Outcomes of Nephron-sparing Surgery in T1 vs T2 Renal Tumours
By Pardeep Kumar, Mudassir Hussain, Mehnaz Jabeen, Rehan Mohsin, Gauhar Sultan, Altaf HashmiAffiliations
doi: 10.29271/jcpsp.2022.05.627ABSTRACT
Objective: To compare the drop in eGFR after nephron-sparing surgery in T1 and T2 renal tumours.
Study Design: Descriptive study.
Place and Duration of Study: Department of Uro-oncology, Sindh Institute of Urology, and Transplantation Karachi, from March 2020 to March 2021.
Methodology: Retrospective data were collected for all patients who underwent nephron-sparing surgery between 2014 to 2019. Eighty-seven patients were divided into two groups based on the T stage of renal tumours (T1 ≤7 cm and T2 >7 cm). The outcomes of the two groups were compared such as eGFR, blood transfusion, hospital stay and complications.
Results: There was a higher drop in eGFR in T2 tumours when compared to T1 tumours at 1 year of follow-up. There were more perioperative complications, higher blood transfusions and longer hospital stays for T2 tumours.
Conclusion: Nephron sparing surgery for T2 renal tumours carries lower eGFR preservation, higher blood transfusions and complications when compared to T1 tumours. The indication for such extensive surgery should be individualized to specific contexts only.
Key Words: Adenocarcinoma kidney, Nephrectomy, Glomerular filtration rate, Length of hospital stay, Blood transfusion.
INTRODUCTION
Nephron-sparing surgery or partial nephrectomy has been shown to preserve long-term renal functions and protect against harmful effects of chronic kidney disease.1 Close assessment of data makes it evident that partial nephrectomy is a trade-off between short-term higher complications with long-term preservation of renal function.2 Nevertheless, this form of treatment has gained popularity in the past few decades and the number of procedures has increased substantially.3 This development is not only driven by the quest to save precious nephrons but also by simultaneous improvements in imaging and surgical techniques.4,5
The question about the limit of nephron-sparing surgery in terms of size and complexity is yet to be answered. According to European urology guidelines, partial nephrectomy is the treatment of choice for T1a tumours (≤ 4 cm) and is preferred for T1b tumours (>4cm and ≤7 cm) though not the treatment of choice.6
The possibility of performing a partial nephrectomy is dictated by size alone as mentioned above in the guideline statement. However, the concept has now shifted to the overall complexity of the tumour taking into consideration factors such as proximity to the hilum and percent of the volume outside or inside the renal tissue.7 This greater understanding and categorization of the anatomy of renal masses and improvement in surgical technique translates into pushing the limits of partial nephrectomy from T1a, to T1b and into T2 tumours.8-10
Although the procedure has been performed successfully in large size tumours, the clinical utility has been questioned.10 With the increasing size of renal mass, the size of residual functioning tissue decreases. This tilts the trade-off between higher complications and preservation of renal functions in the favour of the former. The situation thus put into question the very core rationale of performing partial nephrectomy in the first place. Why face complications if the preservation of renal functions is not worth it?
With the increasing size of excised tissue and decreasing size of residual functioning tissue, it is anticipated that the outcomes might be compromised for larger size tumours. The objective of this study was to compare the drop in eGFR after Nephron-sparing surgery in T1 and T2 renal tumours.
Table I: Descriptive statistics of the groups.
