Peroxinitrite and Malondialdehyde as Biomarkers for Overactive Bladder
By Suleyman Sagir1, Omer Bayrak2, Omer Turgut3, Sule Allahverdi4, Hasan Ulusal5Affiliations
doi: 10.29271/jcpsp.2022.12.1586ABSTRACT
Objective: To measure urine malondialdehyde (MDA) and urine peroxynitrite (ONOO) levels in patients with overactive bladder (OAB), and compare them with healthy individuals; to determine the change of those markers in OAB patients prescribed antimuscarinic drugs.
Study Design: An observational study.
Place and Duration of Study: The Department of Urology, School of Medicine, University of Gaziantep, Gaziantep, Turkey, between August 2021 and February 2022.
Methodology: Patients diagnosed with OAB (Group 1), and healthy controls (Group 2) were compared. Urinary MDA (µmol/L) and ONOO (µmol/L) levels were measured in all participants. The patients diagnosed with OAB were underwent antimuscarinic therapy with propiverine 30 mg. The levels of MDA (µmol/L) and ONOO (µmol/L) were reanalysed during the third month of antimuscarinic therapy. Patients with stress urinary incontinence, neurogenic bladder, pelvic organ prolapse stage ≥3 (POP–Q ≥3), interstitial cystitis (bladder pain syndrome), history of pelvic radiotherapy, symptoms of bladder outlet obstruction, Qmax <10 ml/sec for men and <15 ml/sec for women measured by uroflowmetry, and history of pelvic and incontinence surgery were excluded from the study.
Results: There was no difference in the mean age and or gender distribution of the two groups (p=0.166 and p=0.774, respectively). While the mean MDA levels were significantly higher in patients with OAB, (3.34 ± 1.06µmol/L vs. 2.62 ± 1.45µmol/L, p=0.036), no significant change was detected in ONOO levels between the groups (1.03 ± 0.75 µmol/L vs. 0.71 ± 022 µmol/L, p >0.05). Although no significant change was detected in MDA levels after antimuscarinic therapy (3.50 ± 1.19 µmol/L, p=0.529), there was a statistically significant increase in ONOO levels (1.49 ± 1.45 µmol/L, p=0.013).
Conclusion: MDA might be used in the diagnosis of OAB, as a biomarker, similar to recent studies. ONOO was evaluated for the first time in the literature for the diagnosis of OAB, unfortunately, no significant outcomes were obtained. In addition, both MDA and ONOO had no role in monitoring antimuscarinic therapy.
Key Words: Overactive bladder, Peroxynitrite, Malondialdehyde.
INTRODUCTION
There has been a recent increase in experimental research into the biochemical markers found to be elevated in the urine to reveal the etiopathogenesis of overactive bladder (OAB). Recent studies have shown urinary proteins such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) to be increased in patients with OAB.1-5
Several indicators of oxidative deoxyribonucleic acid (DNA) damage are markers also of oxidative stress such as peroxynitrite (ONOO) and malondialdehyde (MDA). In inflamed tissue, reactions of nitric oxide with superoxide lead to the formation of the reactive ONOO molecule, being a well-known oxidising and nitrating agent with high reactivity at physiological pH. The ONOO that forms can trigger cellular responses that lead to cell signalling, oxidative damage, necrosis or apoptosis.6 Studies have reported the possible involvement of ONOO and MDA – a result of lipid peroxidation –for the pathogenesis of several diseases.7-9
Considering the proof promoting the act of oxidative stress in the pathogenesis of lower urinary tract symptoms, the measurement of urinary biomarkers of oxidative stress such as MDA, and ONOO may provide an understanding of their relationship with OAB and enable the development of targeted therapies. The aim of the present study that to measure urine MDA and urine ONOO levels in patients with OAB, and to compare them with healthy individuals. In addition, it was purposed to examine the change of those biomarkers in OAB patients prescribed antimuscarinic drugs.
METHODOLOGY
After the local EC approval (Decision No. 306, dated: 30.06.2021), 40 patients diagnosed with OAB (Group 1) and 19 healthy controls (Group 2) were compared in the Department of Urology, School of Medicine, University of Gaziantep, Turkey, between August 2021 and February 2022. The patients included in the study were informed about the purpose of the study and were asked to complete the informed consent form. Urinary MDA and ONOO levels were measured in all participants, and within the scope of the study, the patients diagnosed with OAB were initiated on antimuscarinic therapy (Propiverine 30 mg). In addition; the levels of MDA and ONOO were reanalysed during the third month of antimuscarinic therapy.
