Neoadjuvant Immune Checkpoint Inhibitors in Non-small Cell Lung Cancer
By Chongxiang Xue1, Huijing Dong1, Ying Chen1, Xingyu Lu1, Shuyue Zheng2, Huijuan Cui3Affiliations
doi: 10.29271/jcpsp.2022.06.779ABSTRACT
Lung cancer is the leading cause of cancer-related death worldwide. A meta-analysis was conducted to assess the benefits and risks of neoadjuvant immune checkpoint inhibitors (ICIs) in non-small cell lung cancer (NSCLC). Online databases, including PubMed, Embase, Web of Science, Cochrane Library, and clinicaltrials.gov, were retrospectively and systematically searched for eligible trials from database inception to May 2021. A total of 792 patients from 21 clinical trials were included. For surgical data, the pooled operation rate and R0 resection rate were 92% (95% CI 87-96%) and 97% (95% CI 94-99%). Additionally, neoadjuvant ICIs achieved a major pathological response (MPR) of 39% (95% CI 25-53%), including 25% (95% CI 16-36%) pathological complete response (pCR). With radiological response assessment, the pooled objective response rate (ORR) and disease control rate (DCR) were 44% (95% CI 21-68%) and 88% (95% CI 75-98%), respectively. In terms of safety, the pooled rate of any-grade and grade 3–5 treatment-related adverse effects (TRAEs) were 57% (95% CI 38-76%) and 15% (95% CI 6-28%). Eventually, the study concludes that neoadjuvant ICIs are effective and safe for patients with early-stage NSCLC.
Key Words: Neoadjuvant therapy, Immune checkpoint inhibitors, Non-small cell lung cancer, Meta-analysis.
INTRODUCTION
Lung cancer is the leading cause of cancer-related death worldwide.1 In spite of tremendous advances in local and systemic therapies, cure rates of lung cancer have still increased slowly over the last decades. The estimated median 5-year overall survival (OS) rate was 36-92% for early-stage NSCLC patients, and 13-36% for unresectable stage III NSCLC patients.2 Unfortunately, the initial diagnosis of early-stage lung cancer with localized lesion accounted for less than 39%.3 For these patients, complete surgical resection with curative intent remains the most effective therapy.4-6 And neoadjuvant or adjuvant chemotherapy strategies could improve benefits for patients with bulky or high-risk cancer.5,7
In this era of immunotherapy, ICIs have been proven to be a breakthrough and revolutionized approach to the treatment of advanced NSCLC.8
However, it is unclear whether neoadjuvant ICIs could have similar definite curative effect and controlled toxicity to enhance benefit-risk expectations in early-stage NSCLC. With more available results of related trials on neoadjuvant ICIs, the objective of this meta-analysis was to investigate the efficacy and safety of neoadjuvant ICIs for patients with NSCLC using a well-designed and comparative synthesis.
METHODOLOGY
This study was registered in PROSPERO (International Prospective Register of Systematic Reviews), with the number CRD42020188978. A comprehensive systematic search of PubMed, Embase, Web of Science, Cochrane Library, and clinicaltrials.gov were conducted from database inception to May 2021. Medical Subject Headings (MeSHs) and free text terms were combined with Boolean operators. Details about procedures and methods are described in Figure 1.
Eligible studies had to satisfy all the following inclusion criteria of early-stage NSCLC patients who had received neoadjuvant ICIs and met surgical criteria and the main study outcome directly or indirectly included effects and safety indicators being prospective clinical trials. The most complete and representative studies were included, and when these were equal, the most recent study was included. Any trials with insufficient data and retrospective studies without original data were excluded.