|
Variables |
Total n = 87 |
T1 (≤7 cm) n = 76 |
T2 (>7 cm) n = 11 |
p-value |
|
Age (years) |
48.3 ± 12.4 |
48.62 ± 12.28 |
46.12 ± 14.15 |
0.548 a |
|
Gender Male Female |
64 (73.5%) 23 (26.4%) |
56 (73.6%) 20 (26.3%) |
8 (72.7%) 3 (27.2%) |
0.946 b |
|
Approach Open Robotic |
72 (82.8%) 15 (17.2%) |
63 (82.9%) 13 (17.1%) |
8 (81.8%) 2 (18.2%) |
0.930 b |
|
Pathology RCC, Clear cell RCC, Papillary RCC, Chromophobe RCC, others others |
63 (72.4%) 8 (9.2%) 4 (4.6%) 2 (2.3%) 10 (11.4%) |
58 (76.3%) 5 (6.57%) 3 (4%) 2 (2.63%) 8 (10.5%) |
5 (45.4%) 3 (27.2%) 1 (9.1%) 0 (0) 2 (18.1%) |
0.032 b 0.026 b 0.446 b 0.586 b 0.457 b |
|
Margins Status Negative Positive |
76 (87.3%) 11 (12.6%) |
67 (88.2%) 9 (11.8%) |
9 (81.8%) 2 (18.2%) |
0.554 b |
|
T substage |
|
T1a, 31 (40.8%) T1b, 45 (59.2%) |
T2a, 5 (45.4%) T2b, 6 (54.5%) |
0.756 b |
|
Nephrometry Score |
7.17 ± 1.8 |
6.95 ± 1.6 |
8.73 ± 2.2 |
0.002 a |
|
Nephrometry complexity High Intermediate Low |
10 (11.5%) 42 (48.3%) 35 (40.2) |
4 (5.3%) 40 (52.6%) 32 (42.1%) |
6 (54.5%) 2 (18.2%) 3 (27.3%) |
0.001 b |
|
(a) Test of significance, student T-test; (b) Test of significance, Chi-square. |
||||
METHODOLOGY
This retrospective descriptive study was conducted at the department of uro-oncology, Sindh Institute of Urology and transplantation between March 2020 and March 2021. All patients who underwent nephron-sparing surgery from 2014 to 2019 and matched the inclusion criteria were included for analysis while those who had a subsequent Radical nephrectomy after initial nephron-sparing surgery were excluded. The patients were divided into two groups, the first group had tumours staged as T1, No, Mo i.e. size ≤ 7 cm and confined to the kidney while the second group had tumours staged as T2, No, Mo i.e. size >7 cm and confined to the kidney. Both groups were compared for parameters that include age, gender, surgical approach, pathology, eGFR (calculated from MDRD formula), ischemia time, hospital stay, blood transfusion, surgical margins, complications and nephelometry score.
The data were analyzed using SPSS version 20 (SPSS: An IBM Company, IBM Corporation, Armonk, NY, USA). The normality of data was checked using Shapiro-Wilk and Kolmogorov–Smirnov tests. Some of the normally distributed continuous variables such as age, nephrometry score, and eGFR, were expressed as mean ± SD and compared using student t test. The non-normally distributed continuous variables such as ischemia time, hospital stay and blood transfusion were expressed as median (IQR) and compared using non-parametric Mann-Whitney U-test. The categorical variables including gender, approach, margin status, pathology, T-stage, complexity, complications and Grade of complications were expressed as proportion and compared using Chi-square test. A p-value of less than 0.05 was considered significant.
RESULTS
A total of 87 patients were included in this study among whom 76 (87.3%) were in the first group i.e. T1 disease and 11 (12.6%) were in the second group i.e. T2 disease. The two groups were significantly different from each other in terms of complexity on R.E.N.A.L nephrometry score, 6.95 ± 1.6 vs. 8.73 ± 2.2 (p = 0.002) however they were comparable in terms of gender, approach, pathology and margin status. Among the outcome measures, the T2 group had significantly higher blood transfusion rates as compared to T1 group (p = 0.023).