Forty patients (Group 1) over 18 years old (male and female), diagnosed OAB, had episodes of urgent urination, frequent urination, and urge urinary incontinence were included in the study. Patients with stress urinary incontinence, planning pregnancy, neurogenic bladder, and pelvic organ prolapse stage ≥3 upon physical examination [Pelvic Organ Prolapse Quantification System (POP–Q)≥3], and those with a diagnosis of interstitial cystitis (bladder pain syndrome), history of pelvic radiotherapy, symptoms of bladder outlet obstruction, Qmax <10 ml/sec for men and <15 ml/sec for women measured by uroflowmetry, and history of pelvic and incontinence surgery were excluded from the study. Patients with urinary tract infections were included in the study after undergoing treatment, and after no growth was detected in their urine cultures.
The ONOO levels in the urine samples were measured using the method as previously stated in the literature. Accordingly, 10 µL of urine was integrated to 5 mM phenol in 1.990 mL of a 50 mM sodium phosphate buffer (pH 7.4). After incubation for 2 h in a dark environment at 37°C, 15 µL of 0.1 M NaOH was supplemented. The suck of the specimen was then analysed at a wavelength of 412 nm, and the supply of ONOO was evaluated by the molar extinction parameter (ε = 4400/M/cm) of the yield of nitrophenol. The outcomes were stated in µM/L.10,11
Malondialdehyde levels were measured as described by Jain et al. Accordingly, 0.5 mL of 30% TCA was integrated to a 0.2 mL sample, and the tubes were vortexed and kept in ice for 2 h. The tubes were then centrifuged at 2000 rpm for 15 min, after which 1 mL of the supernatant was transferred to another tube to which 0.075 mL of 0.1 M EDTA and 0.025 mL of 1% TBA were supplemented. The tubes were mixed and kept in a boiling water bath for 15 min. Following the cooling of room temperature, the suck was read at 532 nm by spectrophotometry. The required measurements were evaluated by multiplying the absorbance values by the molar extinction parameter (1.56x105cm-1), and the outcomes were stated as nmol/L.12
The normality of the data was analysed with Kolmogorov-Smirnov and Shapiro-Wilk tests. Normally distributed continuous variables were compared between groups using a Student's t-test and not normally distributed continuous variables were compared between groups using a Mann-Whitney U and Wilcoxon tests. Categorical variables were compared between groups using a Chi-square test. Spearman correlation test was used for correlation. Statistical analyses were made using IBM SPSS Statistics for Windows (Version 22.0. Armonk, NY: IBM Corp.) and a p-value <0.05 was considered statistically significant.
RESULTS
There was no difference in the mean age or gender distribution between the OAB (n=40) patients and the control group (n=19, p=0.661, p=0.603, Table I). No significant change was detected in ONOO levels between the OAB patients and the control group (1.03 ± 0.75 µmol/L vs. 0.71 ± 022 µmol/L, p>0.05). The mean MDA level was significantly higher in patients with OAB than in the control group (3.34 ± 1.06µmol/L vs. 2.62 ± 1.45µmol/L, p=0.036, Table I, Figure 1).
While there was a statistically significant increase in ONOO levels after antimuscarinic therapy (1.49 ± 1.45 µmol/L, p=0.013), no significant change was detected in MDA levels after antimuscarinic therapy (3.50 ± 1.19 µmol/L, p=0.529, Table II, Figure 2).
