Percutaneous Biopsy of Metastatic Bone Lesions Invisible on Conventional Computed Tomography
By Cennet Sahin1, Fevziye Kabukcuoglu2Affiliations
doi: 10.29271/jcpsp.2022.10.1350ABSTRACT
Early detection and histopathological diagnosis of bone lesions with suspected metastasis are crucial for planning treatment of cancer patients. We present computed tomography (CT) findings of three cases with bone metastases that were diagnosed by CT-guided biopsy. All three bone lesions were highly suspicious for metastasis on magnetic resonance (MR) and fluoro-deoxy-glucose-positron-emission-tomography-CT (FDG-PET-CT); although they were not visible on conventional CT. Hence, all the biopsies were performed with reference to MR and FDG-PET-CT imaging findings. As a result of histopathological examinations, all three lesions were diagnosed as metastases of primary cancers. Bone lesions with positive MR and FDG-PET-CT findings in patients with a primary known cancer may be metastasis although they are invisible on conventional CT. These lesions should be biopsied with reference to MR and PET-CT findings for treatments of cancer patients.
Key Words: Bone, Metastasis, Computed tomography, PET scan, Biopsy.
INTRODUCTION
Bone is a common site of metastasis.1,2 Diagnosis of a newly detected bone lesion is crucial in treatment planning.3 Although, computed tomography (CT) has an important role in imaging bone lesions due to its high resolution, magnetic resonance (MR) and fluoro-deoxy-glucose (FDG) positron-emission-tomography (PET) are superior modalities.4-9 For lesions invisible on conventional CT, PET and MR may be served as a guide to obtain a diagnostic percutaneous biopsy. In this study, we aimed to share three cases with bone metastases diagnosed by percutaneous biopsy under CT-guidance with reference to PET-CT and MR findings since they were invisible on conventional CT.
CASE 1:
A 57-year male patient, with a history of left toe malignant melanoma diagnosed 10 years ago, was admitted to the hospital with pelvic pain. Since, abdominopelvic ultrasound (US) and antero-posterior (AP) and lateral pelvic radiography examinations were inconclusive, FDG-PET-CT examination was performed to rule out any involvement of visceral organs and bone marrow by the tumour. The PET-CT revealed a suspicious bone lesion on the right iliac wing.
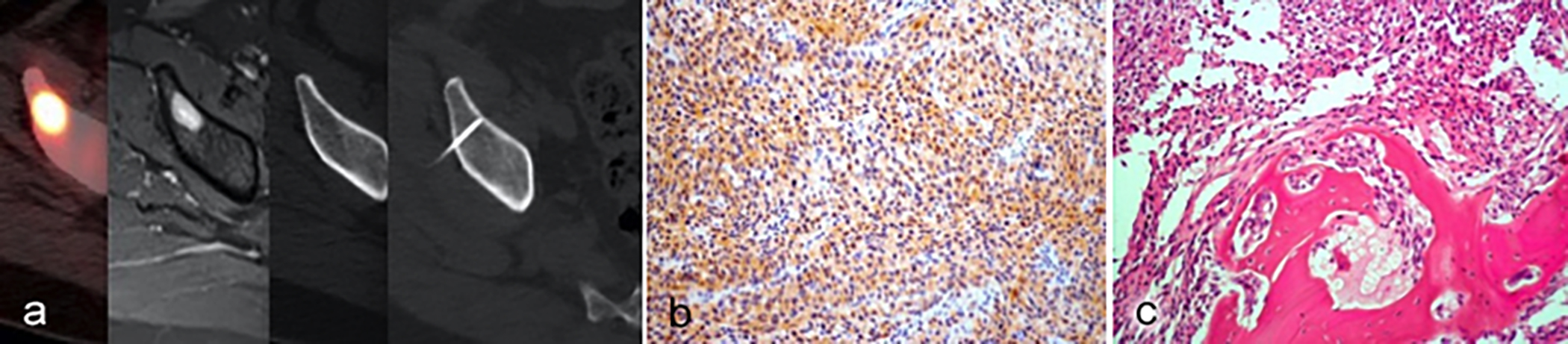 Figure 1a,b,c: (a): The lesion could not be clearly visualised on conventional CT. Thus, the percutaneous biopsy was performed with reference to PET-CT and MR images under CT-guidance. (b). S-100 positivity with immunohistochemical study in malignant melanoma metastasis (S-100 on IHC, ×200). (c). Malignant melanoma metastasis consists of spindle-shaped pleomorphic cells adjacent to bone lamellae (HE×200).
