Patellofemoral Anatomical Measurements on MRI in Patient with Suprapatellar Fat Pad Edema and Mass Effect
By Suna Sahin Ediz1, Senem Senturk2, Adnan Kabaalioglu3Affiliations
doi: 10.29271/jcpsp.2022.12.1600ABSTRACT
Objective: To evaluate the impact of patellofemoral joint anatomical measurements of patients with anterior knee pain who were diagnosed with suprapatellar fat pad (SPFP) impingement syndrome (SP-FPIS) by magnetic resonance imaging (MRI).
Study Design: A prospective, descriptive study.
Place and Duration of Study: Goztepe Education and Research Hospital, between March 2015 and June 2019.
Methodology: The study included 34 patients (Group 1) and 34 healthy volunteers (Group 2) who were referred to the radiology clinic with anterior knee pain; they underwent MRI and diagnosed with SP-FPIS. Twenty-three anatomical measurements such as SPFP cranio caudal length (CC), anteroposterior length (AP), oblique length (OBL), patellar length (PL), patellar tendon length (PTL), Insall Salvati Index (ISI), patellar cartilage distal-tibial tubercle (TT), patellar cartilage length (PCL), Modified Insall Salvati Index (MISI), patellotrochlear cartilage length (TCL), lateral trochlear inclination angle (LTI), etc. related to the morphological structure of SPFP and patellofemoral joint were evaluated and compared in both groups.
Results: The mean age of groups 1 and 2 was 45.62±10.87 and 41.47±11.98 years, respectively. There was a statistically significant difference in patients with SP-FPIS in PL, PT, TT, PC, MISI, TC, PTI, MF, PHY, PPI, MT, LTI, CC, AP, and OBL measurements compared with the control group (p<0.05). In the logistic regression analysis performed to evaluate the effect of statistically significant parameters on anterior knee pain, the probability of SP-FPIS increases 1.5 times as PTL increases among the groups, while the risk of SP-FPIS decreases 0.78 times as LTI decreases (p<0.05).
Conclusion: There is a predisposing effect of PTL increase in SP-FPIS development, while LTI decrease has a protective effect. These results will guide future studies for the development and/or modification of treatment methods.
Key Words: Patellofemoral joint, Suprapatellar fat pad impingement syndrome, Anterior knee pain, Knee magnetic resonance imaging, Knee anatomy.
INTRODUCTION
The knee joint consists mainly of two parts (patellofemoral (PFJ) and tibiofemoral joint), and PFJ has a multifunctional complex structure in which the alignment of the patella and the trochlear groove plays an extremely effective role.1
Other components of the knee joint, the importance of which has become evident in recent years, are fat pads. As the joint moves, fat pads act as flexible and elastic protective pillows
that adapt to the changing shape and volume. They support the increase of synovial surface and thus the lubricant property of the joint surface is distributed over the entire surface.2
The suprapatellar fat pad (quadriceps) (SPFP) is located between the quadriceps tendon (anterior-superior), the suprapatellar recess (posterior) and the upper pole (inferior) of the patella SPFP’s thickness is 6 mm (4–8 mm) in women and 7 mm (5–9 mm) in men and it joins with trochlea, increasing the adaptation of the extensor mechanism during knee flexion.3 SPFP has a rich vascularisation and innervation; therefore, they are considered extremely sensitive structures. The pathology that occurs as a result of inflammation in SPFP is defined as SP-FPIS.3 The exact cause of SP-FPIS is not known.4,5 Predictive factors for SP-FPIS development include direct trauma or excessive use of the knee; anatomy of the extensor mechanism or possible abnormal mechanical structure; high knee flexion angles; adjacent knee abnormalities such as chondromalacia, tendon abnormalities, or synovitis.6
The aim of the present study was to evaluate the structures of the patellofemoral joint by anatomical measurements on MRI to understand more clearly SP-FPIS aetiology and find out possible predisposing anatomical factors.
METHODOLOGY
Patients who underwent MRI for anterior knee pain in the Radiology Clinic, between March 2015 and June 2019 at Goztepe Education and Research Hospital, were prospectively evaluated and included in the study. Thirty-four patients with SP-FPIS and no additional knee pathologies detected on MRI were included as the patient group, and 34 volunteers without active complaints and have normal knee according to orthopaedic and radiological examination on MRI were included as the control group. Patients under 18 years of age, and patients with a history of surgery, trauma, and additional knee pathologies on MRI were excluded from the study.
