africa, India, and Pakistan.46 This is because chronic illnesses become more prevalent as people age. The current systematic review included more men (50.4%) than in previous studies (39.10% and 68.0%).47,45 The sampling methodology, study subjects, study year(s), geographical location, variability within the studied subpopulation, and representation of sex and age groups in the population sample may have contributed to this heterogeneity.
The study also found that HbA1c and venous glucose levels of study participants were high on hospital admission, as in the previous study.43,44 HbA1c values were reported for all newly diagnosed diabetic patients in COVID-19 patients in nineteen studies. On admission, a higher fasting blood glucose (FBG) level was a significant predictor of COVID-19 fatality. Diabetes was found to be associated with worsening disease severity and a worsening prognosis in patients with COVID-19.44,48
In 27 observational studies, patients infected with SARS-CoV-2 had a 16.6% prevalence of newly diagnosed diabetes mellitus (1 to 29%). A proportion of 7.4% of COVID-19 hospitalised patients had DM according to Guan et al. From 8 studies involving 3700 patients, 14.4% of COVID-19 hospitalised patients had recently been diagnosed with diabetes.49,50 Eleven studies report Type 1 diabetes, while only three report Type 2. This finding is in line with other research showing type 1 diabetes outweighs type 2.51 It is currently unknown whether the COVID-19-associated new-onset diabetes is type 1, type 2, or a complicated type. Insulin deficiency caused by SARS-CoV-2 infection may cause -cell death. Only a few case reports have been published on islet cell antibodies in newly diagnosed diabetes.50,52 Perhaps some of the patients had a subclinical COVID-19 or ignored mild symptoms, triggering T1DM in genetically susceptible individuals. Another explanation is that some of these studies were done in areas where COVID-19 was common, while it was rare elsewhere.50
The developed world reported 109/1119 new cases of diabetes (9.74 %), while the developing world reported (415/2122) new cases of diabetes (19.5%). Diabetes is a significant public health problem as well as a clinical concern. The prevalence of diabetic type 1 diabetes is high in the world's poorest countries, but data on disease prevalence are scarce.50,52 Noncommunicable diseases account for the majority of deaths in both developed and developing countries. Westernization and urbanization are held responsible for the increase. The current gradually shift from communicable diseases toward noncommunicable diseases upwards the burden in developing countries.50,46 In developed countries, obesity, population ageing, and hypertension are all risk factors for diabetes. Variations in DM risk factors may also contribute to regional variation in prevalence.46 This heterogeneity may have been exacerbated by population representation by gender and age group. Healthcare providers must educate diabetic patients on personal and environmental hygiene. The emphasis is on early diabetes detection, blood glucose control, patient education, diabetic programmes, and public awareness seminars.
Table III: Characteristics of included studies to assess new-onset diabetes mellitus and clinical outcomes among the COVID-19 patients.
|
Study type |
First author (Ref) |
Country |
Number of newly diagnosed diabetes cases |
Type of diabetes |
Time of diagnosis |
Definition of newly diagnosed diabetes |
Clinical outcomes |
|
Discharge / Death |
|||||||
|
Case report |
Al-Naami 202017 |
Saudi Arabia |
1 |
Type 1 |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l and HbA1c>6.5 |
Death |
|
Case report |
Soliman 202018 |
Qatar |
1 |
Type 1 & DKA |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l and HbA1c>6.5 |
Discharge |
|
Case report |
Rabizadeh 202019 |
Iran |
1 |
N/R |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l and HbA1c>6 |
Discharge |
|
Case report |
Daniel 202020 |
India |
1 |
Type 1 & DKA |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l and HbA1c>6 |
Discharge |
|
Case report |
Marchand 202021 |
France |
1 |
Type 1 |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l and HbA1c>6 |
N/R |
|
Case report |
Ali 202122 |
Qatar |
1 |
Type 1 & DKA |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l and HbA1c>6 |
Death |
|
Case report |
Alfishawy 202123 |
Egypt |
1 |
Type 1 & DKA |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l and HbA1c>6.5 |
Discharge |
|
Case report |
Albuali & AlGhamd202124 |
Saudi Arabia |
1 |
Type 1 & DKA |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l and HbA1c>6.5 |
Discharge |
|
Case report |
Ordooei 202125 |
Iran |