Figure 1a,b,c: (a): The lesion could not be clearly visualised on conventional CT. Thus, the percutaneous biopsy was performed with reference to PET-CT and MR images under CT-guidance. (b). S-100 positivity with immunohistochemical study in malignant melanoma metastasis (S-100 on IHC, ×200). (c). Malignant melanoma metastasis consists of spindle-shaped pleomorphic cells adjacent to bone lamellae (HE×200).
Although the lesion was FDG avid, it was invisible on the conventional CT series of PET-CT. Thus, a contrast-enhanced pelvic MR examination was performed for further evaluation to confirm presence of a lesion in that location. A bone lesion of 20 mm was detected on the right iliac wing that had a bright enhancement on contrast-enhanced T1-weighted MR study (Figure 1). Considering the primary malignant melanoma history of the patient in the background, the lesion was found highly suspicious for metastasis according to PET-CT and MR findings. The patient was referred to our interventional radiology outpatient clinic for a percutaneous bone biopsy with the presumptive diagnosis of metastasis.
In interventional radiology clinic, the patient was informed about the biopsy procedure and biopsy related-complications. Written informed consent was obtained. The biopsy was performed by a radiologist with 6 years of experience in interventional radiology. The patient was positioned on the left lateral decubitus position in CT (Toshiba, Alexion, Japan and Siemens, Somatom, Emotion, Germany) table. The most suspicious part on MR and the most FDG avid portion on PET-CT were accessed to obtain an adequate sample of tissue for diagnosis. The lesion could not be clearly visualised on conventional CT. Thus, the percutaneous biopsy was performed with reference to PET-CT and MR images under CT-guidance. The biopsy was performed under local anaesthesia and aseptic conditions with an 11 G 10 cm bone biopsy needle. The lesion was diagnosed as metastasis of malignant melanoma as a result of histopathological examination (Figure 1).
CASE 2:
A 37-year female patient, with a history of left breast conservative surgery for Luminal A breast cancer two years ago, was admitted to our hospital with pelvic pain. The patient underwent FDG-PET-CT scan since she had cancer history. A bone lesion, 4 mm in size, was detected on a PET-CT scan which had high FDG uptake on the right anterior superior iliac spine. Although, the lesion was FDG avid, it was invisible in the conventional CT series of PET-CT. Thus, the patient had contrast-enhanced MR for further evaluation to confirm the presence of a lesion in that location. The lesion that had contrast enhancement on T1-weighted images was obvious on MRI (Figure 2). Although, the lesion was invisible on the conventional CT series of PET-CT, considering the primary breast cancer history of the patient in the background, it was found highly suspicious for metastasis according to PET-CT and MR imaging findings. The patient was referred to an interventional radiology outpatient clinic for percutaneous bone biopsy with the presumptive diagnosis of metastasis.
A CT-guided biopsy was performed by the same interventional radiologist with the same materials and methods.
The most suspicious part on MR and the most FDG-avid portion on PET-CT was accessed to obtain sufficient tissue sample for diagnosis under CT-guidance. The lesion was diagnosed as metastasis of breast carcinoma on histopathological examination (Figure 2).