Examinations were conducted with GE Signa Excite HD 1.5 Tesla MRG device in a supine position, with the knee in full extension and neutral rotation. The standard knee MRI protocol at the department includes the following sequences: sagittal fat saturated PDW Fast Spin Echo (FSE) (EN/TE, 588/14; echo-train length, 1; slice thickness, 3 mm; interslice gap, 5 mm; matrix, 320 × 256; FOV 16 cm), axial and coronal T2-weighted FSE (EN/TE: 2000-4000/65 ms, Nex = 2, FOV 16 cm, 3 mm-thickness, 0.5 spacing, 20 slices) and sagittal fat saturated Proton Density Weighted (PDW) images (TR/TE: 2360/45 ms, Nex = 2, FOV 16cm, 3 mm-thickness, 0.5 spacing, 24 slices). The sagittal T1-weighted (T1WI) FSE, axial T2-weighted (T2WI) FSE MR images were used in the present study for quantitative anatomical measurements of the patellofemoral joint. Knee MRI examinations were evaluated by one radiologist with 15 years of experience. Oedema and mass effect in SPFP were examined in all patients, and SP-FPIS diagnosis was made in the presence of both. Mass effect was defined as the structurally increased convexity on the posterior joint surface of the suprapatellar fat pad, and oedema was defined as an abnormally high signal on fat-saturated PDW images for SPFP and a low signal on T1-weighted images. On fat-saturated PDW images, the signal intensity of SPFP was evaluated as a large increase in muscle or fluid signal and was considered to be in favour of oedema. Twenty-three anatomical measurements are obtained to assess the effect of knee anatomy in the formation of SP-FPIS and summarised in Table I.
SPSS and Microsoft Excel computer programs were used in the analysis of the data obtained in the study. Kolmogorov–Smirnov test was used to evaluate the suitability of data to normal distribution. Descriptive statistical methods were used to represent data. Categorical variables are expressed as counts and percentages and continuous variables as mean and standard devaitons. Student t-test was used to compare differences between two groups for normally distributed quantitative variables. Chi-square test was used to compare qualitative data. Logistic regression analysis was applied to assess the impact of existing parameters. Results were evaluated within 95% confidence range at p<0.05 significance level.
RESULTS
General characteristics and statistical analysis of both groups based on patellofemoral measurements are summarised in Table II. The mean PL, PTL, ISI, TT, PCL, MISI, TCL, PTI, MFL, LFL, PFA, PA, PHY, PHI, TD, MTL, LTL, TFA, SA, LTI values and statistical analysis of both groups based on patellofemoral measurements is summarised in Table III.
The difference in PL, PTL, TT, PCL, MISI, TCL, PTI, MFL, PHY, PPI, MTL AND LTI values between groups was statistically significant (p<0.05). However, there was no statistically significant difference in terms of ISI, LFL, PFA, PA, TD, LTL, TFA and SA values (p>0.05, Table III).
In logistic regression analysis conducted to assess the effect of statistically significant parameters on anterior knee pain, the probability of SP-FPIS increased by 1.5 times as PTL increased between the groups (p = 0.036), and the risk of SP-FPIS formation decreased by 0.78 times as LTI decreased (p= 0.001, Figure 1).