Table II: Comparison of outcomes (T1 Vs T2).
|
Variables |
Total n = 87 |
T1 (≤7 cm) n = 76 |
T2 (>7 cm) n = 11 |
p-value |
|
Pre-op GFR (ml/mins/1.73m2) |
95.6 ± 32.2 |
95.6 ± 33.34 |
95.37 ± 24.28 |
0.980 a |
|
POD 1 GFR (ml/mins/1.73m2) |
73.3 ± 32.5 |
74.86 ± 33.71 |
63.32 ± 21.13 |
0.274 a |
|
POD 2 GFR (ml/mins/1.73m2) |
74.9 ± 35.4 |
77.12 ± 36.68 |
60.46 ± 21.40 |
0.147 a |
|
POD 3 GFR (ml/mins/1.73m2) |
78.3 ± 33.2 |
79.87 ± 32.41 |
68.39 ± 38.91 |
0.313 a |
|
1 year, GFR (ml/mins/1.73m2) |
69.0 ± 41.6 |
72.35 ± 41.47 |
46.58 ± 37.28 |
0.055 a |
|
Ischemia time (mins) Median (IQR) |
15 (10-20) |
15 (10.3 – 20.0) |
14 (8.5 – 23) |
0.278 c |
|
Hospital Stay (days) Median (IQR) |
4 (3-5) |
4 (3-5) |
4 (3-7) |
0.660 c |
|
Blood transfusion (units) Median (IQR) |
2 (1-2.75) |
2 (1-2) |
3 (1.5 -5.0) |
0.023 c |
|
Complications Yes No |
22 (25.3%) 65 (74.7%) |
17 (22.4%) 59 (77.6%) |
5 (45.5%) 6 (54.3%) |
0.136 b |
|
Grade of complication Grade 1 Grade 2 Grade 3a Grade 3b Grade 4 Grade 5 |
4 (18.1%) 4 (18.1%) 4 (18.1%) 8 (36.3%) 1 (4.54%) 1 (4.54%) |
3 (17.6%) 4 (23.5%) 2 (11.7%) 6 (35.9%) 1 (5.88%) 1 (5.88%) |
1 (20%) 0 2 (40%) 2 (40%) 0 0 |
0.146 b |
|
(a) Test of significance, student t-test; (b) Test of significance, Chisquare; (c) Test of significance, Mann-Whitney U-test. |
||||
The overall mean age of all patients was 48.3 ± 12.4 years with 64 males (73.6%) and 23 females (26.4%, Table I). The male to female ratio was 2.7: 1. The majority of patients were operated through a retroperitoneal flank incision 72 (82.8%) while the rest underwent trans-peritoneal robotic-assisted surgery 15 (17.2%).
The pathological evaluation of resected tissue was reported as Renal cell carcinoma in 77 cases (88.5%) while the rest were mostly benign tumours. Among all of the resections, a positive surgical margin was reported in 11 (12.6%) of cases (Table I). Some of the cases 21 (33.8%) were performed off clamp i.e. at zero ischemia, while the median ischemia time for on clamp cases was 15 (10-20) minutes. A higher percentage of complications were found in the T2 renal tumour group, although not statistically significant from the T1 group (Table II).
The groups were significantly different in terms of complexity with mean nephrometry scores of 6.95 ± 1.6 and 8.73 ± 2.2 respectively (p=0.002). The renal functions were measured with serum creatinine before and after surgery on 1st, 2nd, 3rd and most recent follow up (at least one year). The two groups had a comparable eGFR before surgery (95.6 ± 33.34 vs. 95.37 ± 24.28 mL/min/1.73m2) however after the surgery the T1 group had a lower decline in eGFR compared to the T2 group (72.35 ± 41.47 vs. 46.58 ± 37.28 mL/min/1.73m2, p =0.053), (24.6% vs. 48.8% Figure 1).