Table I: Demographic data and comparison of peroxynitrite and malondialdehyde levels between groups.
|
|
OAB patients |
Control group |
p |
|
Age (mean ± SD) |
|
|
0.661* |
|
Female |
48.6 ± 17.0 |
42.0 ± 17.3 |
|
|
Male |
40.6 ± 17.0 |
44.3 ± 16.5 |
|
|
Gender (n, %****) |
|
|
0.603** |
|
Female |
26(%65) |
7(%37) |
|
|
Male |
14(%35) |
12(%63) |
|
|
Peroxynitrite (mean ± SD) |
1.03 ± 0.75 |
0.71 ± 0.22 |
0.097* |
|
Malondialdehyde (mean ± SD) |
3.34 ± 1.06 |
2.62 ± 1.45 |
0.036*** |
|
n: number of patients, *Mann-Whitney U test, **Chi-Square test, *** Student’s t-test ****column percentage. OAB: Overactive bladder, SD: Standard deviation. |
|||
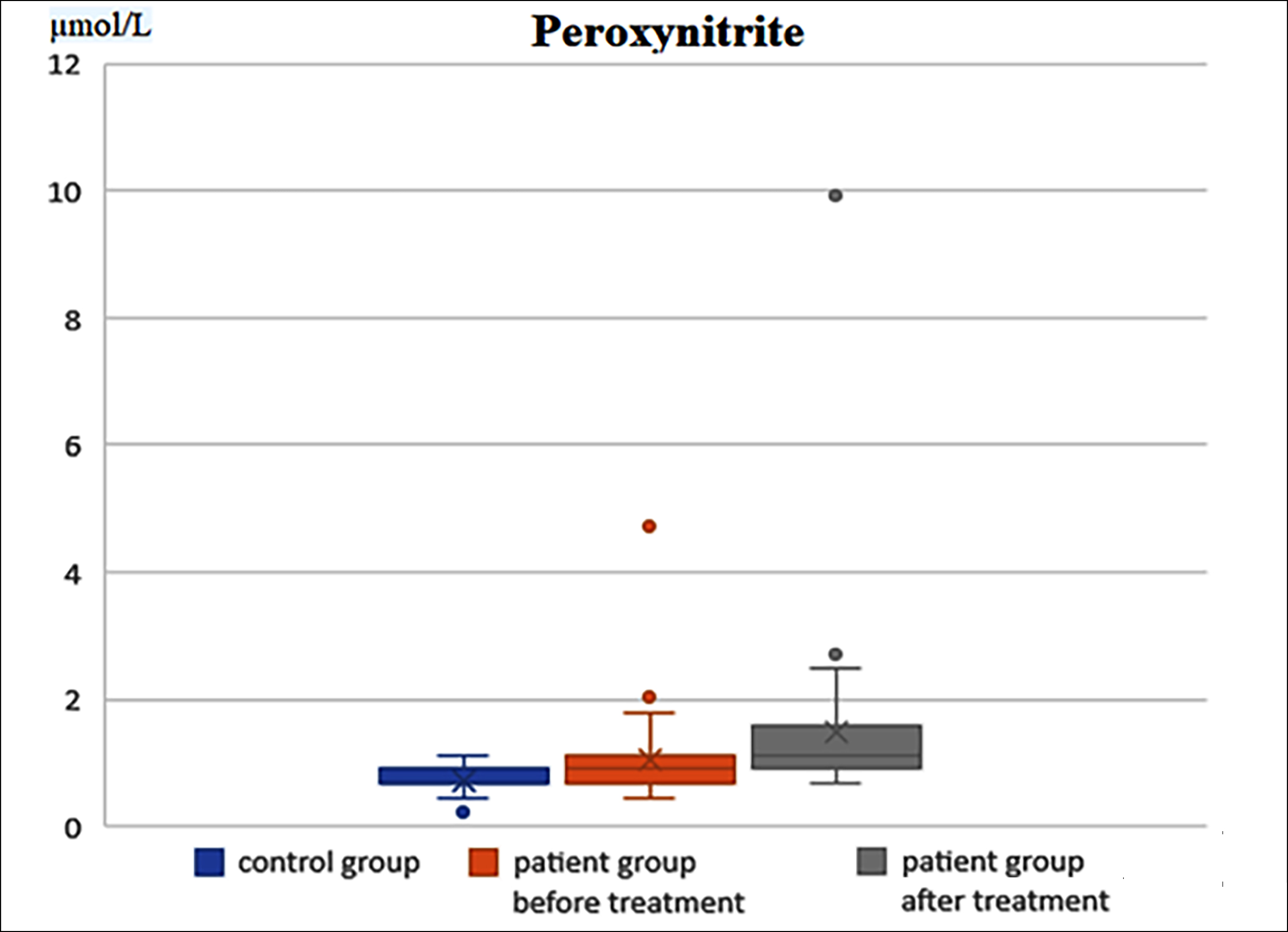 Figure 1: Comparison of peroxynitrite levels between the control group and the OAB patients before and after treatment.
