One-year Visual and Refractive Outcomes of Deep Anterior Lamellar Keratoplasty (DALK) in Patients with Advanced Keratoconus
By Muhammad Abdul Moqeet, Warda Ali, Saad Alam Khan, Aruba Zafar, Hassan MansoorAffiliations
doi: 10.29271/jcpsp.2022.09.1160ABSTRACT
Objective: To determine the one-year visual and refractive outcomes of deep anterior lamellar keratoplasty (DALK) in patients with advanced keratoconus (KC) using inexpensive readily available instruments.
Study Design: An observational study.
Place and Duration of Study: Department of Cornea and Refractive Surgery, Al-Shifa Trust Eye Hospital, from November 2016 to March 2021.
Methodology: The authors evaluated different types of big bubble (BB) formation and the conversion rate of intended DALK (n=120) to the penetrating keratoplasty (PK) in patients with grade 4 KC. For analysis, only those patients were included in whom DALK was completed. Main outcome measures at 1-year follow-up were best-corrected visual acuity (BCVA), maximum keratometry (Kmax) reading, spherical equivalent (SE), and topographic astigmatism (TA). All the postoperative complications were recorded.
Results: Type 1 BB was formed in 68% (n=82) of the patients. An incomplete type 1 BB and type 2 BB were observed in twenty and eighteen patients, respectively. DALK was completed successfully in 102 patients. Whereas, the conversion rate to PK was 15% (n=18). The mean preoperative BCVA improved from 1.11±0.23 logMAR to 0.46±0.20 logMAR, at 1-year follow-up. The mean Kmax, SE, and TA readings reduced from a preoperative value of 62.1±4.60 D, -11.6±2.62 D, and 4.63±1.82 D, respectively, to 49.1±3.10 D, -5.65±0.84 D, and 2.78±1.35 D, respectively, at 1-year follow-up. Stromal rejection was recorded in two patients, but it responded well to topical therapy.
Conclusion: Inexpensive readily available instruments can be used to perform DALK in patients with advanced KC with favourable visual and refractive outcomes.
Key Words: Big bubble, Deep anterior lamellar keratoplasty, Keratoconus, Perforation, Penetrating keratoplasty.
INTRODUCTION
Deep anterior lamellar keratoplasty (DALK) has been suggested as an alternative to conventional penetrating keratoplasty (PK) for visual rehabilitation in patients with keratoconus (KC).1,2 Compared to PK, the DALK preserves the patient's descemet’s membrane (DM) and the endothelium, leading to a tectonically stronger postoperative-globe and reducing the risk of immunological reactions.3,4 However, interface irregularities and stromal scarring remain a challenge with the DALK if the DM is not exposed, yielding inferior results.5
The DM-baring DALK is a time-consuming and technically challenging procedure with a steep learning curve compared to PK, especially when the host stromal tissue over the DM is removed manually layer-by-layer. Not long ago, Anwar and Teichmann introduced the big-bubble (BB) technique for the DM-baring DALK. In this technique, the air is injected into the deep stroma to separate posterior stroma from the DM. Subsequently, the surgeon, curtailing the surgical time, can gain direct access to the cleavage plane, achieving an optimal interface with a reduced risk of DM perforation.6,7
Many surgeons have modified the Anwar and Teichmann’s BB-DALK technique. They have used specialised air-injection cannulas and stromal dissectors to improve the rate of BB formation, thus reducing the learning curve of the BB-DALK procedure.8,9 However, surgical-training constraints and costly instrumentation have limited its adoption in Pakistan. The rationale of this study is to highlight practical aspects of DALK surgery and provide some helpful tips for the novice surgeons wishing to convert from the traditional PK technique to DALK procedure using inexpensive readily available instruments. The aim of the study was to determine the 1-year postoperative visual and refractive outcomes of DALK in patients with advanced KC.
METHODOLOGY
This observational study evaluated the one-year visual and refractive outcomes of the DALK in patients with advanced KC at the Department of Cornea, Al-Shifa Trust Eye Hospital, Rawalpindi, from November 2016 to March 2021. The institutional ethical and research board approved the study.