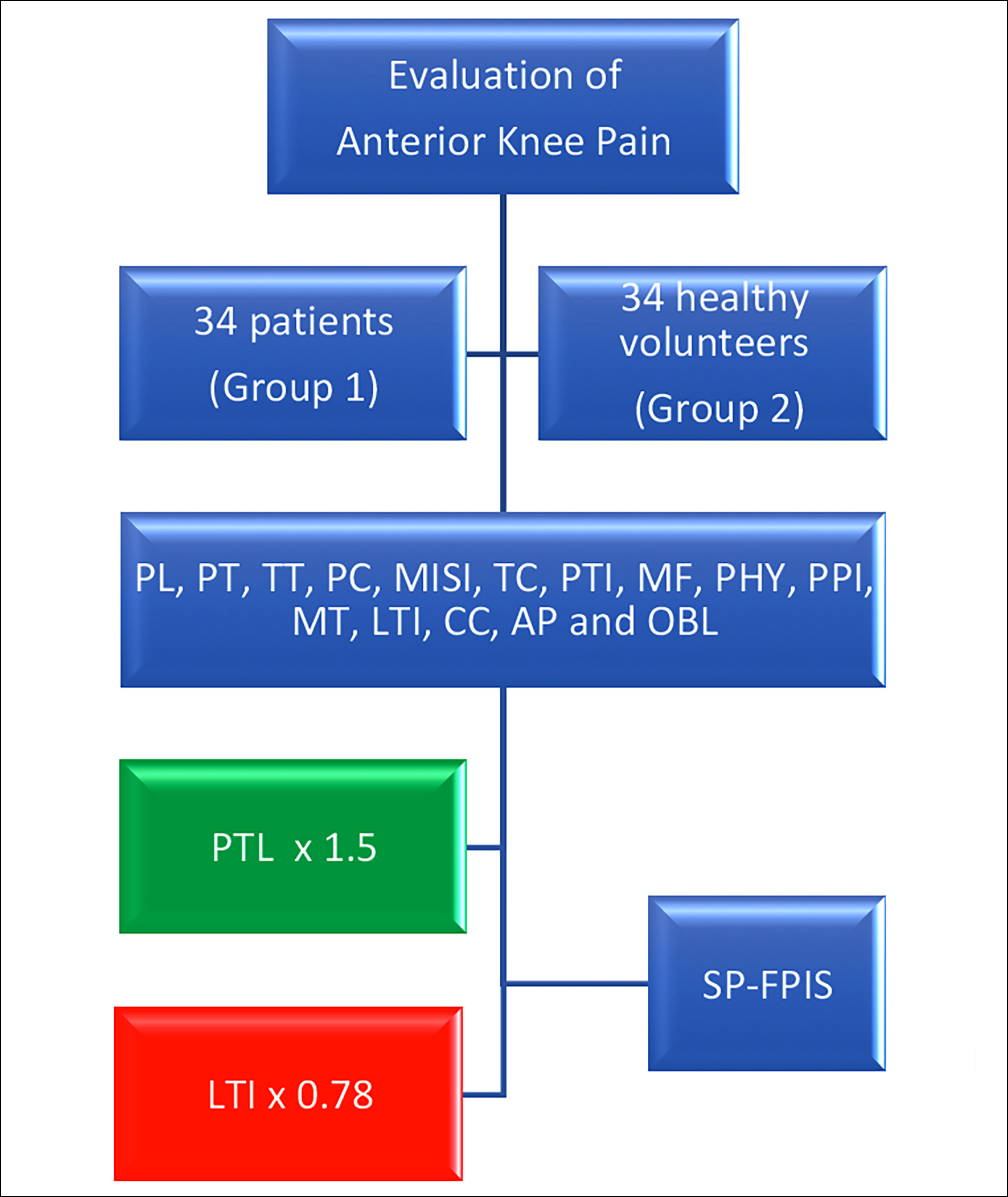 Figure 1: Evaluation of the impact of patellofemoral joint anatomical measurements of patients with anterior knee pain in both groups. PTL and LTI are main factors in the development of SP-FPIS.
Figure 1: Evaluation of the impact of patellofemoral joint anatomical measurements of patients with anterior knee pain in both groups. PTL and LTI are main factors in the development of SP-FPIS.