Figure 1: Comparison of peroxynitrite levels between the control group and the OAB patients before and after treatment.
Table II: Comparison of peroxynitrite and malondialdehyde levels before and after antimuscarinic therapy.
|
|
Before treatment (µmol/L) |
After treatment (µmol/L) |
p |
|
Peroxynitrite (mean ± SD) |
1.03 ± 0.75 |
1.49 ± 1.45 |
0.013* |
|
Malondialdehyde (mean ± SD) |
3.34 ± 1.06 |
3.50 ± 1.19 |
0.529** |
|
*Wilcoxon test, **Student’s t-test. |
|||
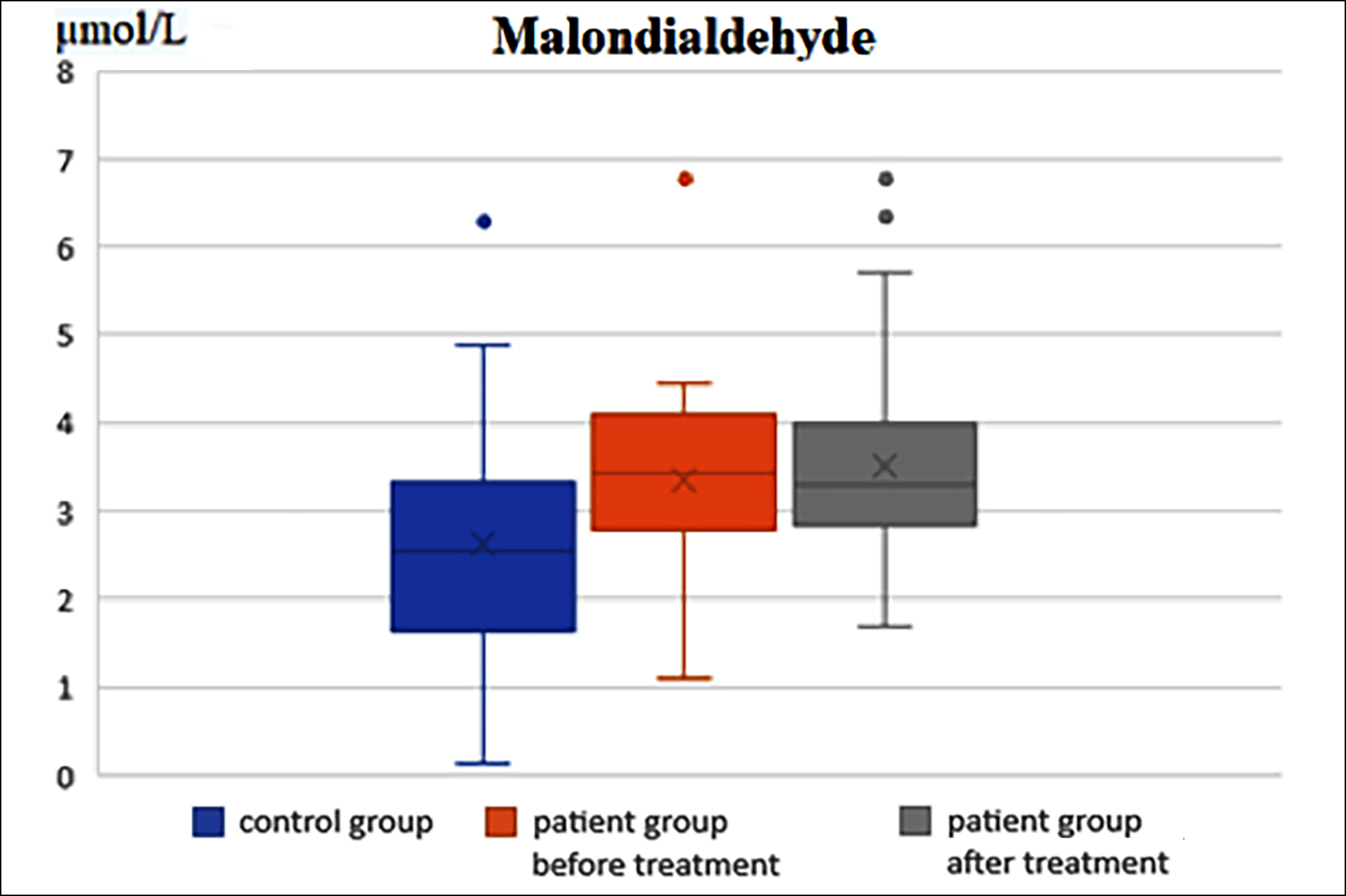 Figure 2: Comparison of malondialdehyde levels between the control group and the OAB patients before and after treatment.
