Predictive Value of HBsAg for Off-treatment Response in HBeAg-negative Chronic Hepatitis B Patients
By Xiaoping Fan1,2, Tao Li1, Feng Liu3, Lixin Zhang1, Baohua Yang1, Lei Wang1Affiliations
doi: 10.29271/jcpsp.2023.01.51ABSTRACT
Objective: To investigate the virological relapse (VR) rate and prognostic value of the HBsAg level at treatment completion for predicting sustained off-treatment response in HBeAg-negative patients after neucleos(t)ide analog (NA) therapy.
Study Design: Prospective observational cohort study.
Place and Duration of Study: The Second Hospital of Shandong University, Ji’nan, China, between December 2001 and January 2020.
Methodology: Eighty-one HBeAg-negative chronic hepatitis B patients who stopped NA treatment were included. Factors associated with the VR were identified using univariate and multivariate Cox regression models.
Results: Of the 81 patients, 42 had sustained off-treatment response with a median follow-up of 60.0 months (interquartile range [IQR] 33.0–111.0 months). Thirty-nine patients relapsed and 32 relapsed within the first year. The cumulative VR rates were 34.6%, 41.0%, 42.5%, 48.1%, and 55.8% at 1, 2, 3, 5, and 10 years off-therapy, respectively. For patients with end-of-treatment (EOT) HBsAg <250 IU/mL, the 10-year cumulative VR rate was 26.0%. Time to HBV DNA negativity (median, 2 months [IQR 1.0–3.0 months]) and age at EOT were also independent predictors of sustained off-treatment response.
Conclusion: Discontinuing long-term NA treatment is a feasible option for HBeAg-negative chronic hepatitis B patients whose HBsAg levels are low, and HBsAg <250 IU/mL may be an acceptable cut-off value. Younger age at EOT and shorter time to HBV DNA negativity are also independent factors associated with sustained off-treatment response.
Key Words: Hepatitis B surface antigen, Nucleos(t)ide analogs, Cessation, Relapse.
INTRODUCTION
Nucleos (t)ide analogs (NAs) were originally developed to block HBV DNA polymerase enzyme reverse transcriptase activity, and they rapidly inhibit the replication of HBV.1-3 Furthermore, NAs minimize liver inflammation, significantly reducing liver carcinogenesis in chronic hepatitis B (CHB) patients.1-3 As NAs cannot directly reduce covalently closed circular DNA (cccDNA) levels, which are the reservoir of HBV proliferation,4,5 virological relapse (VR) is common after NA of-therapy, especially in HBeAg-negative patients.1-3,6 Therefore, the timing of cessation of NAs remains controversial.
cccDNA levels reflect HBV replication activity in liver cells, where low levels of cccDNA in liver cells at end of treatment (EOT) can predict sustained viral inhibition.4 However, as cccDNA in liver cells can only be evaluated via liver biopsy, and there is no standardized detection method, cccDNA level measurement is limited in clinical practice. Although several promising new biomarkers (e.g., HBV RNA and hepatitis B core-related antigen) have been evaluated for the off-treatment response to NAs in CHB patients, controversy remains and the detection methods have not been popularised on a large scale. The specific situation of patients is difficult to evaluate with a single indicator.7-9
The quantitative serum HBsAg level is demonstrably associated with host immune control of HBV and the intrahepatic level of cccDNA.10 Therefore, the definition of functional cure, which is characterised by sustained loss of HBsAg, has been recognised as the ideal treatment endpoint at the current stage.11,12
HBsAg may be expressed from cccDNA and the integrated HBV DNA sequences in the hepatocytes genome; reduction in the level of HBsAg or seroconversion is rare, which is difficult to achieve.13 Studies have shown that lower quantitative serum HBsAg levels at cessation are associated with a lower cumulative VR rate.6,11 A low quantitative serum HBsAg level at EOT seems to be a useful surrogate endpoint for sustained off-treatment response and has been examined in several studies. Nevertheless, the quantitative values are still debatable.6,14
The authors set to evaluate a prospective, observational cohort involving CHB patients with NA of-therapy according to stringent criteria at our medical centre in 2001. With the advantage of long-term follow-up, the present study aimed to investigate the VR and prognostic value of the HBsAg levels at treatment completion for predicting sustained off-treatment response in HBeAg-negative patients after NA therapy.
METHODOLOGY
This prospective observational cohort included CHB patients who were administered NA treatment (lamivudine, adefovir dipivoxil, telbivudine, and entecavir) and stopped NA treatment according to stringent cessation criteria. Patients were recruited from December 2001 to January 2020 from the Second Hospital of Shandong University. This study was approved by the ethical committee of the hospital. All patients signed written informed consent forms. All procedures complied with the tenets of the Declaration of Helsinki.