DISCUSSION
The core aim of performing nephron-sparing surgery or partial nephrectomy is to preserve renal functions by saving healthy nephrons in the ipsilateral renal unit. PN has been shown to conserve renal functions as compared to Radical nephrectomy in several studies.2,11-13 The way this preservation translates into a benefit to the patient, is a matter of debate. Some studies have shown that saving additional nephrons leads to lower cardiovascular morbidity and mortality.14,15 Others suggest that the need for renal replacement therapy in the long term is reduced.16 However, the reported advantage of overall survival is controversial. Most of the available data is retrospective with some suggesting no OS advantage and others showing an advantage over RN and still more showing better OS for RN compared to PN.17-19
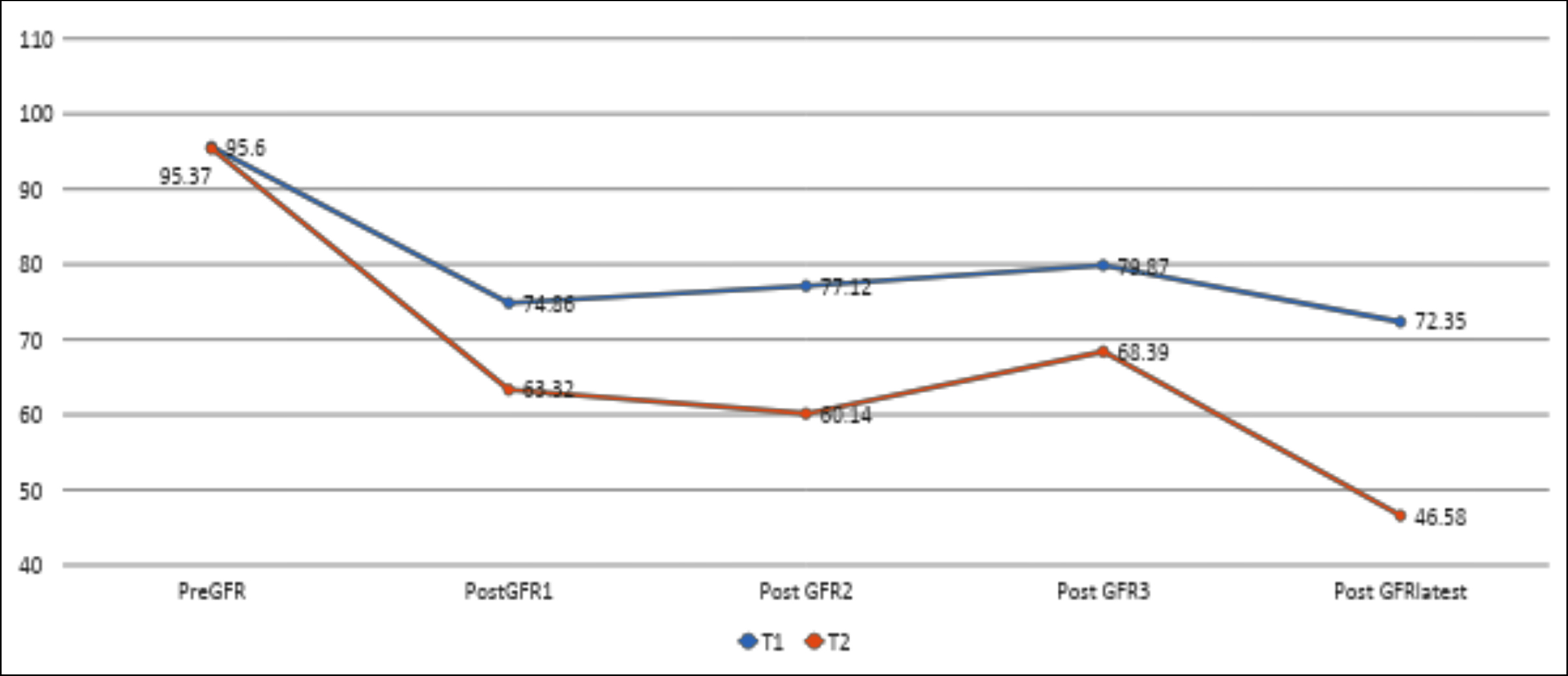 Figure 1: Comparison of renal functions.
Figure 1: Comparison of renal functions.