Figure 2: Comparison of malondialdehyde levels between the control group and the OAB patients before and after treatment.
DISCUSSION
The overactive bladder has been defined by the International Continence Society (ICS) as a storage symptom syndrome of the lower urinary tract symptoms. It is characterised by urinary urgency with or without urge incontinence, usually with frequency and nocturia, in the absence of confirmed infection or other pathology.13,14 The overall prevalence of overactive bladder was 27.4% in Pakistan, and it does not differ by gender, hypertension, pelvic surgery, smoking, constipation, and sleep while it has a significant association with age, body mass index, diabetes mellitus, income, parity, and urinary tract infections.15 Biochemical marker studies in literature and current meta-analyses have reinforced the belief that urinary biochemical markers can serve as a diagnostic and predictive test in the future. Oxidative stress-related biomarkers have been assessed in studies conducted to understand the pathophysiology of OAB, although there is still a lack of consensus on the value of these biomarkers.16 It is believed oxidative stress products can provide valuable information on the neurogenic and non-neurogenic phenotypes of OAB and their associations with specific lower urinary tract symptoms.
Topol et al. reported that OAB caused oxidative harm to the bladder, resulting in the increased formation of reactive oxygen species (ROS), and the formation of large quantities of ROS has been reported to have a negative effect on the components of biological systems. After the bladders of 48 rabbits were experimentally subjected to ischemia, the MDA levels in the bladder muscle and mucosa were measured, and the authors reported the MDA levels in the bladder mucosa to be approximately twice as high as in the bladder smooth muscle (1.06 ± 0.2 vs. 0.56 ± 0.11).17 In Dokumacioglu et al.’s analysis of MDA and 8-OHdG levels, which are used as indicators of oxidative stress in humans, higher values of both indicators were reported in OAB patients than the control group, with MDA levels found to be approximately 8 times higher (3.30 ± 1.29 nmol/L vs. 0.46 ± 0.29 nmol/mL) and 8-OHdG 7 times higher in OAB patients (66.03 ± 16.49 nmol/L vs. 9.22 ± 5.75 nmol/L).18 In the current study, similarly the mean MDA level was high in OAB patients with statistical significance. The mean MDA level was 3.34 ± 1.06 µmol/L and 2.62 ± 1.45 µmol/L in the OAB and control groups, respectively (p=0.036).
ONOO, which is a product of the reaction between nitric oxide and superoxide, is an extremely reactive molecule with a very short half-life that causes oxidative tissue damage. The formation of ONOO not only leads to the generation of pro-oxidants but also reduces the bioavailability of NO by changing its physiological effects and strong antioxidant properties. As it has a short biological half-life and reacts with multiple target molecules, the role of ONOO should be investigated alongside its constituent radicals and its decomposition products.19 ONOO causes cell injury by damaging mitochondria, DNA damage through DNA strand breakage, impaired vascular structures, coronary atherosclerosis, myocardial injury in the heart and ischemia-reperfusion injury in many tissues.20-24
The pathophysiology of OAB is multifactorial, and myogenic, neurogenic and urotheliogenic factors may be involved. Preclinical studies report that chronic pelvic ischemia may contribute to the pathogenesis of OAB. According to reports based on experimental animal bladder samples, chronic ischemia caused by arterial damage and high-fat diet constructs oxidative stress indicators and proinflammatory cytokines in the urothelium and lamina propria of the bladder and also guide to a raised deposition of NGF. All of this results in increased stimulation to the bladder and consequent frequent urination, which can cause bladder hyperactivity.25 In light of this information, the authors aimed to reveal the role of ONOO, which has never been demonstrated to be associated with OAB, in the pathogenesis of OAB treatment with antimuscarinics. This study revealed no difference in ONOO levels between the OAB patients and the control group (1.03 ± 0.75 µmol/L vs. 0.71 ± 0.22 µmol/L, p=0.097). However, a significant increase was noted in the ONOO levels of OAB patients after antimuscarinic therapy (p=0.013). According to Andersson et al., studies showing an association between chronic ischemia and OAB would allow for recent treatment choices that target chronic pelvic ischemia.25
Additional studies are needed to investigate the prevention of free radical formation through alpha-1 blockers, phosphodiesterase type-5 inhibitors, and beta-3 agonists with a view to arresting the morphological, biochemical, and functional alterations provoked by chronic bladder ischemia reported in preclinical trials.