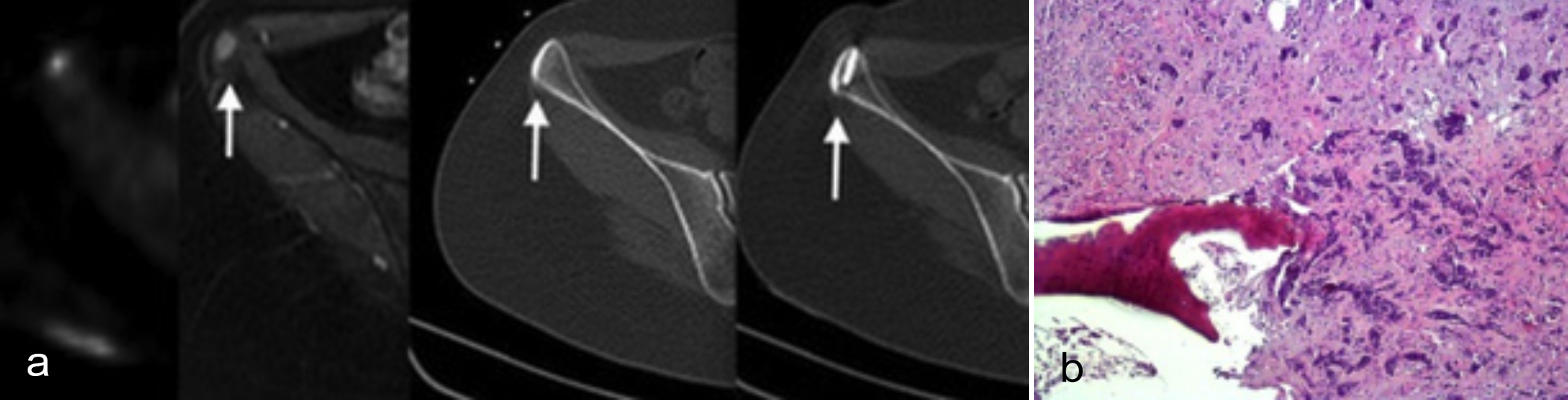 Figure 2 (a, b): (a) Since the lesion could not be clearly visualised on conventional CT image, percutaneous biopsy was performed with reference to MR image under CT-guidance. (b) The lesion was diagnosed as metastasis of primary breast cancer on histopathological examination. Breast carcinoma metastasis is composed of epithelial cell groups adjacent to bone lamellae (HE ×200).
Figure 2 (a, b): (a) Since the lesion could not be clearly visualised on conventional CT image, percutaneous biopsy was performed with reference to MR image under CT-guidance. (b) The lesion was diagnosed as metastasis of primary breast cancer on histopathological examination. Breast carcinoma metastasis is composed of epithelial cell groups adjacent to bone lamellae (HE ×200).
CASE 3:
A 46-year female, with a history of breast cancer diagnosed 2 years ago, presented with a bone mass on D10th vertebra, incidentally detected on a routine follow-up FDG-PET-CT scan. The patient did not have any pain or complaint related to this bone lesion. Although, the lesion was invisible on conventional CT images, it was found suspicious for metastasis according to PET-CT findings (Figure 3). Since the treatment procedure would be changed depending on this bone lesion, she was referred for the percutaneous biopsy to rule out probable metastasis. The patient had the same pre-biopsy preparation procedures as for cases 1 and 2. A CT-guided biopsy was performed by the same interventional radiologist.
The most FDG-avid portion on PET-CT was approached to obtain an adequate tissue sample for diagnosis. The lesion could not be clearly visualised on conventional CT image and the percutaneous biopsy was performed with reference to PET-CT image under CT-guidance (Figure 3). The lesion was diagnosed as metastasis of primary breast cancer on histopathology.