DISCUSSION
One of the most valuable diagnostic methods in investigating the relationship between anterior knee pain and SPFP is the evaluation of the knee with MRI.2,7-10 In this study, the signal features of SPFP were consistent with findings on MRI in the literature.3,5
Table I: Definition of patellofemoral anatomical measurement parameters and presentation of measurement techniques with MRI images.|
|
Abbreviations |
Definition of measurements |
|
Cranio-caudal Length |
CC |
Cranio-caudal length of the suprapatellar fat pad |
|
Antero-posterior Length |
AP |
Antero-posterior length of the suprapatellar fat pad |
|
Oblique Length |
OBL |
Oblique length of suprapatellar fat pad |
|
Patellar Length |
PL |
Measurement of the length between supero-posterior edge and inferior apexia of the patella |
|
Patellar Tendon Length |
PTL |
Measurement of the length between patella inferior and tibial adhesion of the patellar tendon |
|
Insall Salvati Index |
ISI |
PTL/PL |
|
Distal Patella Cartilage – Tibial Tubercle |
TT |
The distance between the most distal point of the articular surface of the patella and the point of adhesion of the patellar tendon to the tuberocytas tibia |
|
Patella Cartilage Length |
PCL |
Patellar articular cartilage length measurement |
|
Modified Insall Salvati Index |
MISI |
TT/PCL |
|
Patella–Trochlea Cartilage Length |
TCL |
The length of the trochlear cartilage overlapping with the patellar cartilage |
|
Patellotrochlear Index |
PTI |
TCL/PCL |
|
Medial Facet Length |
MFL |
Medial patellar facet length |
|
Lateral Facet Length |
LFL |
Lateral patellar facet length |
|
Patellar Facet Asymmetry |
PFA |
MFL/LFL |
|
Patellar Angle |
PA |
The angle between the lines drawn in parallel to the medial and lateral patellar facets |
|
Patellar Length on Anterior Physis Line |
PHY |
Patellar length remaining in the superior of the anterior physis line |
|
Patella Physeal Index |
PPI |
PHY/PCL |
|
Trochlear Depth |
TD |
Trochlear depth is measured by drawing a perpendicular line from the sulcus central point to the line connecting the medial and lateral femoral trochlear facets |
|
Medial Trochlear Length |
MTL |
Trochlear medial length |
|
Lateral Trochlear Length |
LTL |
Trochlear lateral length |
|
Trochlear Facet Asymmetry |
TFA |
MTL/LTL |
|
Sulcus Angle |
SA |
Angle between the lines drawn in parallel to the medial and lateral trochlear facets |
|
Lateral Trochlear Inclination Angle |
LTI |
The angle between the line connecting the femoral condyle posteror cortical segments and the line parallel to the lateral trochlear facet |
Table II: General characteristics and statistical analysis (t-test and chi-square test) of both groups based on patellofemoral measurements.
|
|
Group 1 (n=34) Mean±SD or % |
Group 2 (n=34) Mean±SD or % |
p-value |
|
Age |
45.62±10.87 |
41.47±11.98 |
0.14 |
|
Gender |
15 women (44.12%) 19 men (55.88%) |
22 women (64.71%) 12 men (35.29%) |
0.088 |
|
Side |
23 right (67.65%) 11 left (32.35%) |
14 right (41.18%) 20 left (58.82%) |
0.028* |
Table III: Patellofemoral anatomical measurements in both groups and statistical analysis (t-test) of the results.
|
Anatomical measurements |
Group 1 (n=34) Mean±SD |
Group 2 (n=34) Mean±SD |
p-value |
|
PL |
41.47±4.77 |
38.32±4.5 |
0.01* |
|
PTL |
44.38±7.92 |
38.76±5.85 |
0.0015* |
|
ISI |
1.08±0.21 |
1.01±0.14 |
0.15 |
|
TT |
47.79±6.58 |
42.29±6.65 |
0.0011* |
|
PCL |
28.47±3.26 |
23.29±3.23 |
<0.0001* |
|
MISI |
1.69±0.27 |
1.83±0.29 |
0.04* |
|
TCL |
12.79±4.15 |
15±2.99 |
0.01* |
|
PTI |
0.45±0.16 |
0.65±0.12 |
<0.0001* |
|
MFL |
20.06±3.48 |
18.44±2.16 |
0.02* |
|
LFL |
23.68±2.72 |
23.24±2.39 |
0.48 |
|
PFA |
0.86±0.17 |
0.8±0.11 |
0.12 |
|
PA |
135.65±7.09 |
132.06±8.02 |
0.0549 |
|
PHY |
15.44±4.61 |
18.62±5.42 |
0.01* |
|
PPI |
0.54±0.15 |
0.8±0.23 |
<0.0001* |
|
TD |
6.09±1.16 |
5.82±1.19 |
0.36 |
|
MT |
14.38±2.75 |
12.85±2.63 |
0.02* |
|
LT |
21.94±2.66 |
20.59±3.18 |
0.06 |
|
TFA |
0.66±0.13 |
0.63±0.12 |
0.34 |
|
SA |
136.74±7.52 |
136.09±6.56 |
0.71 |
|
LTI |
19.53±3.17 |
23.06±4.4 |
0.0003* |
|
CC |
18.24±3.78 |
11.82±1.96 |
<0.0001* |
|
AP |
10.82±1.73 |
5.5±0.9 |
<0.0001* |
|
OBL |
13.38±2.16 |
7.21±1.27 |
<0.0001* |
|
*p<0.05. |
|||
SPFP is the smallest of peripatellar fat pads. In a study conducted by Staeubli et al., SPFP dimensions in healthy individuals were found to be 7±2 mm in women and 8±2 mm in men.11 The average 1-mm difference between men and women was determined to be 0.9 mm in the present study. In a study conducted by Sirvanci and Ganiyusufoglu, the SPFP dimensions measured in the patient and control group were consistent with the findings of the present study.12
SPFP oedema is often unilateral. Contrary to the information in the literature, when both knees of patients with oedema in SPFP were examined in a study conducted by Shabshin et al., the rate of existing oedema in both knees was reported as 80%.5 However, the results in the present study do not support Shabshin et al.