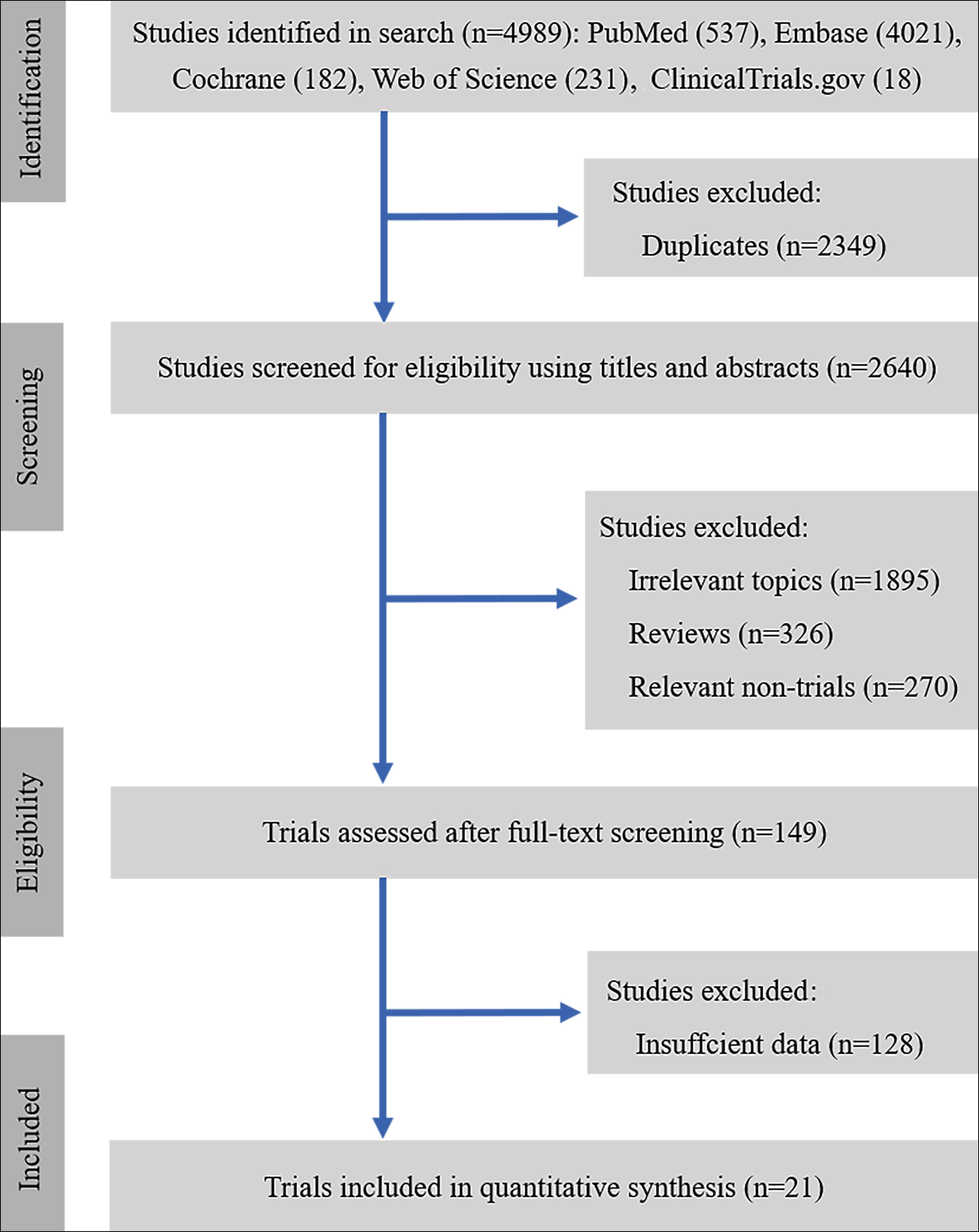 Figure 1: Flow chart for study selection.
Figure 1: Flow chart for study selection.
Two independent investigators (CX and HD) performed study screening and further exploration. Any discrepancies were solved by a discussion with a third author (HC) until consensus was achieved. The variables extracted using a standardized extraction sheet included details of publications, phase of clinical study, participant characteristics, tumor histology and stage, interventions, duration of follow up, and endpoint measures. Feasibility outcomes of interest were defined as operation completion rates, R0 resection rates, pCR, MPR, ORR, DCR. Safety outcomes of interest were defined as TRAEs% of any-grade and grade 3-5.
Newcastle Ottawa Scale (NOS) was applied to assess the quality of included studies and validated independently by two authors (CX, and HD).9 Potential publication bias among the main outcome was assessed by Begg’s test.
STATA software (version 14.0) was used for all statistical analyses and the generation of the forest plots. The pooled estimates were considered statistically significant if the 95% CI did not include 1.0, with a p value of<0.05 (two-sided).10 Statistical heterogeneity across studies was assessed using the I2statistic and forest plots. An I2 value of <50% indicated a low heterogeneity.11 On the assumption that incidence data was close to 0 or 1, log-transformed event rates by the double arcsine method need to be restored to reach the final conclusion. Random-effect models were applied to reduce the influence of inter-study heterogeneity. Subgroup analyses were conducted according to the area, arms, intervention, and immune target.
RESULTS
A total of 792 patients in 3 RCTs and 18 no comparative clinical trials were deemed comparatively high quality and eligible for inclusion.12-32 The characteristics of the included studies were showed in Table 1. But even then, large methodological heterogeneity might have existed for lack of matched groups in these single-arm trials. No publication bias was observed in those studies.