1 |
Type 1 |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l and HbA1c>6.5 |
Discharge |
|
Case report |
Ghosh 202126 |
India |
1 |
Type 1 & DKA |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l |
Discharge |
|
Case series |
Kuchay 202027 |
India |
3 |
Type 1 |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l and HbA1c>6.5 |
Discharge |
|
Case series |
Yang 202028 |
China |
N/R |
N/R |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l |
Death: 16 |
|
Case series |
Alsadhan 202029 |
Saudi Arabia |
2 |
N/R |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l and HbA1c>6.5 |
Death: 1 |
|
Case series |
Zavaleta 202030 |
Peru |
4 |
N/R |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l and HbA1c>6.5 |
Death:2 |
|
Case series |
Reddy 202031 |
India |
2 |
N/R |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l and HbA1c>6 |
Discharge:2 |
|
Case series |
Plasencia-Dueñas 202132 |
Peru |
4 |
N/R |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l |
N/R |
|
Case series |
Shankar 202133 |
India |
3 |
N/R |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l |
Discharge:5 |
|
Case series |
Suwanwongse & Shabarek 202134 |
United States |
3 |
N/R |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l and HbA1c>6 |
Discharge:3 |
|
Cohort |
Li 202035 |
China |
94 |
N/R |
Third day of hospital admission |
No prior diabetes history, FPG ³7.0 mmol/l |
Discharge:74 Death:20 |
|
Cohort |
Zhou 202036 |
China |
22 |
N/R |
N/R |
No prior diabetes history, RBG ≥11.1 mmol/l |
N/R |
|
Cohort |
Wang 202037 |
China |
16 |
Type 2 |
First day of hospital admission |
No prior diabetes history, RBG ≥11.1 mmol/l and HbA1c>6.5% |
Death:16 |
|
Cohort |
Zhang 202038 |
China |
21 |
N/R |
Within 3 days after hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/l and HbA1c>6.5% |
Discharge: 127 (76.5%) Death: 24 (14.5%) |
|
Cohort |
Wang 202039 |
China |
176 |
Type 2 |
First day of hospital admission |
No prior diabetes history, FBG ≥7.0 mmol/ |
Death: 114(18.8%) |
|
Cohort |
Fadini 202040 |
Italy |
21 |
N/R |
Exact time of diagnosis not reported |
No prior diabetes history, HbA1c ³6.5% or RBG |
Discharge: 298 (72.2%) Death: 48 (11.6%) |
|
Cohort |
Lampasona 202041 |
USA |
29 |
N/R |
Exact time of diagnosis was not reported |
No prior diabetes history, HbA1c ³6.5% or RBG |
Death: 102 |
|
Cohort |
Smith 202142 |
Italy |
49 |
NR |
Exact time of diagnosis was not reported |
No prior diabetes history |
Death:63 Discharge:57 |
|
Cross-sectional |
Farag 202143 |
Egypt |
65 |
Type 1 & 2 |
First day of hospital admission |
No prior diabetes history, fasting plasma glucose [FPG] ≥126 mg/dL or random blood glucose [RBG] ≥200 mg/dL and HbA1c <6.5% |
Death:62 |
|
Total=27 |
|
|
524 |
|
|
|
|
COVID-19 had a mortality rate of 470/3241 (14.50%). According to one study, patients with newly diagnosed diabetes died at a higher rate than patients with no newly diagnosed diabetes (18.2% vs. 9.7%).43 Similarly, an American study discovered that diabetic COVID-19 patients died at a higher rate than non-diabetic COVID-19 patients (288.8% vs. 6.2%).53 COVID-19 death was reported as a single or composite outcome in a few studies. According to Barron et al.54 COVID-19 caused the death of 1.50 percent (364/23,698 patients) of T1DM patients. In comparison, O'Malley et al. reported a 4% (5/113) mortality rate.55 Chronic hyperglycemia has been associated with impaired immune function and a poor prognosis. This is the first study to demonstrate that diabetes exacerbates disease severity and impairs prognosis in patients with COVID-19.54 Diabetes patients have a higher mortality and morbidity rate when it comes to serious medical conditions such as myocardial infarction. Additionally, elevated FBG predicts hospitalization in critically ill patients who do not have diabetes. Additionally, diabetes has been linked to severe disease following respiratory infections. Patients with COVID-19 and T2D who had well-controlled glycaemia had better outcomes, according to a recent study.54,55