The clinical and surgical records of patients with a diagnosis of advanced KC were reviewed; defined as grade 4 disease,10 having inadequate corneal pachymetry for corneal collagen cross linking or intracorneal ring segment insertion, rigid gas permeable lens intolerance and/or a best corrected visual acuity (BCVA) of <0.6 logMAR. The patients with a prior history of coexisting ocular disease, acute or healed hydrops, previous intraocular surgery, and those patients in whom the surgical procedure had converted from DALK to PK due to the macroperforation of the DM, were excluded from the study analysis. A puncture in the DM was labeled as a microperforation if the anterior chamber could be maintained with air or balanced salt solution injection (BSS). Contrarily, if the anterior chamber collapsed even after the injection of the air or BSS following a DM perforation, it was termed as macroperforation. While DALK was performed in microperforations, macroperforations of the DM were converted to PK.
All the patients were operated on under general anaesthesia by a single surgeon (MAM). The centre of the recipient cornea was marked with a gentian violet surgical marker. The surgeon chose the diameter of the trephine, considering the cone size and the topography-measured horizontal corneal diameter to include as much as possible of the cone within the partially trephined area (Figure 1a). Subsequently, a crescent knife was used for the superficial keratectomy, aiming to debulk the corneal stroma. A 0.5 mm iris repositor with the tapering ends was introduced into the deep stroma, starting at the trephination groove’s margin and later advanced it towards the corneal centre for 3 to 4 mm, following the curvature of the recipient cornea. Later, a standard air cannula attached to 5 ml syringe was introduced into the stromal tunnel, and air injection was performed to achieve a BB formation. The type 1 BB appears like a white disk that originates from the corneal centre and gradually enlarges towards the corneal periphery (8-9 mm), separating Dua's layer (DL) from the deep stroma (Figure 1b). On the contrary, type 2 BB is formed between the posterior surface of DL and the DM.11 If a type 1 BB was formed, the air injection was stopped as soon as the BB reached the trephination margin. A No.11 Bard-Parker® blade was used to slash the BB open, allowing the air to escape and collapse the formed BB. Blunt-tipped Vannas scissors were used to divide the anterior stroma into four quadrants from the centre to the periphery (Figure 1c), which was then excised at the trephination margin, exposing the smooth, transparent, and glistening DM (Figure 1d). However, if the surgeon did not achieve a type 1 BB after more than one attempt, or a type 2 BB was formed, the surgeon performed debulking using the same divide and conquer technique described above. Subsequently, a layer-by-layer manual stromal dissection was performed with the help of standard and inexpensive surgical instruments. A pocket was created between the residual stroma and DM with the help of the 0.5 mm iris repositor, guided by the residual microbubbles in the deep stroma. Following this, a flat spatula was introduced into the pocket. The surgeon experienced no resistance if the proper plane just above the DM was achieved. If that was not the case, the steps described above were repeated, aiming to access the intended plane. The vannas scissors then excised the residual stroma above the DM. In case of a microperforation during the final deep lamellar dissection, the anterior chamber was formed again with an air or BSS injection, and the procedure continued. Contrarily, the surgeon converted the procedure to PK, if there was a macroperforation. Nonetheless, the trephination edges were trimmed, if required, to reduce postoperative astigmatism.
The donor cornea was punched out on a Teflon block from the endothelial side with a long-handle corneal trephine (CORONET®, Network Medical Products Ltd, UK), having a diameter that was 0.25 mm larger than that used on the recipient. The endothelium was stained with trypan blue 0.06% and gently scraped off with a dry Weck-Cel sponge (Figure 1e). The donor corneal tissue was then sutured onto the recipient bed with 10-0 nylon interrupted sutures (Figure 1f).