For feasibility data, the primary endpoints included operation completion rate, R0 resection rate, pCR, MPR, ORR and DCR. Just as demonstrated in Figure 2, the pooled operation completion rate and R0 resection rate were 92% (95% CI 87-96%) and 97% (95% CI 94-99%). Additionally, neoadjuvant ICIs achieved an MPR of 39% (95% CI 25-53%) including 25% (95% CI 16-36%) pCR. With radiological response assessment, the pooled ORR and DCR were 44% (95% CI 21-68%) and 88% (95% CI 75-98%), respectively.
For safety data, the pooled result of any grade and grade 3–5 TRAEs% were 57% (95% CI 38-76%) and 15% (95% CI 6-28%), respectively (Figure 3). The rate of surgical complications and operation delay was 10% (95% CI 1-26%) and 3% (95% CI 0-9%), respectively.
The main grade 3–4 AEs were blood or lymphatic system disorders (14%, 95% CI 5-23%), skin reaction (4 %, 95% CI -1-10%), diarrhea/colitis (5%, 95% CI 0-10%). Relatively common toxicities of any grade and grades 3–4 are presented in Table II in detail.
To confirm the variables attributable to heterogeneity, subgroup analysis was performed using the following classification variables: area, arms, intervention, and immune targets types. The final subgroup analysis results are demonstrated in Tables III-VI.
DISCUSSION
At present, neoadjuvant chemotherapy is an acceptable practice to reduce tumor burden for patients with operable and locally advanced NSCLC.7 Nonetheless, the role of neoadjuvant immunotherapy is not defined. Recent preclinical studies have demonstrated that neoadjuvant ICIs could eradicate chances of distant micro-metastases by modulating the breadth and durability of tumor-specific CD8+ T-cell response.33,34 Hence, improved antitumor efficacy help patients acquire better long-term survival.35,36 For inoperable patients, neoadjuvant ICIs strategy, either monotherapy or in combination, could have enormous potential to improve the downstage rate and eventually improve the feasibility of surgery.7,37-39
In the case of neoadjuvant ICIs, assessing tumor response according to conventional radiological criteria may underestimate the pathological response. Pathological response, as an outcome measure, correlates with improved PFS and OS data.7 And immune-related pathologic response criteria (irPRC) have been developed in the completely resected specimen.40-42
 Figure 2: Forest plots depicting surgical data (A. pCR; B. MPR; C. ORR; D. DCR; E. surgical resection rate; F. R0 resection rate).
Figure 2: Forest plots depicting surgical data (A. pCR; B. MPR; C. ORR; D. DCR; E. surgical resection rate; F. R0 resection rate).
In this regard, having standardized and thorough protocols for tumor response assessment after neoadjuvant ICIs to grade responses and guide further data collection is crucial.43-45
To the best of the authors’ knowledge, this is thus far the most comprehensive meta-analysis that has evaluated the feasibility and safety of neoadjuvant ICIs for NSCLC patients. Based on the data we collected, neoadjuvant ICIs achieved relatively improved rates of operation completion and R0 resection inoperable NSCLC patients. According to previous studies,25,46 the median rate of pCR reviewed from 15 trials of neoadjuvant chemotherapy was 4% (range 0–16%), and MPR reported in GLCCG trials was only 7%.