The indication criteria for NA treatment and cessation criteria have been described in the author’s previous study.15 Briefly, the criteria for administration of NA treatment were: serum HBsAg positive ≥6 months, ALT levels >1.5 times of the ULN (40 IU/L), and HBV DNA loads ≥104copies/mL for HBeAg-negative patients. When NAs were discontinued, patients were prescribed no less than 18 months of additional consolidation treatment after HBV DNA could not be detected and ALT normalisation plus no less than 24 months of NA treatment.15
The present study started when NA treatment was discontinued. Patients were excluded when NA resistance appeared and/or complications associated with liver cirrhosis (including ascites, jaundice, variceal bleeding, hepatic encephalopathy, or portal hypertension) occurred. Pretreatment characteristics were recorded retrospectively. Patients with inadequate HBsAg data at EOT were also excluded.
One hundred and five HBeAg-negative CHB patients discontinued NAs at the medical centre. Twenty-four patients were excluded because of a non-available HBsAg value. Finally, the data of 81 patients were included in the analysis. Forty-two patients maintained sustained off-treatment response, of whom 23 were followed up for no less than 5 years. Thirty-nine patients experienced VR. The authors analysed the off-treatment response in pre-protocol populations.
During the first 4 months after cessation of treatment, all patients were followed up monthly. Subsequently, they were followed up every 3 months for the first year and every 6 months thereafter. Patients were clinically evaluated at each visit, while liver biochemistry, serum HBV DNA quantification, and HBV serologies (mainly HBsAg and anti-HBsAb) were tested routinely. VR was the endpoint of this study, defined as serum HBV DNA loads ≥104 copies/mL after NA discontinuation with an additional visit 2 weeks later for confirmation.
HBsAg quantification was tested with the i2000 Chemical Luminescent Immunoassay (Abbott, Abbott Park, IL, USA) and Abbott reagents (0.05–250 IU/mL). Before 2010, when the HBsAg value was >250 IU/mL, the sample was not diluted, and the value was recorded as >250 IU/mL. And the value ≤250 had the exact quantification. After 2010, a special diluent was used to dilute the sample by 100 to 1000 times when HBsAg was >250 IU/mL, and more precise HBsAg quantifications were obtained. In accordance with the results of previous studies,6 and the diagnostic range of quantitative tests, 100 and 250 IU/mL were set as the cut-off values of HBsAg at EOT.
The Kolmogorov–Smirnov test was used to examine the normality of continuous variables. Continuous data were presented as mean ± standard deviation and median (interquartile range; IQR). Categorical data are expressed as numbers and percentages. The difference in means was tested with the two-sample t-test or Mann–Whitney U test, as appropriate. Categorical data were tested with the chi-squared test or Fisher’s exact test. Cox regression models were used to test factors associated with VR. When the p-value of a variable was lower than 0.1 in the univariate analyses, the variable was entered into the multivariate analyses. A p-value <0.05 was considered statistically significant. All statistical analyses were performed with IBM SPSS software, version 22.0 (IBM Corp., Armonk, NY, USA).
Receiver operating characteristic (ROC) curves were used to describe the diagnostic performance of several markers and combinative indicators. The above analyses were performed using MedCalc software, version 12.7.0.0 (MedCalc Software bvba, Ostend, Belgium).
RESULTS
The average age of patients at cessation of treatment in the total population was 36.8±12.2 years and most participants were male (62 patients, 76.5%). The median total treatment time was 40.0 months (IQR 26.5–61.5 months). The median ALT baseline level was 157.0 U/L (IQR 100.0–273.5 U/L), and the median HBV DNA load was 6.18 log 10 copies/mL (IQR 5.10–7.00 log 10 copies/mL) (Table I). All patients were initially administered NA monotherapy, including 48 (48/81, 59.3%) patients with lamivudine, 22 (22/81, 27.2%) with adefovir dipivoxil, two (2/81, 2.5%) with telbivudine, and nine (9/81, 11.1%) with entecavir. No other NAs were added throughout the treatment.
For patients with sustained off-treatment response, the median follow-up time was 60.0 months (IQR 33.0–111.0 months). Thirty-nine (48.1%) patients relapsed. Twenty-three of the 39 patients (59.0%) relapsed within the first 6 months after cessation, while 32 patients (82.1%) relapsed within 1 year off-therapy. Two patients relapsed more than 5 years after the cessation of therapy (88 and 91 months, respectively).