Diabetic ketoacidosis (DKA) was common in COVID-19 and T1DM patients (6/11 studies, 54%). Other studies found higher rates of DKA in COVID-19 and T1DM patients, with O'Malley et al. reporting 24% (27/113),55 Unsworth et al. reporting 36% (12/33),47 and Atlas et al. reporting 51.7 percent (30/58).56 DKA has been seen in people with COVID-19, indicating SARS-CoV-2 directly affects pancreatic cells. In a SARS-CoV-1 hospitalized patient, immunostaining for ACE2 protein was strong in pancreatic islets but weak in exocrine tissues.50,52 This should worry anyone concerned. This means that diabetic patients would seek care after complications started to occur if no diabetic survey was conducted among COVID-19 patients. After that, diabetic complications would be irreversible. Diabetes mellitus severely limits patients' activities.50 Many studies have linked sub-acute viral injury to T1DM, an autoimmune disease characterized by pancreatic beta cell loss. Acute islet injury has been reported rarely. Acute diabetes has been linked to pancreatic islet viruses.50 Hyperglycemia has been linked to SARS-CoV-2 mortality. Virus-ACE2 receptor coupling may impair ACE2 function, linked to DM in ACE2 mouse models.48,49 The virus can use the ACE2 receptor to replicate inside pancreatic islets, damaging cells like insulin-producing beta cells. Insulin deficiency leads to acute DM in SARS-CoV-2 patients. Newly diagnosed diabetes has a worse COVID-19 prognosis than no diabetes or pre-existing diabetes.50
Unhealthy lifestyle choices like excessive calorie consumption and lack of physical activity have been linked to rising non-communicable disease prevalence in low-resource settings. Multidisciplinary diabetes management is not readily available in low-resource settings. Obtained normoglycaemia and normolipidaemia require careful dietary management.51,52 To prevent full-blown diabetes and other cardiometabolic disorders, COVID-19 patients with newly diagnosed diabetes should be closely monitored. With chronic inflammation and impaired immune response, diabetics appear to be more susceptible to severe illness from COVID-19, with poorer outcomes. Acute anti-inflammatory therapy with immunomodulation may be effective in COVID-19 diabetic patients, but further research is required.53 Patient education on personal and environmental hygiene is needed. Education, programmes for diabetic patients and public awareness talks are all emphasised. Delays in taking action to flatten the COVID-19 pandemic curve will have disastrous consequences.49 Dementia, diabetes, hypertension, and cardiac diseases will necessitate special protection for the elderly in developing countries it’s time to use tele-consultation and tele-medicine to better control their blood sugars.50
The study has some strengths and limitations. This is the first study to consider new onset DM in COVID-19 patients from developed and developing countries. Two researchers independently searched, screened, selected, extracted, and assessed most studies. Unlike many other COVID-19 systematic reviews and meta-analyses, we excluded overlapping cohorts. Secondly, the authors were able to show the effect of glycemic control on COVID-19 mortality in terms of elevated HbA1c. The authors conducted a thorough literature search and carefully selected and scored studies. Finally, the review assessed new onset in COVID-19 patients. As a result, most studies correctly defined DM and inquired about the assessment method.
A meta-analysis was not possible due to a lack of relevant data. Clinical heterogeneity in the studied groups (some studies included adult patients and others included pediatric populations). The burden of new diabetes in COVID-19 patients varies by region and country. All included studies were not designed to estimate new-onset diabetes in COVID-19 patients. The small number of studies that screened for diabetes during serology SARS-CoV-2 testing and the study sample size make it difficult to confirm that SARS-CoV-2 solely causes DM. The clinical characteristics of COVID-19 diabetic patients could not be fully summarised due to a lack of published data.
This study included only a few patients with type 2 diabetes (T1DM). Then, more research will be done with more comprehensive data. These limitations highlight the need for larger global studies with community-based treatment of mild cases. With limited research, we could only use observational studies, case reports and series. Their sample sizes were small and their power was weak.
CONCLUSION
The prevalence of new-onset diabetes mellitus in COVID-19 patients ranged from 1% to 29%. Symptoms that were frequently observed included elevated blood glucose levels, fever, polyuria and polydipsia, shortness of breath, arthralgia and myalgia, nausea and vomiting in patients with newly diagnosed diabetes. The mean HbA1c, and venous glucose levels were all elevated in participants. From 27 studies, 524 patients with newly diagnosed diabetes were identified among 3241 COVID-19 patients. The developed world reported (109/1119) new diabetes cases (9.74%), while the developing world reported (415/2122) (19.5%). COVID-19 had a mortality rate of 470/3241 (14.50%). These patients should receive prompt treatment and should be closely monitored for the development of type 2 diabetes and other cardio-metabolic disorders.