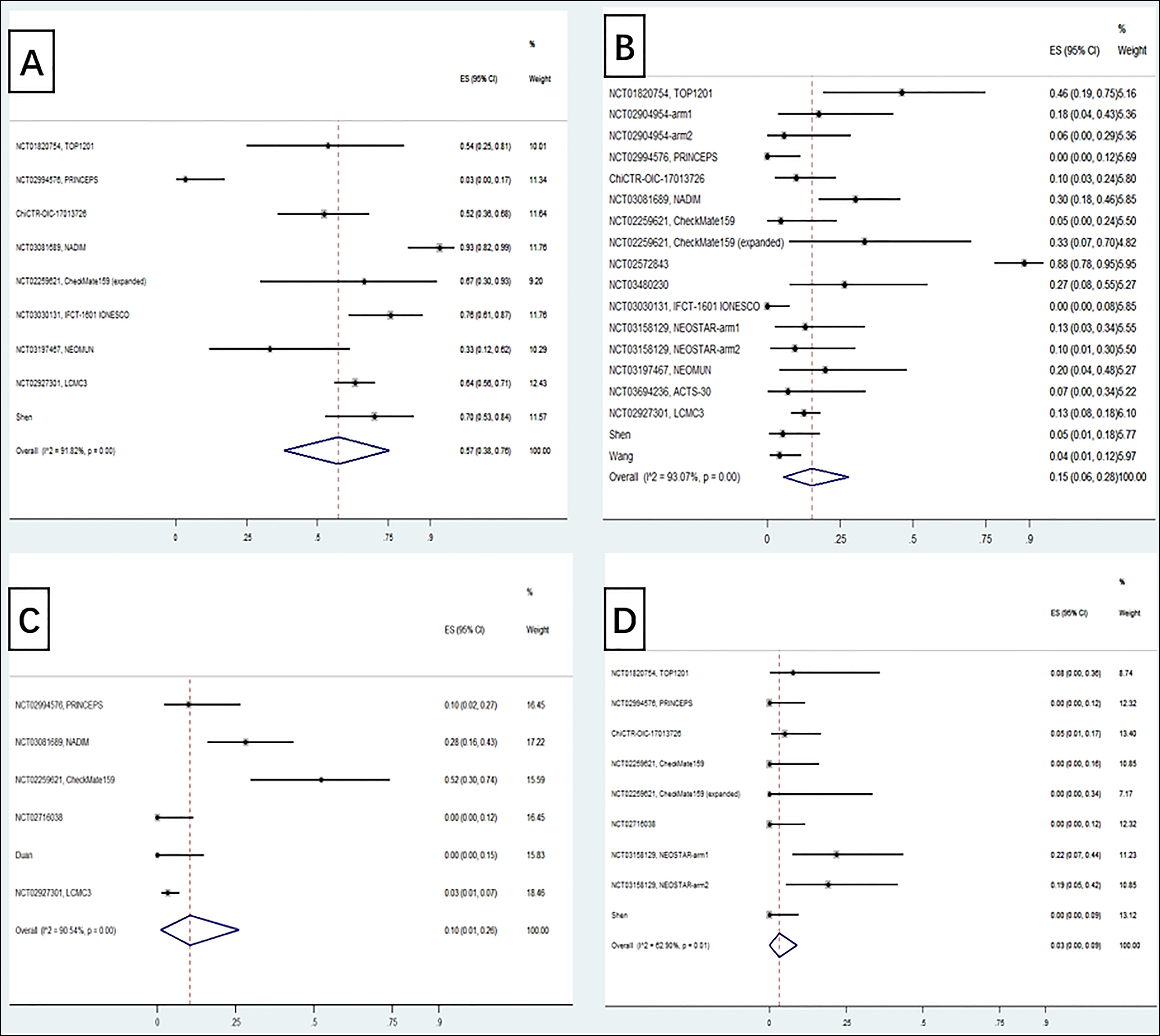 Figure 3: Forest plots depicting safety data (A. any-grade TRAEs; B. G3-5 TRAEs; C. complications; D. operation delay rate).
Figure 3: Forest plots depicting safety data (A. any-grade TRAEs; B. G3-5 TRAEs; C. complications; D. operation delay rate).
By comparison, our meta-analysis indicated higher pCR and MPR in neoadjuvant ICIs settings, no matter immune therapy was used alone or in combination with chemoradiotherapy. The outcome of ORR and DCR data also proved to be favorable, supporting the feasibility and obvious effects of neoadjuvant ICIs.
Another issue that we studied is the occurrence of adverse events associated with neoadjuvant ICIs modality, particularly immune-induced diarrhea/colitis and accompanying pulmonary surgical complications. Translationally, early trials also highlighted the importance of appropriate doses and schedules of neoadjuvant ICIs.47 Currently, determining the optimal timeline and combination strategy of ICIs to achieve the highest cure rates possible requires further investigation.6,34,48 In the trials published to date, immune-related adverse events (irAEs) induced by neoadjuvant ICIs did rarely delay the preplanned surgery, demonstrating that neoadjuvant ICIs is relatively safe.13
As a result, this study might have a meaningful impact on clinical practice, especially in early-stage NSCLC patients being planned to receive ICIs treatment.
This study, nonetheless, still had several potential limitations. Generally, the RCTs with high methodological quality reported better results than the noncomparative studies. To a great extent, a single-arm meta-analysis is subject to subjectivity and heterogeneity. Considerable heterogeneity across studies limited actual evidence-based recommendation grades for neoadjuvant therapy with ICIs for lack of controlled arms.