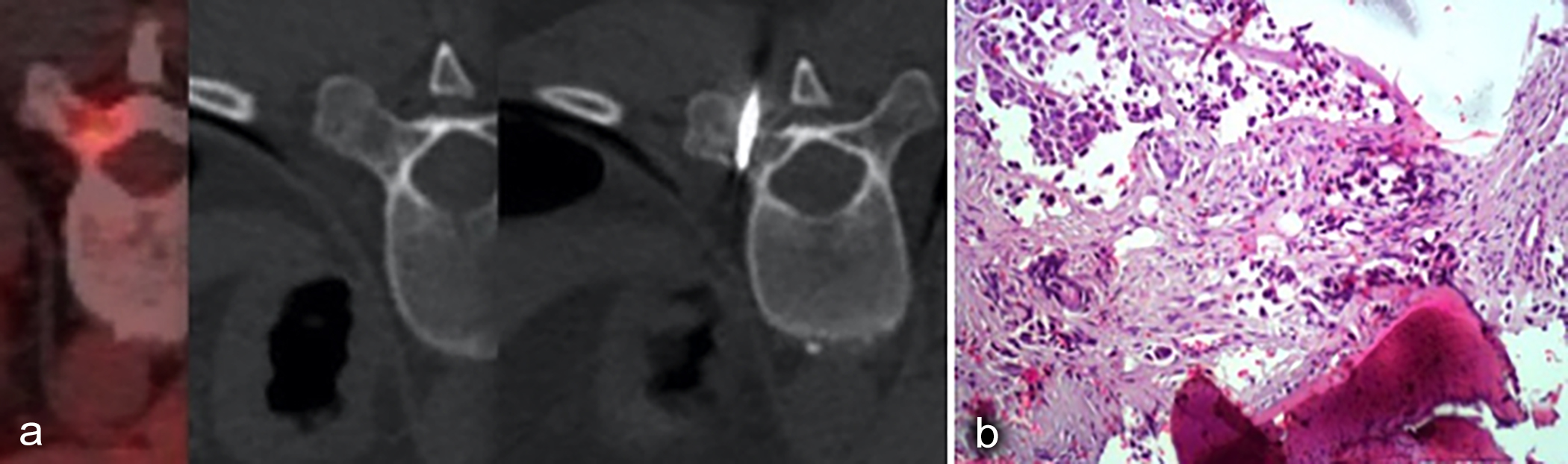 Figure 3 (a, b): (a) Since the lesion could not be clearly visualised on conventional CT image, the percutaneous biopsy was performed with reference to PET-CT image under CT guidance. (b) The lesion was diagnosed as metastasis of primary breast cancer on histopathology. Breast carcinoma metastasis composed of epithelial cell groups adjacent to bone lamellae can be seen (HE ×200).
Figure 3 (a, b): (a) Since the lesion could not be clearly visualised on conventional CT image, the percutaneous biopsy was performed with reference to PET-CT image under CT guidance. (b) The lesion was diagnosed as metastasis of primary breast cancer on histopathology. Breast carcinoma metastasis composed of epithelial cell groups adjacent to bone lamellae can be seen (HE ×200).
DISCUSSION
Bone is one of the common sites of metastasis and bone metastasis may be encountered in almost all cancers.1,2 Almost 20% of newly detected bone lesions in patients with known cancer may not be associated with their primary cancers and almost 3.4% of these may be new malignant bone tumours rather than metastases.3 Thus, an accurate diagnosis of a newly detected bone lesion, especially when it is solitary and there is a background history of cancer, is crucial for further treatment.3
Image-guided percutaneous bone biopsy, as an efficient, safe, and cheap method, plays an important role in the diagnosis of a newly detected bone lesion and planning an accurate treatment method. It provides a minimally invasive and a direct approach to the target lesion, accurate positioning of the needle into the most suspicious area of the lesion and a high diagnostic value of sampling compared to open surgical biopsies that may require wide excisions and bone grafting.10
Despite its high specificity, CT has limited diagnostic capability in imaging of early deposits of bone metastasis.4 At least 25% of bone metastases may be invisible on CT, although they are visible FDG-avid in PET-CT.11 In the early phases of bone metastasis, if the bone cortex is intact and the lesion is small, it may be invisible on conventional CT, although the lesion is avid on FDG PET-CT and obviously visible on MR. FDG PET-CT is widely used in the staging and restaging of cancer patients due to its high ability to detect early deposits of cancer cells. Although, FDG PET-CT is very sensitive (>90%) in imaging of bone metastases, its specificity is not very high. MR has been shown to be the most sensitive method with almost 100% sensitivity and 97% specificity in imaging of bone lesions.6 Both MR and PET-CT are superior to CT to detect the early infiltration bone marrow by metastatic cancer.5-9 In this study, although the solitary new bone lesions were visible in all three cases on FDG PET-CT images and in 2 cases were visible on MR, these lesions could not be visualised on conventional CT (Figures 1-3). Thus, all three biopsies were performed according to MR and PET-CT coordinates under CT-guidance in these patients and all had a positive diagnostic histopathological results.