Table I: Enrolled patients’ clinical characteristics.|
|
Total |
Relapse group (n = 39) |
Non-relapse group (n = 42) |
p-value* |
|
Age at EOT (years) |
36.8±12.2 |
41.5±10.7 |
32.5±11.9 |
0.001 |
|
Male/Female (n, %) |
62 (76.5%) / 19 (23.5%) |
30 (76.9%) / 9 (23.1%) |
32 (76.2%) / 10 (23.8%) |
0.938 |
|
Pretreatment HBV DNA (log 10 copies/mL)# |
6.07±1.16 |
6.18±1.12 |
5.97±1.20 |
0.438 |
|
Total treatment duration (months)# |
40.0 (26.5–61.5) |
36.0 (29.0–51.0) |
49.0 (24.0–67.8) |
0.262 |
|
Pretreatment ALT# |
157 (100–274) |
188 (117–321) |
143 (93–237) |
0.176 |
|
Pretreatment AST# |
85 (56–144) |
97 (60–141) |
71 (54–155) |
0.392 |
|
HBV DNA negativity last time (months)# |
37.0 (24.0–59.5) |
35 (27–48) |
46 (23–67) |
0.371 |
|
Time to HBV DNA negativity (months)# |
2.0 (1.0–3.0) |
2.0 (1.0–3.0) |
2.0 (1.0–3.0) |
0.152 |
|
HBsAg at cessation less than 100 IU/mL (n, %) |
21 (25.9%) |
4 (10.3%) |
17 (40.5%) |
0.002 |
|
HBsAg at cessation less than 250 IU/mL (n, %) |
32 (39.5%) |
8 (20.5%) |
24 (57.1%) |
0.001 |
|
# Median (IQR); * t-test, Mann–Whitney U test or chi-squared test. IQR, interquartile range. HBV DNA negativity last time (months) refers to the time from HBV DNA negativity after antiviral treatment to VR after NA cessation. Time to HBV DNA negativity refers to the time from initiation of antiviral treatment to HBV DNA negativity. |
||||
Table II: Factors associated with VR.
|
|
Univariate |
Multivariate |
||||
|
HR |
95%CI |
p-values |
HR |
95%CI |
p-values |
|
|
Age at EOT |
1.050 |
1.021–1.080 |
0.001 |
1.069 |
1.038–1.100 |
<0.001 |
|
Male/Female |
1.045 |
0.496–2.202 |
0.908 |
|
|
|
|
Pretreatment ALT |
1.000 |
0.998–1.002 |
0.969 |
|
|
|
|
Pretreatment AST |
0.999 |
0.996–1.002 |
0.465 |
|
|
|
|
Total treatment duration |
0.996 |
0.985–1.007 |
0.474 |
|
|
|
|
HBV DNA negativity last time |
0.995 |
0.984–1.007 |
0.410 |
|
|
|
|
Time to HBV DNA negativity |
1.209 |
1.021–1.432 |
0.028 |
1.221 |
1.048–1.423 |
0.010 |
|
HBsAg at EOT (≥250 IU/ml vs. <250 IU/ml) |
2.898 |
1.331–6.312 |
0.007 |
4.016 |
1.821–8.858 |
0.001 |
|
Pretreatment HBV DNA (log 10 copies/ml) |
1.068 |
0.811–1.406 |
0.639 |
|
|
|
The cumulative VR rates were 34.6%, 41.0%, 42.5%, 48.1%, and 55.8% at 1, 2, 3, 5, and 10 years of-therapy, respectively. Compared with the patients who did not relapse, the patients who relapsed were significantly older (32.5±11.9 years vs. 41.5±10.7 years, p=0.001). Moreover, the non-relapse group with sustained off-treatment response had a higher percentage of HBsAg values <100 (40.5% vs. 10.3%, p=0.002) and 250 (57.1% vs. 20.5%, p=0.001) IU/mL at EOT (Table I).
Cox regression analyses were performed to explore factors related to VR. Finally, the quantitative serum HBsAg level at EOT (≥250 IU/ml vs. <250 IU/ml) (hazard ratio (HR) 4.016, p=0.001), age at EOT (HR 1.069, p<0.001), and time to HBV DNA negativity (HR 1.221, p=0.010) were identified as independent predictors for VR (Table II).
Similarly, the quantitative serum HBsAg level at EOT (≥100 IU/mL vs. <100 IU/mL) was also identified as a predictor for cessation of NAs in both the univariate (HR 3.156, p=0.030) and multivariate (HR 4.739, p=0.004) Cox regression analyses.
At EOT, a quantitative serum HBsAg level <250 IU/mL was observed in 32 (39.5%) patients. Twenty-four of the 32 patients had SVR of-therapy, while eight patients relapsed. Of the eight relapsed patients, six (75%) had VR within the first 6 months off-therapy.
At EOT, a quantitative serum HBsAg level <100 IU/mL was observed in 21 (25.9%) patients. Four patients had VR within 6 months after NA discontinuation.
The authors also compared the cumulative VR rates between patients with quantitative serum HBsAg levels <100 and 100–250 IU/mL at EOT. The log-rank test revealed no difference between the groups (p=0.380, Figure 1).