The relationship between knee pain and SPFP pathologies is controversial. In a previous study, Tsavalas and Karantanas found no statistically significant relationship between knee pain and SPFP oedema.13 However, the pain was completely resolved after a US-guided injection of corticosteroids for oedema in SPFP in these patients with no other detectable cause. In studies by Roth et al. and Shabshin et al. it has been reported that there is a correlation between anterior knee pain and mass effect in SPFP.4,5 Another study that supports the fact that the presence of oedema in SPFP leads to knee pain was conducted by Ozdemir et al.14 Their results proved that the treatment with 1-ml local corticosteroid and 1-ml local anaesthetic injection into the fat pad was extremely effective, which further supported the relationship with pain. The authors believe that the role of oedema and mass effect on the development of pain in suprapatellar fat pads may be owing to stress and inflammation due to a significant increase in size.
When examining the factors affecting the formation of oedema in suprapatellar fat pads, the study conducted by Roth et al. showed a relationship between PL, PTL, patellar joint length, and SA.4 In the present study, a statistically significant relationship was found between PL, PTL, patellar cartilage length, and oedema formation in SPFP (p=0.01, 0015, and <0.0001 respectively). However, no significant relationship with SA was detected (p=0.71).
In the present study, the effect of anatomical etiopathogenesis on the formation of SP-FPIS was investigated and the largest number of anatomical factors in the literature was reviewed, making the results one of the strengths of the study. Statistically significant difference in parameters such as SPFP PL, PTL, TT, PCL, MISI, TCL, PTI, MFL, PHY, PPI, MTL, and LTI between both groups shows the importance of anatomical evaluation. In particular, increasing PTL values increase the likelihood of SP-FPIS formation, while decreasing LTI values reduce the likelihood of SP-FPIS formation. This anatomical background can affect the rich vascular circulation and neural network in SPFP and lead to an inflammatory process resulting in anterior knee pain.
SPFP is known to contain a rich vascular and neural network as well as an intensive localisation of pluripotent mesenchymal stem cells. In addition to preventing friction during knee flexion, mesenchymal cells contained in the SPFP are used in autologous stem cell transfer with their high proliferative power, and they are experimentally used in the treatment of osteoarthritis as a source of mesenchymal cells obtained from fat tissue.15 High proliferative and repair capabilities suggest that SPFP should not be functionally perceived only as support tissue. Its relationship with knee pain and the current potential of SPFP deserves thorough research. The authors believe that future studies investigating the SPFP in terms of knee pain should be standardised with measurement techniques determined according to international criteria, the aetiology of inflammation should be illuminated by samples obtained from the SPFP, and the studies should include large series of patient populations treated with local injection therapies accompanied by imaging methods.
CONCLUSION
PTL and LTI are significant factors in the development of SP-FPIS and these findings should be considered in the decision of medical or interventional treatment options for SP-FPIS.
ETHICAL APPROVAL:
This study was approved by Istanbul Medeniyet Unıversity Goztepe Education And Research Hospital Clinical Trials Ethics Committee (Project Decision No. 0059).
PATIENTS’ CONSENT:
Informed consent forms were obtained from the patients and healthy volunteers participating in the study.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
SSE: Conception and design, analysis or interpretation of data for the work, discussion, literature review, and critical revision of the manuscript.