ETHICAL APPROVAL:
Ethics approval for this study is not necessary under Pakistani law as no patient data were collected.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
ND, MD, KJ: The conception and design of the study.
MKY, MSA, KJ: Acquisition, analysis and interpretation of data.
FAR, MD, KJ: Drafting the article.
MD, MSA, KJ: Revising it critically for important intellectual content.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol 2020; 92(6): 568-76. doi: 10.1002/jmv.25748.
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020; 395(10224): 565-74. doi: 10.1016/ S0140-6736(20)30251-8.
- Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020; 395(10223): 514-23. doi: 10.1016/S0140-6736(20)30154-9.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395(10223):497-506. doi: 10.1016/ S0140-6736(20)30183-5.
- Pal R, Yadav U, Grover S, Saboo B, Verma A, Bhadada SK. Knowledge, attitudes and practices towards COVID-19 among young adults with Type 1 Diabetes Mellitus amid the nationwide lockdown in India: A cross-sectional survey. Diabetes Res Clin Pract 2020; 166:108344. doi: 10.1016/ j.diabres.2020.108344.
- Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: Assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol 2016; 4(2):148-58. doi: 10.1016/ S2213-8587(15)00379-4.
- Gentile S, Strollo F, Mambro A, Ceriello A. COVID-19, ketoacidosis and new-onset diabetes: Are there possible cause and effect relationships among them? Diabetes Obes Metab 2020; 22(12):2507-8. doi: 10.1111/dom.14170.
- Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol 2020; 8(9):782-92. doi: 10.1016/ S2213-8587(20)30238-2.
- Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab 2020; 22(10): 1935-41. doi: 10.1111/dom. 14057.
- Maddaloni E, Buzzetti, R. COVID-19 and diabetes mellitus: Unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020; 36: e3321. DOI: 10.1002/dmrr.3321.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J Clin Epidemiol 2021; 134(3):103-12. doi: 10.1016/j.jclinepi. 2021.02.003.
- Critical Appraisal Tools. Joanna Briggs Institute. [Cited 10 September 2021]. Available from: https://jbi.global/critical- appraisal-tools.
- Ottawa Hospital Research Institute. Ohri.ca. 2022 cited 10 September 2021. Available from: http://www.ohri.ca/ programs/clinical_epidemiology/oxford.asp.
- Developed Countries List 2022. Worldpopulationreview.com. 2022 [cited10 September 2021]. Available from: https:// worldpopulationreview.com/country-rankings/developed-countries.
- Developing Countries 2022. Worldpopulationreview.com. 2022 [cited 2610 September 2021]. Available from: https:// worldpopulationreview.com/country-rankings/developing-countries
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13. doi: 10.1186/1471- 2288-5-13.
- Al-Naami AQ, Khan LA, Zaidan FI, Al-Neami IA, Awad AS, Hobani AI, et al. Hyperglycemic hyperosmolar state (HHS) with new-onset diabetes mellitus in a patient with SARS CoV-2 Infection. Authorea Preprints 2020; 2020:1-3. doi: 10.22541/au.160611667.79425739/v1.
- Soliman A, Al-Amri M, Ellithy K, Alaaraj N, Hamed N, De Sanctis V. Newly-onset type 1 diabetes mellitus precipitated by COVID-19 in an 8-month-old infant. Acta Bio Medica Atenei Parmensis 2020; 91(3):e2020046. doi: 10.23750/abm.v91i3.10074.
- Rabizadeh S, Hajmiri M, Rajab A, Kouchak HE, Nakhjavani M. Severe diabetic ketoacidosis and coronavirus disease 2019 (COVID-19) infection in a teenage patient with newly diagnosed diabetes. J Pediatr Endocrinol Metab 2020; 33(9): 1241-3. doi: 10.1515/jpem-2020-0296.
- Daniel S, Gadhiya B, Parikh A, Joshi P. COVID-19 in a child with diabetic ketoacidosis: An Instigator, a deviator or a spectator. Indian Pediatr 2020; 57(10):969. doi: 10.1007/ s13312-020-2008-2.