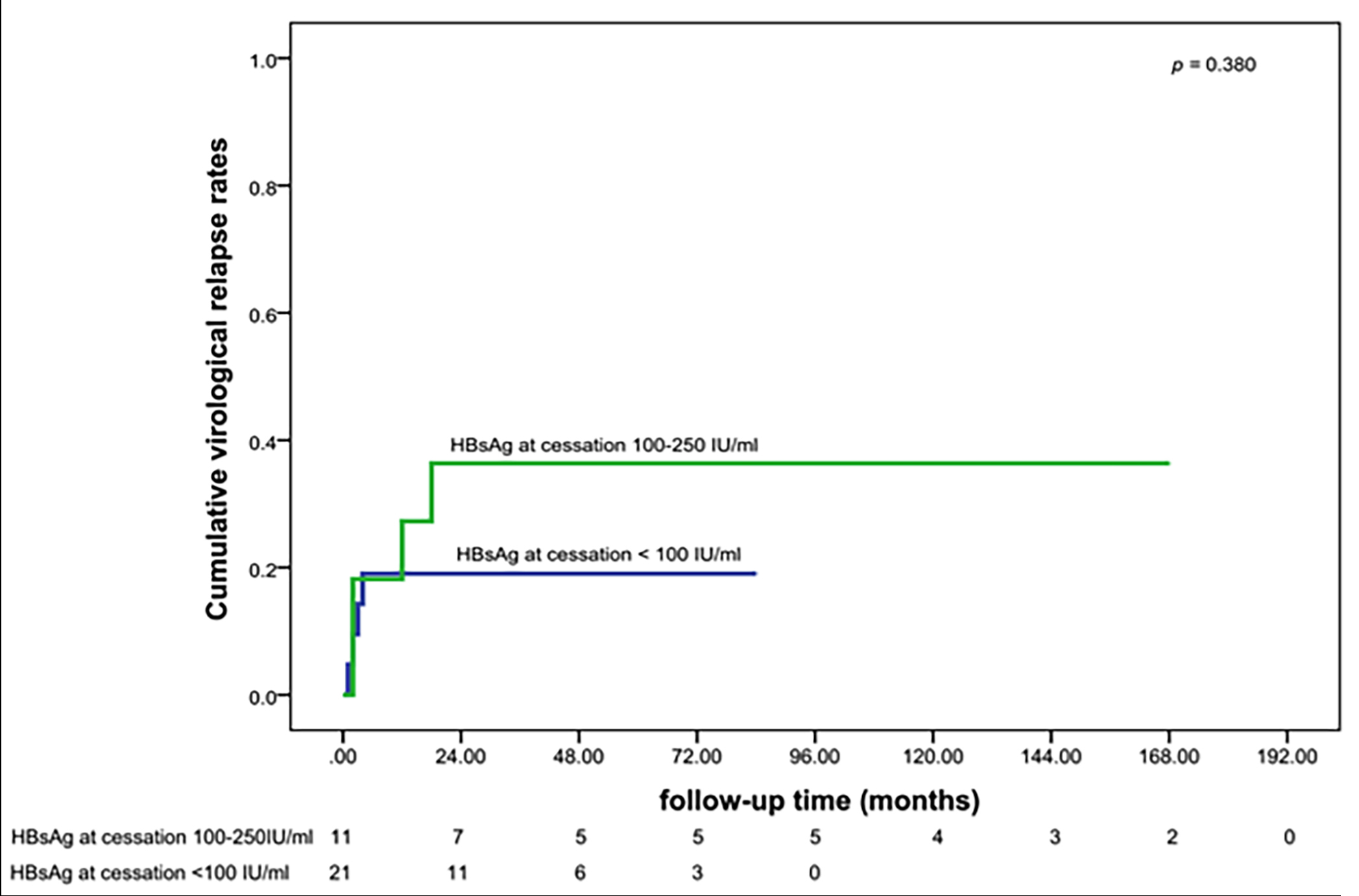 Figure 1: Cumulative VR rates of patients with HBsAg <100 IU/mL at cessation and HBsAg 100–250 IU/mL at cessation.
Figure 1: Cumulative VR rates of patients with HBsAg <100 IU/mL at cessation and HBsAg 100–250 IU/mL at cessation.