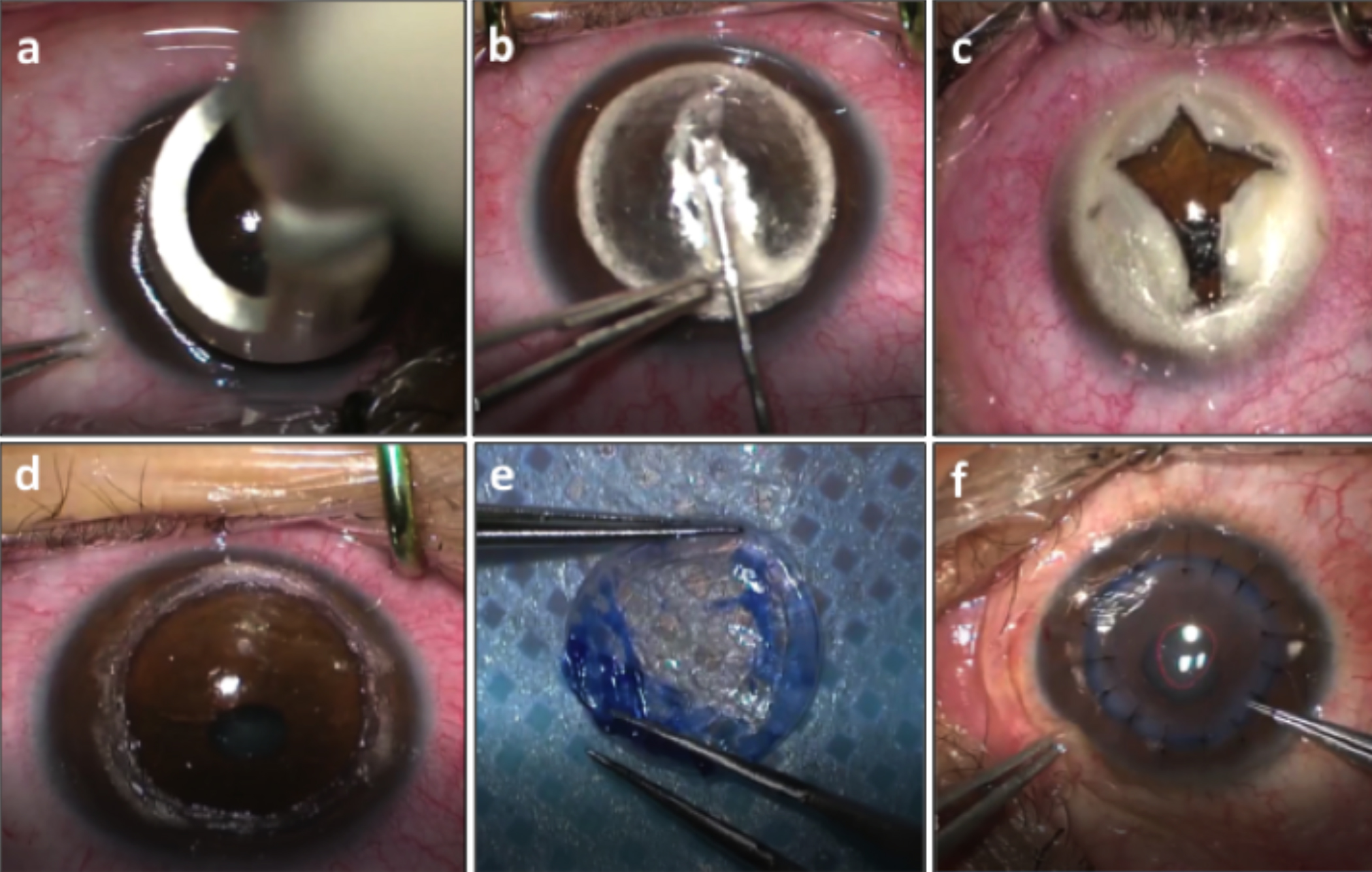 Figure 1a-f: Surgical steps of deep anterior lamellar keratoplasty (DALK). (a) Trephination of the recipient cornea by a long-handle trephine of a chosen diameter. (b) Type-1 big bubble formation. (c) Blunt-tipped vanna scissors used to divide the anterior stroma into four quadrants from the centre to the periphery. (d) Baring of the descemet’s membrane. (e) Trypan blue (0.06%) stained endothelium scraped off with a dry Weck-Cel sponge. (f) Donor button sutured onto the recipient bed with 10-0 nylon-interrupted sutures.