- Marchand L, Pecquet M, Luyton C. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol Springer 2020; 57(1): 1265-1266. doi: 10.1007/s00592-020-01570-0.
- Ali E, Badawi M, Ahmed A, Abdelmahmuod E, Ibrahim W. Severe SARS-CoV-2 infection presenting with acute kidney injury and diabetic ketoacidosis complicated by pancreatitis in a 53-year man with hypertension. Clin Case Rep 2021; 9(3):1202-6. doi.org/10.1002/ccr3.3731.
- Alfishawy M, Nassar M, Mohamed M, Fatthy M, Elmessiery RM. New-onset type 1 diabetes mellitus with diabetic ketoacidosis and pancreatitis in a patient with COVID-19. Sci Afr 2021; 13:e00915. doi: 10.1016/j.sciaf.2021.e00915.
- Albuali WH, AlGhamdi NA. Diabetic ketoacidosis precipitated by atypical coronavirus disease in a newly diagnosed diabetic girl. J. Taibah Univ. Medical Sci. 2021; 16(4): 628-31. doi.org/10.1016/j.jtumed.2021.01.011.
- Ordooei M, Behniafard N, Soheilipour F, Akbarian E. New onset of diabetes in a child infected with COVID-19: A case report. J. Diabetes Metab Disord 2021; 20(2):2129-32. doi: 10.1007/s40200-021-00900-5.
- Ghosh R, Dubey S, Roy D, Ray A, Pandit A, Ray BK, Benito-León J. Choreo-ballistic movements heralding COVID-19 induced diabetic ketoacidosis. Diab Metabol Synd 2021; 15(3):913. doi: 10.1016/j.dsx.2021.04.010.
- Kuchay MS, Reddy PK, Gagneja S, Mathew A, Mishra SK. Short term follow-up of patients presenting with acute onset diabetes and diabetic ketoacidosis during an episode of COVID-19. Metab Syndr Clin Res Rev 2020; 14(6):2039-41. doi: 10.1016/j.dsx.2020.10.015.
- Yang JK, Jin JM, Liu S, Bai P, He W, Wu F, et al. Blood glucose is a representative of the clustered indicators of multi-organ injury for predicting mortality of COVID-19 in Wuhan, China. Chin Med Rxiv Print 2020; 2020:1-23. doi.org/10.1101/ 2020.04.08.20058040.
- Alsadhan I, Alruwashid S, Alhamad M, Alajmi S, Alshehri S, Alfadhli E, et al. Diabetic ketoacidosis precipitated by coronavirus disease 2019 infection: Case series. Curr Therap Res 2020; 93(1):100609. doi: 10.1016/j.curtheres.2020.100609.
- Zavaleta MJC, Flórez CDA, Dueñas EAP, Arroyo JCC. Diabetic ketoacidosis during COVID-19 pandemic in a developing country. Diab Res Clin Pract 2020; 168(1):108391. doi: 10. 1016/j.diabres.2020.108391.
- Reddy PK, Kuchay MS, Mehta Y, Mishra SK. Diabetic ketoacidosis precipitated by COVID-19: A report of two cases and review of literature. Diabetes Metab Syndr 2020; 14(5):1459–62. doi: 10.1016/j.dsx.2020.07.050.
- Plasencia-Dueñas EA, Concepción-Zavaleta MJ, Gonzáles-Yovera JG. Pancreatic enzyme elevation patterns in patients with diabetic ketoacidosis: Does severe acute respiratory syndrome coronavirus 2 play a role? Pancreas 2021; 50(2):e19. doi: 10.1097/MPA.0000000000001728.
- Shankar GH, Sharma V, Sarangi B, Walimbe A, Markal KP, Reddy VS. Surge in diabetic ketoacidosis in children with Type 1 diabetes during COVID-19 pandemic-a report from a tertiary care center in Pune, India. J Pediatr Crit Care. 2021; 8(2):110.
- Suwanwongse K, Shabarek N. Newly diagnosed diabetes mellitus, DKA, and COVID-19: Causality or coincidence? J Med Virol 2021; 93(1): 1150-3. doi: 10.1002/jmv.26339.
- Li H, Tian S, Chen T, Cui Z, Shi N, Zhong X, et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes Metab 2020; 22(1): 1897-06. doi: 10.1111/ dom.14099.
- Zhou W, Ye S, Wang W, Li S, Hu Q. Clinical Features of COVID-19 Patients with Diabetes and Secondary Hyperglycemia. J Diabetes Res 2020; 2020(1): 3918723. doi: 10. 1155/2020/3918723.