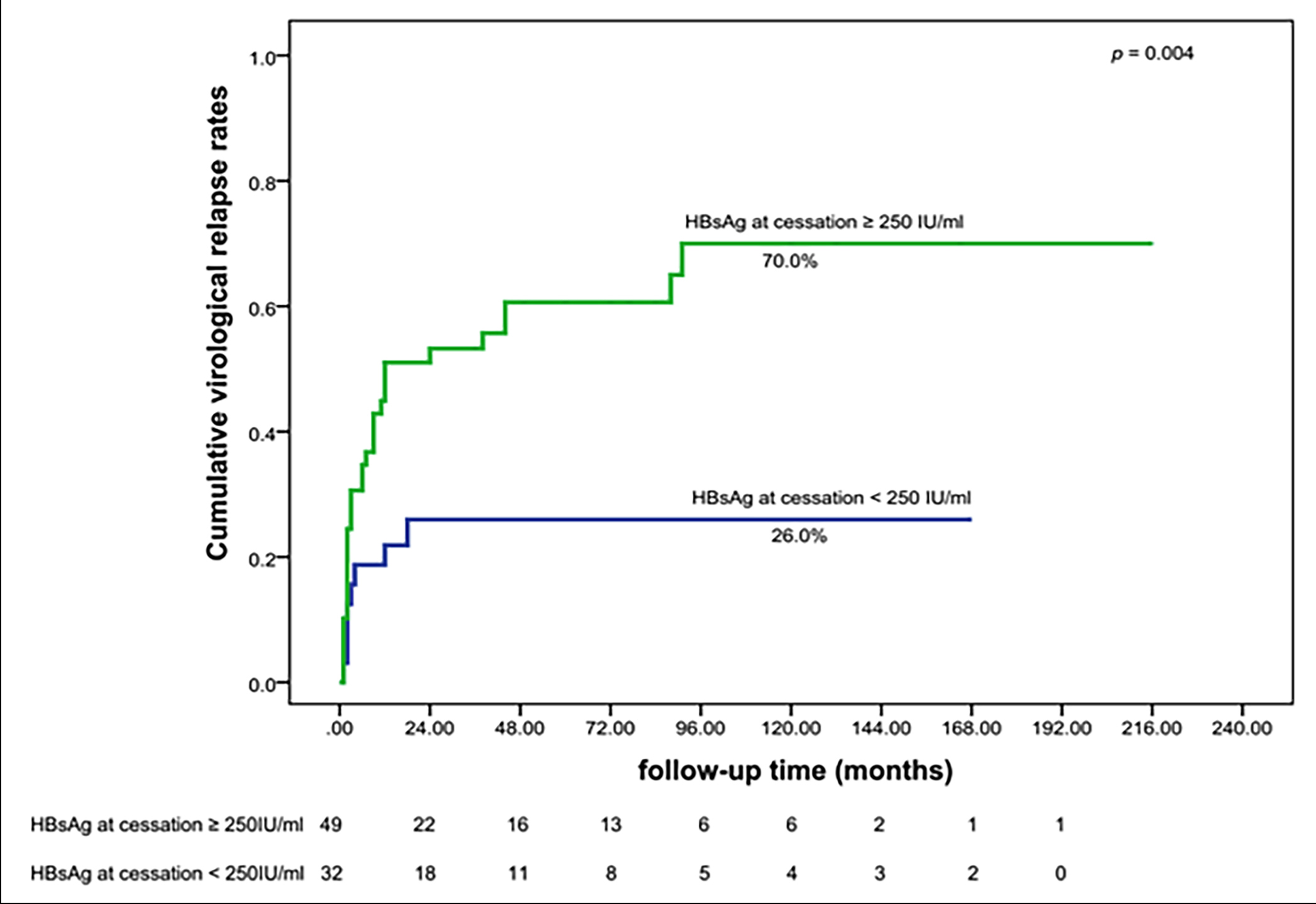 Figure 2: Cumulative VR rate of patients with HBsAg <250 IU/mL at cessation and with HBsAg ≥250 IU/mL at cessation.
Figure 2: Cumulative VR rate of patients with HBsAg <250 IU/mL at cessation and with HBsAg ≥250 IU/mL at cessation.
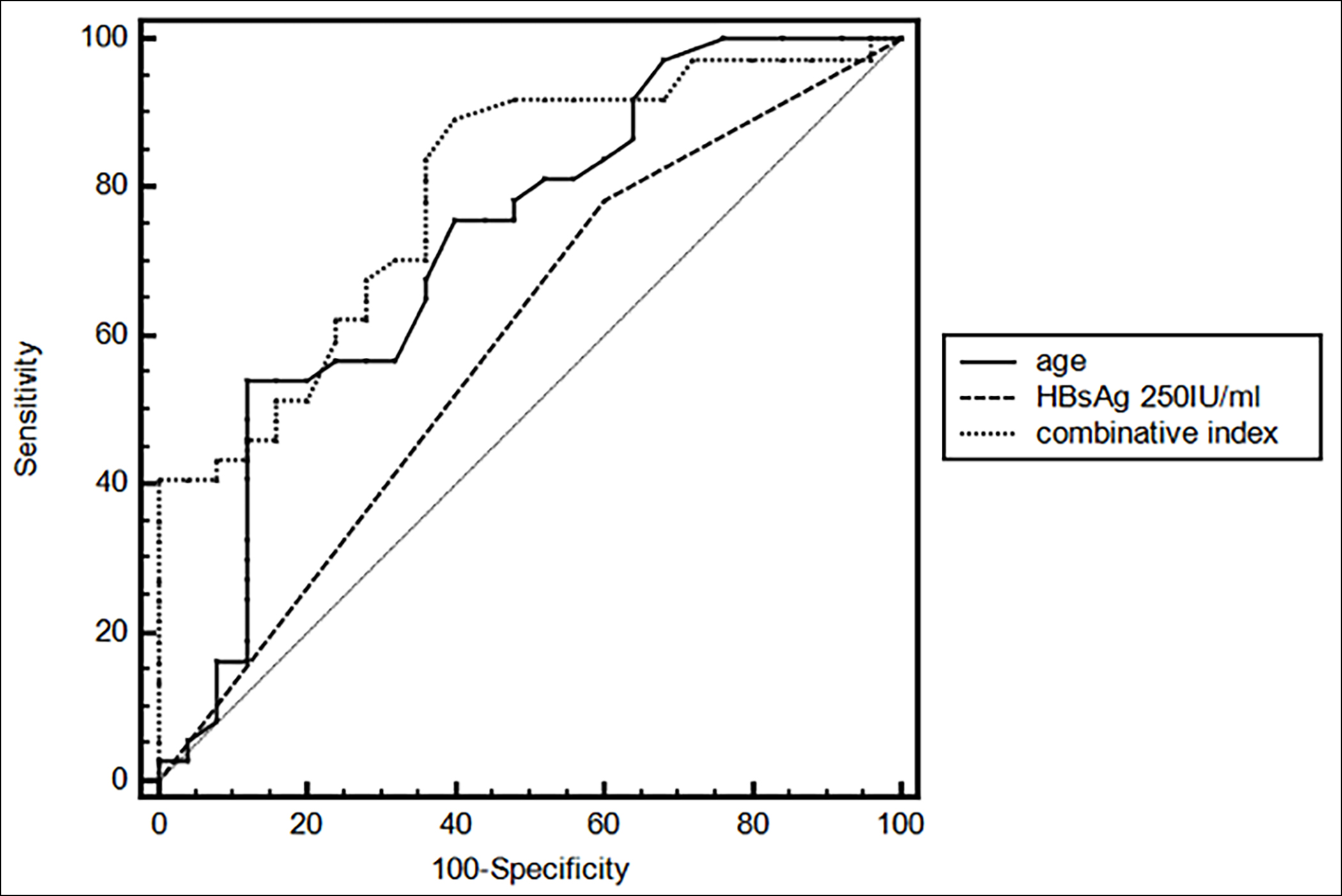 Figure 3: AUROC curves describing the diagnostic performance of HBsAg, age, and combinative index (HBsAg and age) for 5-year VR in HBeAg-negative CHB patients.
Figure 3: AUROC curves describing the diagnostic performance of HBsAg, age, and combinative index (HBsAg and age) for 5-year VR in HBeAg-negative CHB patients.
The cumulative VR rates in patients with quantitative serum HBsAg levels <250 IU/mL at EOT and quantitative serum HBsAg levels ≥250 IU/mL at EOT were also compared. The cumulative VR rates in the groups were 18.7% and 44.9% at 1 year, 26.0% and 51.0% at 2 years, 26.0% and 60.6% at 5 years, and 26.0% and 70.0% at 10 years off-therapy, respectively. The long-rank test revealed significantly lower cumulative VR rates in patients with a serum quantitative HBsAg level <250 IU/mL at EOT (p=0.004) (Figure 2).
Sixty-two patients (39 relapsed patients and 23 patients non-relapsed) were followed up for at least 5 years. When considering the 5-year cumulative VR rate as an observational endpoint, an area under the ROC curve (AUROC) of 0.592 was obtained for an HBsAg value <250 IU/mL at EOT. An AUROC of 0.723 was obtained for age at cessation. For the combinative diagnostic value of HBsAg and age, an AUROC of 0.794 was obtained (Figure 3).
Twenty patients whose quantitative serum HBsAg level at EOT was <250 IU/mL were ≤40 years old and observed 5-year and 10-year cumulative VR rates as low as 15.0%.
DISCUSSION