The available data on PN for T1 tumours is substantial to make comparisons on various parameters with RN for the same indication. However, for T2 tumours there is limited available data for PN.8-10 Performing PN for T2 tumours is a recent practice facilitated by improvement in imaging, surgical techniques and postoperative care. However, the utility of such surgery is not well established and EUA guidelines recommend individualization of such decisions in patients with preexisting CKD only.6
To the best of authors’ knowledge, this is the first study with a direct comparison of the peri-operative outcomes of PN for T1 and T2 tumours. The compared outcomes included eGFR, ischemia time, blood transfusion, operative time, hospital stay and complications in the two groups.
Since the comorbidities and split function of the contralateral kidneys were not included in the analysis, it is difficult to say that the decline in eGFR was purely due to higher loss of nephrons in the second group or this group contained more functionally impaired nephrons, to begin with. Jang et al. compared RN and PN for T1b tumours (4-7 cm) and reported a decline in eGFR of 11.1% in the PN group while our patients with T2 tumours recorded a 48.8% decline in renal functions.20
A retrospective comparison of PN and RN for T2 tumours reported that the PN group had a lower decline in renal functions when compared to RN at the expense of higher complications.8 A meta-analysis of 21 case-control studies including 11204 patients with larger tumours i.e. T1b and T2 concluded that PN for T2 tumours is associated with higher blood loss and complications with lower recurrence rate and cancer-specific mortality in comparison to RN.9
The perioperative outcomes such as blood transfusion for T2 tumours undergoing partial nephrectomy are found to be inferior to those with T1 tumours. The median blood transfusion was significantly higher in the T2 group 3.
Surgical complications were recorded in 22 cases (25.2%) with 45.4% occurring in the T2 group and 22.3% in the T1 group. The majority of these were grade 3b (9.1%) followed by 4.5 % each of Grade 1, 2 and 3a according to Clavin-Dindo grade of complications. One patient died after surgery due to an unknown cause (0.6%).
There were low numbers of T2 tumours included in the study that might have affected the results. Comorbid conditions that affect global renal functions after surgery were not included in the analysis.
CONCLUSION
Nephron sparing surgery for T2 renal tumours carries lower eGFR preservation, higher blood transfusion and complications when compared to T1 tumours. The indication for such extensive surgery should be individualized to specific contexts only.
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
PK, MH: Conception of design, data collection, analysis and manuscript writing.
MJ, RM: Data collection and analysis.
GS: Revision of the manuscript and critical appraisal.
AH: Final approval of the version to be published.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Mehra K, Manikandan R, Dorairajan LN, Sreerag S, Jain A, Bokka SH. Trifecta outcomes in open, laparoscopy or robotic partial nephrectomy: Does the surgical approach matter? J Kidney Cancer VHL 2019; 6(1):8-12. doi: 10.15586/jkcvhl. 2019.115.
- MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TBL, Hilvano-Cabungcal AM, et al. Systematic review of perioperative and quality-of-life outcomes following surgical management of localised renal cancer. Eur Urol 2012; 62(6): 1097-17. doi: 10.1016/j.eururo.2012.07.028.
- Poon SA, Silberstein JL, Chen LY, Ehdaie B, Kim PH, Russo P. Trends in Partial and Radical Nephrectomy: An Analysis of Case Logs from Certifying Urologists. J Urol 2013; 190(2): 464-9. doi: 10.1016/j.juro.2013.02.0.
- Park DS, Hong YK, Lee SR, Hwang JH, Kang MH, Oh JJ. Three-dimensional reconstructive kidney volume analyses according to the endophytic degree of tumours during open partial or radical nephrectomy. Int Braz J Urol Off J Braz Soc Urol 2016; 42(1):37-46. doi: 10.1590/S1677-5538.IBJU. 2014.0417.
- Westerman ME, Matsumoto JM, Morris JM, Leibovich BC. Three-dimensional printing for renal cancer and surgical planning. Eur Urol Focus 2016; 2(6):574-6. doi: 10. 1016/j.euf.2016.12.009.