Figure 1a-f: Surgical steps of deep anterior lamellar keratoplasty (DALK). (a) Trephination of the recipient cornea by a long-handle trephine of a chosen diameter. (b) Type-1 big bubble formation. (c) Blunt-tipped vanna scissors used to divide the anterior stroma into four quadrants from the centre to the periphery. (d) Baring of the descemet’s membrane. (e) Trypan blue (0.06%) stained endothelium scraped off with a dry Weck-Cel sponge. (f) Donor button sutured onto the recipient bed with 10-0 nylon-interrupted sutures.
Upon completion of the procedure, the surgeon administered sub-conjunctival injections of 14 mg/mL gentamicin and dexamethasone 0.1%. A bandage contact lens (BCL) was also placed, and kept until the epithelium had healed completely. The patients were advised to instill topical dexamethasone 0.1% and moxifloxacin 0.5% 3 hourly, for one month. The topical steroid regimen was tapered over 8-10 months to avoid steroid-induced complications, such as glaucoma and cataract. To manage post keratoplasty astigmatism, the individual suture removal was done at least 6 months after the surgery, if required. However, loose, broken, vascularised, or infected sutures were removed or replaced earlier, if deemed necessary.
The pre- and postoperative ocular examinations at 1, 6, and 12 months follow-up visits, included Snellen BCVA (converted to logMAR notation), manifest refraction to determine spherical equivalent (SE), slit-lamp examination, tonometry, dilated fundus examination, corneal tomography (Galilei G4; Ziemer Ophthalmic Systems AG), and non-contact specular microscopy (Konan Medical, USA) for endothelial cell density (ECD). All the intraoperative and postoperative complications as well as secondary interventions were documented.
The data analyses were done using SPSS version 20 (SPSS, Chicago, Illinois, USA). The quantitative data were described as mean ± standard deviation and nominal data as frequencies. The repeated measures ANOVA test analysed the change in the parameters over baseline and at the postoperative test points of 1, 6, and 12 months. A p-value of <0.05 was considered statistically significant.
RESULTS
During the study period, one hundred and twenty (n=120) patients underwent the intended DALK procedure. A successful type 1 BB was formed in 68% (n=82) of the cases. Contrarily, an incomplete type 1 BB and type 2 BB were reported in twenty and eighteen patients, respectively. Macroperforations of the DM occurred in 15% of the cases (n=18), regardless of the type of BB formation, and as many as 11.7% of the patients (n=14) had microperforations. The macroperforations of the DM were converted intraoperatively to PK (n=18). Contrarily, DALK was completed successfully in all the other patients (n=102) including those with microperforations.
For analysis, only those patients were included in whom DALK was completed (n=102). While the male participants constituted 64.7% (n=66) of the study population; 35.3% (n=36) patients were females. The mean age of the patients at the time of surgery was 17.9±3.4 years. The mean follow-up period was 16 months, with all of the patients completing at least a 1-year review. Anterior stromal scarring not involving the DM was observed in 11 patients. The minimum and maximum host trephination diameters were 7.5 mm and 8.25 mm, respectively, with the donor button measuring 0.25 mm larger than that of the host trephination in each case.
In this study, three patients were complicated by the formation of a double anterior chamber that was managed successfully by an air-injection tamponade on the first postoperative day. The mean preoperative BCVA (1.11±0.23 logMAR) improved to 0.46±0.20 logMAR at 12 months (p<0.001). Correspondingly, the mean maximum keratometry (Kmax) reading, SE, and topographic astigmatism (TA) reduced from a preoperative value of 62.1±4.60 D, -11.6±2.62 D, and 4.63±1.82 D, respectively, to 49.1±3.10 D, -5.65±0.84 D, and 2.78±1.35 D, respectively, at 1-year follow-up (all p<0.001). Table I describes the mean preoperative and postoperative central corneal thickness (CCT) and ECD values at different time points.