This study investigated the prognostic value of the quantitative serum HBsAg level at EOT in HBeAg-negative CHB patients with NA therapy discontinuation for predicting the sustained off-treatment response. An HBsAg value <250 IU/mL at EOT independently predicted sustained off-treatment response. Relatively low cumulative VR rates at year 10 (26.0%) were observed in HBeAg-negative CHB patients with HBsAg at EOT <250 IU/mL. Time to HBV DNA negativity and age were also identified as independent predictors.
Patients with a lower HBsAg level at EOT have lower VR rates and higher HBsAg clearance rates;6,16,17 however, the quantitative cut-off values remain debatable. According to a recent meta-analysis, a quantitative serum HBsAg level at EOT <100 IU/mL is a predictable biomarker of sustained off-treatment response,6 and this level was also proven to be a predictable biomarker of sustained off-treatment response in this cohort. However, the present study also confirmed the prognostic value of a quantitative serum HBsAg level at EOT <250 IU/mL for sustained off-treatment response, while the cumulative VR rates between HBeAg-negative CHB patients with HBsAg at EOT <100 IU/mL and 100–250 IU/mL did not differ significantly. Obviously, the latter cut-off is easier to realise in clinical practice. A cumulative VR rate of 26.0% at year 10 revealed acceptable clinical outcomes for HBeAg-negative CHB patients with NA cessation. Moreover, the present study, with the major advantage of a long follow-up period, also considered several potential confounding factors, such as time to HBV DNA negativity and age, in the multivariate Cox regression analyses. Therefore, the above cut-off value is feasible, especially in Asian HBeAg-negative CHB patients.