In conclusion, image-guided biopsy is a useful, reliable, minimally invasive, effective and cheap method for the diagnosis of metastatic bone lesions with low complication and high diagnostic yield rates. Bone lesions with positive MR and FDG PET-CT findings in patients with a primary known cancer may represent metastases although the lesions may be invisible on conventional CT images. These lesions should be biopsied with reference to MR and PET-CT findings so as not to delay the treatment.
PATIENTS’ CONSENT:
Written informed consent were obtained from all patients to perform the biopsies and to share the data.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
CS: Performing the biopsies, conception and design, acquisition and interpretation of the data, and writing the cases.
FK: Performing the histopathological analysis, drafting, revision, and proofreading.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- D'Oronzo S, Coleman R, Brown J, Silvestris F. Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J Bone Oncol 2018; 15:004-4. doi: 10.1016/j.jbo.2018.10.004.
- Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L. Bone metastases: An overview. Oncol Rev 2017; 11 (321):43-49. doi: 10.4081/oncol.2017.321.
- Zhang L, Wang Y, Gu Y, Hou Y, Chen Z. The need for bone biopsies in the diagnosis of new bone lesions in patients with a known primary malignancy: A comparative review of 117 biopsy cases. J Bone Oncol 2018; 14:100213. doi:10.1016/j.jbo.2018.100213.
- O’sullivan GJ, Carty FL, Cronin CG. Imaging of bone metastasis: An update. World J Radiol 2015; 7(8):202-11. doi: 10.4329/wjr.v7.i8.202.
- Wu LM, Gu HY, Zheng J, Xu X, Deng X, Zhang W, et al. Diagnostic value of whole-body magnetic resonance imaging for bone metastases: A systematic review and meta-analysis. J Magn Reson Imaging 2011; 34(1): 128-35. doi: 10.1002/jmri.22608.
- Duo J, Han X, Zhang L, Wang G, Ma Y, Yang Y. Comparison of FDG PET/CT and gadolinium-enhanced MRI for the detection of bone metastases in patients with cancer: A meta-analysis. Clin Nucl Med 2013; 38(5): 343-8. doi: 10.1097/RLU.0b013e3182817af3.
- Jeong D, Bui M, Peterson D, Montilla-Soler J, Gage KL. FDG avid breast cancer bone metastases silent on CT and scintigraphy: A case report with radiologic-pathologic correlation. Acta Radiol Open 2017; 6(10):205846 0117734243. doi:10.1177/2058460117 734243.
- Yang HL, Liu T, Wang XM, Xu Y, Deng SM. Diagnosis of bone metastases: A meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol 2011; 21 (12):2604-17. doi: 10.1007/s00330-011-2221-4.
- Piccardo A, Altrinetti V, Bacigalupo L, Puntoni M, Biscaldi E, Gozza A, et al. Detection of metastatic bone lesions in breast cancer patients: Fused 18F-Fluoride-PET/MDCT has higher accuracy than MDCT. Preliminary experience. Eur J Radiol 2012; 81(10):2632-8. doi: 10.1016/j.ejrad.2011. 12.020.
- Taylor MB, Bromham NR, Arnold SE. Carcinoma of unknown primary: Key radiological issues from the recent National Institute for Health and Clinical Excellence guidelines. Br J Radiol 2012; 85(1014):661-71. doi:10. 1259/bjr/75018360.
- Ahmed F, Muzaffar R, Fernandes H, Tu Y, Albalooshi B, Osman MM. Skeletal metastasis as detected by 18F-FDG PET with negative CT of the PET/CT: Frequency and ımpact on cancer staging and/or management. Front Oncol 2016; 6:208. doi:10.3389/fonc.2016.00208.