The graft host junction opacification was observed in 27.5% (n=28) of the patients in the first 6 months. A clear graft host interface was observed in 72.5% (n=74) patients in the early weeks following surgery. Mild interface opacification resulted in 7 patients; however, none suffered a poor visual outcome. Immunological stromal rejection complicated two eyes in the first year following surgery. These patients responded well to topical dexamethasone 0.1% instillation with subsequent clearance of stromal haze and oedema. Postoperative complications, including cataract, glaucoma, infectious keratitis, and/or graft failure were not observed during the follow-up period.
DISCUSSION
This study evaluated 1-year visual and refractive outcomes of the DALK procedure in patients with grade 4 KC. In this study, the type 1 BB was created in 68% of cases, which was comparable to BB formation reported by Fogla and Padmanabham (69.2%),12 and Fontana et al. (64%).13 However, it was lower than the BB-formation rate observed by Anwar and Teichmann (80 to 90%) in the series of 181 keratoconus patients.6 This difference in the BB-formation rate may be attributed to the learning curve associated with any new surgical technique, considering that the current study included the early patients. To support this, a higher percentage of BB-formation was noticed in the last 42 procedures (80%) than in the first 60 surgical interventions (58%). Another factor that may have influenced the BB- formation rate in this study is the severity of corneal ectasia. It is believed that the stromal lamellae are fewer and loosely spaced at the apex of advanced keratoconic corneas. Hence the injected air easily reaches the DM in patients with advanced KC and cleaves the weak adhesions present between DM and the posterior stroma.14
In this study, microperforations were reported in 12% of the cases, comparable to what has been cited in other studies (8-13%).6,12,13 Nonetheless, the DALK procedure was completed successfully in all these cases. On the other hand, 15% of cases (n=18) had converted to PK due to a macroperforation. While transitioning from PK to lamellar keratoplasty, Mustafa et al. also reported DM perforations in 14/50 (28%) eyes, 8 of which (16%) necessitated the conversion to PK.15 Recently, a multi-centre study evaluated the risk factors linked with conversion of intended DALK to PK, with conversion to PK reported in 16.2% (n=114/705) of the cases. They concluded that type 2 BB-formation, manual layer-by-layer dissection, stromal scarring, and surgeon’s inexperience independently increased the risk of conversion.16 The findings in this study were consistent with the observations mentioned above. A conversion from an intended DALK to PK was noted in those patients who had one or more of the following features: a) an incomplete type 1 BB (n=4/18); b) type 2 BB (n=12/18); c) DM-sparing stromal scarring (n=11/18); and d) manual layer-by-layer dissection (n=18/18). Additionally, the conversion rate decreased to 10% after the initial few cases. Thus, implying that the successful type 1 BB-formation, absence of stromal scarring, and surgeon’s experience may be instrumental in diminishing the conversion rate to PK.
Table I: Visual and refractive outcomes of DALK in advanced keratoconus.|
Study parameters |
Pre-operative (mean values) |
Post-operative (mean values) |
F-calc (dF) |
p-value |
||
|
1 Month |
6 Months |
12 Months |
||||
|
BCVA (LogMAR) |
1.11±0.23 |
0.80±0.20 |
0.60±0.21 |
0.46±0.20 |
305.8 (2.3) |
<0.001 |
|
Kmax (D) |
62.10±4.60 |
51.16±3.14 |
50.33±3.67 |
49.10±3.10 |
331.8 (1.8) |
<0.001 |
|
SE (D) |
-11.60±2.62 |
-7.75±2.11 |
-7.17±0.91 |
-5.65±0.84 |
205.1 (2.1) |
<0.001 |
|
TA (D) |
4.63±1.82 |
3.89±1.42 |
3.34±1.34 |
2.78±1.35 |
94.5 (1.7) |
<0.001 |
|
ECD (cells/mm2) |
2726.8±89.9 |
2696.9±93.2 |
2664.6±111.9 |
2629.3±119.8 |
29.6 (92.3) |
<0.001 |
|
CCT (μm) |
351.57±69.86 |
545.80±31.70 |
521.50±41.10 |
513.60±37.70 |
512.9 (1.6) |
<0.001 |
|
BCVA: Best-corrected visual acuity; Kmax: Maximum keratometry value; D: Diopter; SE: Spherical equivalent; TA: Topographic astigmatism; ECD: Endothelial cell density; CCT: Central corneal thickness. |
||||||
The BCVA reported in this study compares favourably with that reported after DALK and PK for KC.17-19 Most studies have used the percentage of the patients with the postoperative BCVA of ≥20/40 as the desired endpoint to determine surgical success.13 Bearing this in mind, a success rate of 82% was achieved in this study at 1-year follow-up. Additionally, there was no significant difference in the mean postoperative BCVA at different time points amongst the patients who underwent type 1 BB-DALK and manual-DALK. This could be explained as the DM was exposed in all the operated cases, allowing a smooth optical interface with favourable postoperative visual outcomes.