CHB functional cure, which is characterised by sustained loss of HBsAg, has been recognised as the ideal treatment endpoint and has attracted much focus.11,12 Other studies have shown that discontinuing NA therapy can be followed by HBsAg clearance, and the rates are higher with longer follow-ups.18,19 In the present study, several patients without HBsAg loss also achieved functional cure after NA treatment discontinuation, which was consistent with previous findings.17,18,20
For patients who relapsed after NA cessation, regular follow-up and timely treatment guaranteed relatively acceptable outcomes. The relapse time for patients with HBsAg at EOT <250 IU/mL and ≥250 IU/mL was median 2.5 months [IQR2-10 months] and median 6 months [IQR 2–10 months], respectively. Most patients in this study relapsed within the first year after NA discontinuation, predominantly within 6 months, which indicated the importance of closer follow-up in the initial stage after cessation.
Time to HBV DNA negativity and age at EOT were also identified as independent predictors of VR in the current study. The combination of these indicators and HBsAg at EOT might lead to lower cumulative VR rates. Because of the relatively approximate detection method for HBV DNA (lower limit of quantitation 1 × 103 copies/mL), the current study only evaluated the combinative predictive value of age and HBsAg. A 10-year cumulative VR rate of 15.0% was observed in HBeAg-negative CHB patients with a quantitative serum HBsAg level at EOT <250 IU/mL and an age less than or equal to 40 years at cessation, revealing better outcomes than those previously reported.17,21 The above-mentioned low cumulative VR rate was similar to that reported for patients with HBV RNA undetectability and quantitative serum HBsAg level at EOT HBsAg <10 IU/mL (48 weeks, 9.1%) in a recent cohort.14 The AUROC of 0.794 revealed a moderate diagnostic value of the combinative indicators (age and quantitative serum HBsAg level at EOT <250 IU/mL) for the 5-year cumulative VR rate.
Some limitations of this study should be emphasised. First, the usage of low-genetic barrier NAs was a major limitation, which was owing to early usage and cessation of NAs in the prospective cohort. However, this feature enabled longer-term follow-up. More than 75% of the included patients were followed up for at least 5 years, which reinforces the relevance of the present conclusions. The present cohort also excluded patients with NA resistance, which minimised the interference effect of low-genetic barrier NAs. The application of high-genetic barrier NAs is at least as effective as that reported in current research.22,23 However, the generalisation of the current findings is limited. Second, as this was a long-duration observational study, a considerable proportion of EOT blood samples could not be obtained; therefore, some patients without sufficient data were excluded, which might have resulted in a bias. Third, because the AUROC for the combinative index was only moderate, the authors did not develop a predictive model for off-treatment response.
CONCLUSION
Discontinuing NAs after long-term treatment should be a feasible option for HBeAg-negative CHB patients who have low HBsAg levels, and a quantitative serum HBsAg level <250 IU/mL may be an acceptable cut-off value. Younger age at EOT and shorter time to HBV DNA negativity are also important factors associated with sustained off-treatment response. Regular monitoring, especially within the first year, is necessary after NA discontinuation.
FUNDING:
This study was funded by Shandong Province Natural Science Foundation (No. ZR2019PH052), National Key Research and Development Program of China (No. 2017 YFC0908100, No. 2017YFC0908104).
ETHICAL APPROVAL:
The study was approved by the Ethical Committee of the Second Hospital, Cheeloo College of Medicine, Shandong University (Reference No. KYLL-2015(LW)-0002).
PATIENTS’ CONSENT:
Informed consent was obtained from all patients included in the study.
AVAILABILITY OF DATA AND MATERIALS:
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
COMPETING INTEREST:
The authors declared no competing interests.
AUTHORS' CONTRIBUTION:
WL, YBH: Contributed to the conception and design of the study.
WL, YBH, FXP, LT, LF, ZLX: Contributed to patient inclusion and follow-up.
LT, FXP: Contributed to the interpretation of the data.
LT, LF: Contributed to the statistical analysis of the data.
FXP, LT: Drafted the manuscript.
WL, YBH, LF: Revised the manuscript critically.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018; 67(4):1560-99. doi: 10.1002/ hep.29800.
- European association for the study of the liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017; 67(2):370-98. doi: 10.1016/j. jhep.2017.03.021.
- Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-pacific clinical practice guidelines on the manage-ment of hepatitis B: A 2015 update. Hepatol Int 2016; 10(1):1-98. doi: 10.1007/s12072-015-9675-4.
- Guo JT, Guo H. Metabolism and function of hepatitis B virus cccDNA: Implications for the development of cccDNA-targeting antiviral therapeutics. Antiviral Res 2015; 122: 91-100. doi: 10.1016/j.antiviral.2015.08.005.