The current study has certain limitations. The main limitation is the limited sample size. The present study should be supported by large patient series.
CONCLUSION
MDA might be used in the diagnosis of OAB, as a biomarker, similar to recent data. The present study was a premise study of the use of urinary OONO measurement as a biomarker in the diagnosis of OAB, unfortunately, no significant outcome was obtained. In addition, both MDA and ONOO had no role in monitoring antimuscarinic therapy.
ETHICAL APPROVAL:
Approval for the study was granted by the Clinical Research Ethics Committee of Gaziantep University of Health Sciences (Decision No. 306, dated:30.06.2021), prior to initiation of the research work.
PATIENTS’ CONSENT:
The study was conducted following the Declaration of Helsinki, and patients gave their written consent.
COMPETING INTEREST:
The authors declared no competing interests.
AUTHORS' CONTRIBUTION:
SS, OB, OT: Designed, conceptualised, drafted the work, reviewed and edited the work, substantively revised the manuscript, and supervised the work,
SA, HU: Collected data and revised the figures.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Kim J, Kim WT, Kim W‐J. Advances in urinary biomarker discovery in urological research. Investig Clin Urol 2020; 61:8‐22. doi: 10.4111/icu.2020.61.S1.S8.
- Kuo HC. Potential biomarkers utilised to define and manage overactive bladder syndrome. LUTS 2012; 4:32-41. doi: 10. 1111/j.1757-5672.2011.00131.x.
- Liu HT, Chen CY, Kuo HC. Urinary nerve growth factor levels in overactive bladder syndrome and lower urinary tract disorders. J Formos Med Assoc 2010; 109: 862‐78. doi: 10.1016/S0929-6646(10)60133-7.
- Kashyap MP, Pore SK, de Groat WC, Chermansky CJ, Yoshimura N, Tyagi P. BDNF over expression in the bladder induces neuronal changes to mediate bladder overactivity. Am J Physiol Renal Physiol 2018; 315: 45‐56. doi: 10.1152/ ajprenal.00386.2017.
- Magalhaes TF, Baracat EC, Doumouchtsis SK, Haddad JM. Biomarkers in the diagnosis and symptom assessment of patients with bladder pain syndrome: A systematic review. Int Urogynecol J 2019; 30:1‐10. doi: 10.1007/s00192-019-04075-9.
- Rizwan A, Ahtesham H, Haseeb N. Peroxynitrite: Cellular pathology and implications in autoimmunity. J Immunoassay Immunochem 2019; 40:123-38. doi: 10. 1080/15321819.2019.1583109.
- Taysi S, Cikman O, Kaya A, Demircan B, Gumustekin K, Yilmaz A, et al. Increased oxidant stress and decreased antioxidant status in erythrocytes of rats fed with zinc-deficient diet. Biol Trace Elem Res 2008; 123:161-7. doi: 10.1007/s12011-008-8095-x.
- Taysi S, Gul M, Sari RA, Akcay F, Bakan N. Serum oxidant/antioxidant status of patients with systemic lupus erythematosus. Clin Chem Lab Med 2002; 40:684-8. doi: 10.1515/CCLM.2002.117.
- Taysi S, Kocer I, Memisogullari R, Kiziltunc A. Serum oxidant/antioxidant status in patients with Behcet's disease. Ann Clin Lab 2002; 32:377-82.
- Al-Nimer MS, Al-Ani FS, Ali FS. Role of nitrosative and oxidative stress in neuropathy in patients with type 2 diabetes mellitus. J Neurosci Rural Pract 2012; 3: 41-4. doi: 10.4103/0976-3147.91932.