In this study, the donor graft was oversized by 0.25 mm compared to the host trephination diameter, because the usage of same-size donor grafts in DALK in the patients with advanced KC has been found to result in the DM folds’ formation, as the donor tissue exerts a compressive effect on the underlying stromal bed.13 However, oversized donor buttons may induce high postoperative myopia.12 In this study, the mean postoperative SE and TA were -5.65±0.84 D and 2.78±1.35 D, respectively, at 1-year follow-up. Considering that this study included consecutive patients with advanced KC, the results are consistent with the other DALK studies that show greater or equivalent postoperative myopic refraction.5,13,20
Immunologic rejection after DALK is a possible but rare complication, it has been reported in 3% to 14% of the patients.3,13 Stromal rejection occurred in two patients; both had no known risk factors. Nonetheless, they responded well to a short course of topical corticosteroid therapy and both patients had complete resolution of the stromal oedema, resulting in an optically clear graft.
CONCLUSION
DALK is a valuable alternative surgical technique to PK in patients with advanced KC, it has a steep learning curve. In these patients, inexpensive readily available instruments can be used to perform DALK with favourable postoperative visual and refractive outcomes. During DALK, the exposure of the DM may be achieved by both slashing the formed type 1 BB open and/or manual layer-by-layer dissection, showing comparable visual outcomes. Nonetheless, type 1 BB-formation and surgeon’s experience in DALK may reduce the risk of intraoperative complications and the conversion rate to PK.
ETHICAL APPROVAL:
The Institutional Ethical and Research Board approved the study (Ref No. ERC-20/AST-21).
PATIENTS’ CONSENT:
Informed consents were obtained from all the patients.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
MAM: Conception and design, data collection, write-up, and review.
WA: Data collection, write up, and review.
SAK: Data analysis, data collection, and interpretation.
AZ: Manuscript drafting and review.
HM: Final review, guarantor, and write-up.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Liu H, Chen Y, Wang P, Li B, Wang W, Su Y, et al. Efficacy and safety of deep anterior lamellar keratoplasty vs. penetrating keratoplasty for keratoconus: A meta-analysis. PLoS One 2015; 10(1):e0113332. doi: 10.1371/journal.pone. 0113332.
- Nanavaty M, Vijjan K, Yvon C. Deep anterior lamellar keratoplasty: A surgeon's guide. J Curr Ophthalmol 2018; 30(4):297-310. doi: 10.1016/j.joco.2018.06.004.
- Gonzalez A, Price MO, Feng MT, Lee C, Arbelaez JG, Price FW. Immunologic rejection episodes after deep anterior lamellar keratoplasty: Incidence and risk factors. Cornea 2017; 36(9):1076-82. doi: 10.1097/ICO.000000000000 1223.
- Myerscough J, Roberts H, Yu AC, Elkadim M, Bovone C, Busin M. Five-year outcomes of converted mushroom keratoplasty from intended deep anterior lamellar keratoplasty (DALK) mandate 9-mm diameter dalk as the optimal approach to keratoconus. Am J Ophthalmol 2020; 220:9-18. doi: 10.1016/j.ajo.2020.07.009.
- Watson SL, Ramsay A, Dart JKG, Bunce C, Craig E. Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. Ophthalmol 2004; 111(9):1676-82. doi: 10.1016/j.ophtha.2004.02.010.