- Martinez MG, Villeret F, Testoni B, Zoulim F. Can we cure hepatitis B virus with novel direct-acting antivirals? Liver Int 2020; 40 (Suppl) 1:27-34. doi: 10.1111/liv.14364.
- Liu J, Li T, Zhang L, Xu A. The role of hepatitis B surface antigen in nucleos (t)ide analogues cessation among asian patients with chronic hepatitis B: A systematic review. Hepatology 2019; 70(3):1045-55. doi: 10.1002/hep.30474.
- Papatheodoridi M, Papatheodoridis G. Emerging diagnostic tools to decide when to discontinue nucleos(t)ide Analogues in chronic hepatitis B. Cells 2020; 9(2). doi: 10.3390/cells9020493.
- Wang J, Shen T, Huang X, Kumar GR, Chen X, Zeng Z, et al. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. J Hepatol 2016; 65(4):700-10. doi: 10.1016/j.jhep.2016.05.029.
- Inoue T, Tanaka Y. The Role of Hepatitis B Core-Related Antigen. Genes 2019; 10(5):357. doi: 10.3390/genes 10050357.
- Liu YY, Liang XS. Progression and status of antiviral monitoring in patients with chronic hepatitis B: From HBsAg to HBV RNA. World J Hepatol 2018; 10(9):603-11. doi: 10.4254/ wjh.v10.i9.603.
- Yip TC, Lok AS. How do we determine whether a functional cure for HBV infection has been achieved? Clin Gastroenterol Hepatol 2020; 18(3):548-50. doi: 10. 1016/j.cgh. 2019.08.033.
- Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: From discovery to regulatory approval. Hepatology 2017; 66(4):1296-313. doi: 10.1016/j.jhep.2017.05.008.
- Mak LY, Seto WK, Fung J, Yuen MF. Use of HBsAg quantification in the natural history and treatment of chronic hepatitis B. Hepatol Int 2020; 14(1):35-46. doi: 10.1007/s12072- 019-09998-5.
- Seto WK, Liu KS, Mak LY, Cloherty G, Wong DK, Gersch J, et al. Role of serum HBV RNA and hepatitis B surface antigen levels in identifying Asian patients with chronic hepatitis B suitable for entecavir cessation. Gut 2020; 2020; gutjnl-2020-321116. doi: 10.1136/gutjnl-2020-321116.
- Liu F, Liu ZR, Li T, Liu Y, Zhang M, Xue Y, et al. Varying 10-year off-treatment responses to nucleos(t)ide analogues in patients with chronic hepatitis B according to their pretreatment hepatitis B antigen status. J Dig Dis 2018; 19(9): 561-71. doi: 10.1111/1751-2980.12654.
- Su TH, Yang HC, Tseng TC, Liou JM, Liu CH, Chen CL, et al. Distinct relapse rates and risk predictors after discontinuing tenofovir and entecavir therapy. J Infect Dis 2018; 217(8):1193-201. doi: 10.1093/infdis/jix690.
- Jeng WJ, Sheen IS, Chen YC, Hsu CW, Chien RN, Chu CM, et al. Off-therapy durability of response to entecavir therapy in hepatitis B e antigen-negative chronic hepatitis B patients. Hepatology 2013; 58(6):1888-96. doi: 10.1002/ hep.26549.
- Berg T, Simon KG, Mauss S. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients FINITE study. J Hepatol 2017; 67(5): 918-24. doi: 10.1016/j.jhep.2017.07.012.
- Lachlan Hall SA, Vogrin S, Wawryk O. Discontinuation of nucleot(s) ideanalogue therapy in HBeAg-negative chronic hepatitis B: A meta-analysis. Gut 2022; 71(8):1629-41. doi: 10.1136/gutjnl-2020-323979.
- Papatheodoridis GV, Rigopoulou E, Papatheodoriri M, Zachou K, Xourafas V, Gatselis NK, et al. DARING-B: Discontinuation of effective entecavir (ETV) or tenofovir (TDF) therapy in non-cirrhotic HBeAg-negative chronic hepatitis B (CHBeAg-) patients: final results of a pros-pective greek study. Antivir Ther 2018; 23:677-85. doi: 10.3851/IMP3256.
- Patwardhan VR, Sengupta N, Bonder A, Lau D, Afdhal NH. Treatment cessation in noncirrhotic, e-antigen negative chronic hepatitis B is safe and effective following prolonged anti-viral suppression with nucleosides/nucleotides. Aliment Pharmacol Ther 2014; 40(7):804-10. doi: 10.1111/ apt. 12908.
- Marcellin P, Wong DK, Sievert W, Buggisch P, Petersen J, Flisiak R, et al. Ten-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B virus infection. Liver Int 2019; 39(10):1868-75. doi: 10.1111/ liv. 14155.
- Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016; 1(3):196-206. doi: 10. 1016/S2468-1253(16) 30107-8.