- Professionals SO. EAU Guidelines: Renal cell carcinoma. Uroweb. [cited 2021 May 13]. Available from: https://uroweb. org/guideline/renal-cell-carcinoma/#7.
- Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: A comprehensive standardised system for quantitating renal tumour size, location and depth. J Urol 2009; 182(3): 844-53. doi: 10.1016/j.juro.2009.05.035.
- de Saint Aubert N, Audenet F, Mccaig F, Delavaud C, Verkarre V, Le Guilchet T, et al. Nephron sparing surgery in tumours greater than 7cm. Progres En Urol J Assoc Francaise Urol Soc Francaise Urol 2018; 28(6):336-43. doi: 10.1016/j.purol.2018.03.009.
- Mir MC, Derweesh I, Porpiglia F, Zargar H, Mottrie A, Autorino R. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: A systematic review and meta-analysis of comparative studies. Eur Urol 2017; 71(4):606–17. doi: 10.1016/j.eururo.2016.08.060.
- Alanee S, Herberts M, Holland B, Dynda D. Contemporary experience with partial nephrectomy for stage T2 or greater renal tumors. Curr Urol Rep 2015; 17(1):5. doi: 10.1007/ s11934-015-0558-y.
- Jiang YL, Peng CX, Wang HZ, Qian LJ. Comparison of the long-term follow-up and perioperative outcomes of partial nephrectomy and radical nephrectomy for 4 cm to 7 cm renal cell carcinoma: A systematic review and meta-analysis. BMC Urol 2019; 19(1):48. doi: 10.1186/s12894-019- 0480-6.
- Yang C, Liao Z. Comparison of Radical Nephrectomy and Partial Nephrectomy for T1 Renal Cell Carcinoma: A Meta-Analysis. Urol Int 2018; 101(2):175-83. doi: 10.1159/000 490576.
- Scosyrev E, Messing EM, Sylvester R, Campbell S, Van Poppel H. Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol 2014; 65(2):372-7. doi: 10.1016/j.eururo.2013.06.044.
- Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumours--is there a difference in mortality and cardiovascular outcomes? J Urol 2009; 181(1):55–61; discussion 61-62.
- Capitanio U, Terrone C, Antonelli A, Minervini A, Volpe A, Furlan M, et al. Nephron-sparing techniques independently decrease the risk of cardiovascular events relative to radical nephrectomy in patients with a T1a-T1b renal mass and normal preoperative renal function. Eur Urol 2015; 67(4):683-9. doi: 10.1016/j.eururo.2014.09.027.
- Miller DC, Schonlau M, Litwin MS, Lai J, Saigal CS, Urologic Diseases in America Project. Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer 2008; 112(3):511-20. doi: 10.1002/cncr.23218.
- Sun M, Bianchi M, Trinh Q-D, Hansen J, Abdollah F, Hanna N, et al. Comparison of partial vs radical nephrectomy with regard to other-cause mortality in T1 renal cell carcinoma among patients aged ≥75 years with multiple comorbidities. BJU Int 2013; 111(1):67-73. doi: 10.1111/j.1464- 410X.2012.11254.x.
- Shuch B, Hanley J, Lai J, Vourganti S, Kim SP, Setodji CM, et al. Overall survival advantage with partial nephrectomy: A bias of observational data? Cancer 2013; 119(16):2981-9. doi: 10.1002/cncr.28141.
- Van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, Borkowski A, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011; 59(4):543-52. doi: 10.1016/j.eururo.2010.12.013.
- Jang HA, Kim JW, Byun SS, Hong SH, Kim YJ, Park YH, et al. Oncologic and Functional Outcomes after Partial Nephrectomy Versus Radical Nephrectomy in T1b Renal Cell Carcinoma: A Multicenter, Matched Case-Control Study in Korean Patients. Cancer Res Treat 2016; 48(2):612-20. doi: 10. 4143/crt.2014.122.