- Anwar M, Teichmann KD. Big-bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. J Cataract Refract Surg 2002; 28(3):398-403. doi: 10.1016/ s0886-3350(01)01181-6.
- Anwar M, Teichmann KD. Deep lamellar keratoplasty: Surgical techniques for anterior lamellar keratoplasty with and without baring of Descemet’s membrane. Cornea 2002; 21(4):374-83. doi: 10.1097/00003226-200205000-00009.
- Fogla R, Sahay P, Sharma N. Preferred practice pattern and observed outcome of deep anterior lamellar keratoplasty - A survey of Indian corneal surgeons. Indian J Ophthalmol 2021; 69(6):1553-8. doi: 10.4103/ijo.IJO_3067_20.
- Arundhati A, Chew M, Lim L, Mehta J, Lang S, Htoon H, et al. Comparative study of long-term graft survival between penetrating keratoplasty and deep anterior lamellar keratoplasty. Am J Ophthalmol 2021; 224:207-16. doi: 10.1016/j.ajo.2020.11.006.
- Kamiya K, Kono Y, Takahashi M, Shoji N. Comparison of simulated keratometry and total refractive power for keratoconus according to the stage of amsler-krumeich classification. Sci Rep 2018; 8(1). doi: 10.1038/s41598-018- 31008-1.
- Dua H, Katamish T, Said D, Faraj L. Differentiating type 1 from type 2 big bubbles in deep anterior lamellar keratoplasty. Clin Ophthalmol 2015; 9:1155. doi: 10.2147/OPTH. S81089.
- Fogla R, Padmanabhan P. Results of deep lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol 2006; 141(2):254-9.e1. doi: 10.1016/j.ajo.2005.08.064.
- Fontana L, Parente G, Tassinari G. Clinical outcomes after deep anterior lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol 2007; 143(1):117-24. doi: 10.1016/j.ajo.2006.09.025.
- Meek KM, Tuft SJ, Huang Y, Gill PS, Hayes S, Newton RH, et al. Changes in collagen orientation and distribution in keratoconus corneas. Investig Ophthalmol Vis Sci 2005; 46(6):1948-56. doi: 10.1167/iovs.04-1253.
- Unal M, Bilgin B, Yucel I, Akar Y, Apaydin C. Conversion to deep anterior lamellar keratoplasty (DALK): Learning curve with big-bubble technique. Ophthalmic Surg Lasers Imaging. 2010; 41(6):642-50. doi: 10.3928/15428877-20100929-09.
- Myerscough J, Friehmann A, Bovone C, Mimouni M, Busin M. Evaluation of the risk factors associated with conversion of intended deep anterior lamellar keratoplasty to penetrating keratoplasty. Br J Ophthalmol 2020; 104(6):764-7. doi: 10.1136/bjophthalmol-2019-314352.
- Khattak A, Nakhli FR, Al-Arfaj KM, Cheema AA. Comparison of outcomes and complications of deep anterior lamellar keratoplasty and penetrating keratoplasty performed in a large group of patients with keratoconus. Int Ophthalmol 2018; 38(3):985-92. doi: 10.1007/s10792-017-0548-9.
- Henein C, Nanavaty MA. Systematic review comparing penetrating keratoplasty and deep anterior lamellar keratoplasty for management of keratoconus. Cont Lens Anterior Eye 2017; 40(1):3-14. doi: 10.1016/j.clae.2016.10.001.
- Yuksel B, Kandemir B, Uzunel UD, Çelik O, Ceylan S, Kusbeci T. Comparison of visual and topographic outcomes of deep-anterior lamellar keratoplasty and penetrating keratoplasty in keratoconus. Int J Ophthalmol 2017; 10(3): 385-90. doi: 10.18240/ijo.2017.03.10.
- Coombes AGA, Kirwan JF, Rostron CK. Deep lamellar keratoplasty with lyophilised tissue in the management of keratoconus. Br J Ophthalmol 2001; 85(7):788-91. doi: 10.1136/bjo.85.7.788.