- Vanuffelen BE, Van Der Zee J, De Koster BM, Vansteveninck J, Elferink JG. Intracellular but not extracellular conversion of nitroxyl anion into nitric oxide leads to stimulation of human neutrophil migration. Biochem J 1998; 330:719-22. doi: 10.1016/s0024-3205(98)00316-6.
- Jain SK, McVie R Duett J, Herbst JJ. Erythrocyte membrane lipid peroxidation and glycocylated hemoglobin in diabetes. Diabetes 1989; 38:1539-43. doi: 10.2337/diab.38.12.1539.
- Sheikh MA, Fawad A, Rabbani KJ, Mazhar SB, Ali S, Yasmin H, et al. Overactive bladder: A multicentre study in Pakistan. J Pak Med Assoc 2022; 72:17-21. doi: 10.47391/ JPMA.20-1463.
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: Report from the standardisation sub-committee of the international continence society. Neurourol Urodyn 2002; 21:167-78. Doi. 10.1002/nau. 10052.
- Rashid S, Babur MN, Khan RR, Khalid MU, Mansha H, Riaz S. Prevalence and associated risk factors among patients with overactive bladder syndrome in Pakistan. Pak J Med Sci 2021; 37:1185-9. doi: 10.12669/pjms.37.4.4262.
- Ralib AM, Pickering JW, Shaw GM. Test characteristics of urinary biomarkers depend on quantitation method in acute kidney injury. J Am Soc Nephrol 2012; 23: 322‐33. doi: 10.1681/ASN.2011040325.
- Topol T, Schuler C, Leggett RE, Hydery T, Benyamin S, Levin RM. Effect of solifenacin plus and minus antioxidant supplements on the response to experimental outlet obstruction and overactive bladder dysfunction in rabbits. Urological Sci 2011; 22:141-6. doi: 10.1016/j.urols.2011. 04.001.
- Dokumacioglu E, Demiray O, Dokumacioglu A, Sahin A, Sen TM, Cankaya S. Measuring urinary 8‐hydroxy‐2′ deoxyguanosine and malondialdehyde levels in women with overactive bladder. Investig Clin Urol 2018; 59:252‐6. doi: 10.4111/icu.2018.59.4.252.
- Tecder-Unal M, Tufan H. The role of peroxynitrite in biologic systems. Turkey Klinikleri J Cardiol 2003; 16:110-8.
- Leist M, Fava E, Montecucco C, Nicotera P. Peroxynitrite and nitric oxide donors induce neuronal apoptosis by eliciting autocrine excitotoxicity. Eur J Neuro Sci 1997; 9:1488-98. doi: 10.1111/j.1460-9568.1997.tb01503.x.
- Zingarelli B, Cuzzocrea S, Zsengeller Z, Salzman A L, Szabo C. Protection against myocardial ischemia and reperfusion injury by of 3-aminobenzamide, an inhibitor of poly (ADP-Ribosyl) synthetase. Cardiovasc Res 1997; 36:205-15. doi: 10.1016/s0008-6363(97)00137-5.
- Moro MA, Darley-Usmar VM, Lizasoain I, Su Y, Knowles RG, Radomski MW, et al. The formation of nitric oxide donors from peroxynitrite. Br J Pharmacol 1995; 116: 1999-2004. doi: 10.1111/j.1476-5381.1995.tb16404.x.
- White C R, Brock T A, Chang L, Crapo J, Briscoe P, Ku D, et al. Superoxide and peroxynitrite in atherosclerosis. Proc Natl Acad Sci Usa 1994; 91:1044-8. doi: 10.1073/pnas. 91.3.1044.
- Tecder-Unal M, Çakıcı Đ, Demiryürek T, Kanzık Đ. The role of peroxynitrite in ischemia-reperfusion arrhythmias in anaesthetized rats. Eur J Pharmaceu Sci 1998; 6: 584. doi.org/10.1016/S0928-0987 (98)91535-4.
- Andersson KE, Nomiya M, Yamaguchi O. Chronic pelvic ischemia: Contribution to the pathogenesis of lower urinary tract symptoms (LUTS): A new target for pharmacological treatment? LUTS 2015; 7:1-8. doi: 10.1111/luts.12084.