- Wang Z, Du Z, Zhu F. Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID-19 patients. Diabetes Res Clin Pract 2020; 164(1): 108214. doi: 10.1016/j.diabres.2020.108214.
- Zhang Y, Li H, Zhang J, Cao Y, Zhao X, Yu N, et al. The clinical characteristics and outcomes of diabetes mellitus and secondary hyperglycaemia patients with coronavirus disease 2019: A single‐center, retrospective, observational study in Wuhan. Diabetes Obes Metab 2020; 22(8): 1443-54. doi: 10.1111/dom.14086.
- Wang S, Ma P, Zhang S, Song S, Wang Z, Ma Y, et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: A multi-centre retrospective study. Diabetologia 2020; 63(1): 2102-11. doi: 10.1007/s00 125- 020-05209-1.
- Fadini GP, Morieri ML, Boscari F, Fioretto P, Maran A, Busetto L, et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract 2020; 168(1):108374 doi: 10.1016/j.diabres.2020.108374.
- Lampasona V, Secchi M, Scavini M, Bazzigaluppi E, Brigatti C, Marzinotto I, et al. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: An observational cohort study. Diabetologia 2020; 63(12):2548-58. doi: 10.1007/s00125-020-05284-4.
- Smith SM, Boppana A, Traupman JA, Unson E, Maddock DA, Chao K, et al. Impaired glucose metabolism in patients with diabetes, pre-diabetes, and obesity is associated with severe COVID-19. J Med Virol 2020; 93(1):409-15. doi: 10.1002/jmv.26227.
- Farag AA, Hassanin HM, Soliman HH, Sallam A, Sediq AM, Abd Elbaser ES, et al. Newly diagnosed diabetes in patients with COVID-19: Different types and short-term outcomes. Trop Med Infect Dis 2021; 6(3):142. doi: 10.3390/tropicalmed6030142.
- Nassar M, Nso N, Baraka B, Alfishawy M, Mohamed M, Nyabera A, Sachmechi I. The association between COVID-19 and type 1 diabetes mellitus: A systematic review. Metab. Syndr: Clin. Res. Rev. 2021; 15(1):447-54. doi: 10.1016/j.dsx.2021.02.009.
- Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID19: Preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care 2020;43(8):e83e5. doi: 10.2337/dc20-1088.
- Buowari O. Diabetes mellitus in developing countries and case series. 1st ed. Croatia: InTech; 2013.
- Unsworth R, Wallace S, Oliver NS, et al. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care 2020; 43(11):e170e1. doi: 10.2337/dc20-1551.
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181(2):271-80. doi: 10.1016/j.cell.2020. 02.052.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382(18):1708-20. doi: 10.1056/NEJMoa 2002032.
- Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Obes Metab 2021; 23(3):870-4. doi: 10.1111/dom.14269.
- Kostopoulou E, Eliopoulou MI, Gil AR, Chrysis D. Impact of COVID-19 on new-onset type 1 diabetes mellitus-A one-year prospective study. Eur Rev Med Pharmacol Sci 2021; 25(19):5928-35. doi: 10.26355/eurrev_202110_26869.
- Khunti K, Del Prato S, Mathieu C, Kahn SE, Gabbay RA, Buse JB. COVID-19, hyperglycemia, and new-onset diabetes. Diabetes Care 2021; 44(12):2645-55. doi: 10.2337/dc21- 1318.
- Bode B, Garrett V, Messler J, McFarland R, Crowe J, Booth R, Klonoff DC. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J diabetes Sci Technol 2020; 14(4):813-21. doi: 10.1177/ 1932296820924469.
- Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study. Lancet Diabetes Endocrinol 2020; 8(10):813e22. doi: 10.1016/S2213-8587(20)30272-2.
- O’Malley G, Ebekozien O, Desimone M, Pinnaro CT, Roberts A, Polsky S, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance Study. J Clin Endocrinol Metab 2021; 106(2): e936-42. doi: 10.1210/clinem/dgaa825.
- Atlas G, Rodrigues F, Moshage Y, Welch J, White M, O’Connell MA. Presentation of pediatric type 1 diabetes IN Melbourne, Australia during the initial stages of the COVID-19 pandemic. J Paediatr Child Health 2020; 56(10): 1654e5. doi: 10.1111/jpc.15081.