SS, AK: Critical revision of the manuscript and discussion.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Ali SA, Helmer R, Terk MR. Analysis of the patellofemoral region on MRI: Association of abnormal trochlear morphology with severe cartilage defects. AJR Am J Roentgenol 2010; 194(3):721-7. doi.10.2214/AJR.09.3008.
- Jarraya M, Diaz LE, Roemer FW, Arndt WF, Goud AR, Guermazi A. MRI findings consistent with peripatellar fat pad impingement: How much related to patellofemoral maltracking? Magn Reson Med Sci 2018; 17(3):195-202. doi. 10.2463/mrms.rev.2017-0063.
- Bas A, Tutar O, Yanik I, Samanci C. Quadriceps fat-pad impingement syndrome: MRI findings. BMJ Case Rep 2012; 2012. doi. 10.1136/bcr-2012-007643.
- Roth C, Jacobson J, Jamadar D, Caoili E, Morag Y, Housner J. Quadriceps fat pad signal intensity and enlargement on MRI: Prevalence and associated findings. AJR Am J Roentgenol 2004; 182(6):1383-7. doi.10.2214/ajr.182. 6.1821383.
- Shabshin N, Schweitzer ME, Morrison WB. Quadriceps fat pad edema: Significance on magnetic resonance images of the knee. Skeletal Radiol 2006; 35(5):269-74. doi.10.1007/ s00256-005-0043-7.
- Yılmaz B, Alaca R, Goktepe AS, Mohur H, Kalyon TA. Patellofemoral agri sendromunda izokinetik egzersiz programının fonksiyonel kapasite ve ağrı üzerindeki etkisi. Turkish J Physical Medicine Rehabilitation 2001; 47(5).
- Hirschmann A, Hirschmann MT. Chronic knee pain: Clinical value of mri versus SPECT/CT. Semin Musculoskelet Radiol 2016; 20(1):3-11. doi. 10.1055/s-0036-1579674.
- Nacey NC, Geeslin MG, Miller GW, Pierce JL. Magnetic resonance imaging of the knee: An overview and update of conventional and state of the art imaging. J Magn Reson Imaging 2017; 45(5):1257-75. doi. 10.1002/jmri.25620.
- Skiadas V, Perdikakis E, Plotas A, Lahanis S. MR imaging of anterior knee pain: A pictorial essay. Knee Surg Sports Traumatol Arthrosc 2013; 21(2):294-304. doi. 10.1007/s00 167-012-1976-8.
- Wang J, Han W, Wang X, Pan F, Liu Z, Halliday A, et al. Mass effect and signal intensity alteration in the suprapatellar fat pad: Associations with knee symptoms and structure. Osteoarthritis Cartilage 2014; 22(10):1619-26. doi. 10. 1016/j.joca.2014.05.018.
- Staeubli HU, Bollmann C, Kreutz R, Becker W, Rauschning W. Quantification of intact quadriceps tendon, quadriceps tendon insertion, and suprapatellar fat pad: MR arthrography, anatomy, and cryosections in the sagittal plane. AJR Am J Roentgenol 1999; 173(3):691-8. doi.10. 2214/ajr.173.3.10470905.
- Sirvanci M, Ganiyusufoglu AK. Quadriceps fat pad signal intensity and enlargement on MRI. AJR Am J Roentgenol 2005; 184(5):1708. doi. 10.2214/ajr.184.5.01841708.
- Tsavalas N, Karantanas AH. Suprapatellar fat-pad mass effect: MRI findings and correlation with anterior knee pain. AJR Am J Roentgenol 2013; 200(3):W291-6. doi. 10.2214/AJR.12.8821.
- Ozdemir ZM, Aydingoz U, Korkmaz MF, Tunay VB, Ergen FB, Atay O, et al. Ultrasonography-guided injection for quadriceps fat pad edema: Preliminary report of a six-month clinical and radiological follow-up. J Belg Soc Radiol 2016; 100(1):78. doi. 10.5334/jbr-btr.1148.
- Munoz-Criado I, Meseguer-Ripolles J, Mellado-Lopez M, Alastrue-Agudo A, Griffeth RJ, Forteza-Vila J, et al. Human suprapatellar fat pad-derived mesenchymal stem cells induce chondrogenesis and cartilage repair in a model of severe osteoarthritis. Stem Cells Int 2017; 2017:4758930. doi. 10.1155/2017/4758930.