Table I: The characteristics of the included studies.|
Identification |
Authors |
country |
Phase |
Stage |
Arm |
N |
SCC |
Interventions |
Outcomes |
|
NCT01820754, TOP1201 |
Yang, 2017 |
USA |
II |
IB-IIIA |
1 |
13 |
38.46% |
CT; ipilimumab+ CT |
Mortality, safety, OS |
|
NCT02904954 |
Altorki, 2019 |
USA |
II |
I-IIIA |
1 |
17 |
41.18% |
Durvalumab |
DFS, safety, response rates |
|
USA |
II |
I-IIIA |
2 |
17 |
41.18% |
SBRT+Durvalumab |
DFS, safety, response rates |
||
|
NCT02938624, MK3475-223 |
Bar, 2019 |
Israel |
I |
I-II |
1 |
10 |
60.00% |
Pembrolizumab |
DLT, MPR, response rate |
|
NCT02259621, CheckMate159 |
Bott, 2019 |
USA |
I |
I-IIIA |
1 |
21 |
23.81% |
Nivolumab |
Safety, MPR, ORR, DFS, OS |
|
NCT02818920 |
Ready, 2019 |
USA |
II |
IB-IIIA |
1 |
30 |
56.67% |
Pembrolizumab |
Safety, MPR, pCR |
|
NCT02994576, PRINCEPS |
Besse, 2020 |
France |
II |
IA-IIIA |
1 |
30 |
16.67% |
Atezolizumab |
Safety, MPR, respose rate |
|
ChiCTR-OIC-17013726 |
Gao, 2020 |
China |
I |
IA–IIIB |
1 |
40 |
82.50% |
Sintilimab |
Safety, nonoperation delay rate, MPR, ORR, DFS, OS |
|
NCT03081689, NADIM |
Provencio, 2020 |
Spain |
II |
IIIA |
1 |
46 |
34.78% |
Nivolumab+CT |
PFS, OS, MPR, respose rate, surgical outcome, and safety |
|
NCT02259621, CheckMate159 (expanded) |
Reuss, 2020 |
USA |
II |
IB-IIIA |
1 |
9 |
11.11% |
Nivolumab+ipilimumab; nivolumab |
Safety, MPR |
|
NCT04338620 |
Lei, 2020 |
China |
II |
IIIA/IIIB |
2 |
14 |
NA |
Camrelizumab+CT |
pCR, MPR, ORR, DFS and safety |
|
NCT02572843 |
Rothschild, 2020 |
Switzerland |
II |
IIIA-N2 |
1 |
68 |
NA |
CT +durvalumab |
EFS |
|
NCT02716038 |
Shu, 2020 |
USA |
II |
IB–IIIA |
1 |
30 |
40.00% |
Atezolizumab+CT |
MPR, ORR, DFS, Safety |
|
NCT03480230 |
Tfayli, 2020 |
Lebanon |
II |
IB–III |
1 |
15 |
13.33% |
Avelumab |
ORR, pCR, MPR, PFS, OS |
|
NCT03030131, IFCT-1601 IONESCO |
Wislez, 2020 |
France |
II |
IB-IIIA |
1 |
46 |
41.30% |
Durvalumab |
Safety, OS, DFS, ORR, MPR |
|
NCT03158129, NEOSTAR |
Cascone, 2021 |
USA |
II |
I-IIIA |
1 |
23 |
43.48% |
Nivolumab, 3cycles |
Safety, MPR, OS |
|
USA |
II |
I-IIIA |
2 |
21 |
33.33% |
Nivolumab+ipilimumab; nivolumab |
|||
|
Duan |
Duan, 2021 |
China |
II |
IIA–IIIB |
1 |
23 |
17.39% |
PD-1 (Pembrolizumab/Nivolumab/Sintilimab) + CT |
Safety, MPR, pCR,ORR |
|
NCT03197467, NEOMUN |
Eichhorn, 2021 |
Germany |
II |
IIA-IIIA |
1 |
15 |
13.33% |
Pembrolizumab |
Safety, DFS, OS |
|
NCT03694236, ACTS-30 |
Hong, 2021 |
Korea |
I/II |
III |
1 |
14 |
50.00% |
Durvalumab+CRT |
Safety, ORR, R0%, EFS, OS, pCR, MPR |
|
NCT02927301, LCMC3 |
Lee, 2021 |
USA |
II |
IB-IIIB |
1 |
181 |
38.12% |
Atezolizumab |
MPR and safety |
|
Shen |
Shen, 2021 |
China |
II |
IIB–IIIB |
1 |
37 |
100.00% |
Pembrolizumab+CT |
Safety, MPR, pCR |
|
Wang |
Wang, 2021 |
China |
II |
IIIA |
1 |
72 |
91.67% |
Nivolumab/pembrolizumab/camrelizumab+CT |
Safety、pCR、R0、ORR、complications |
|
CT: Chemotherapy; RT: Radiotherapy; EFS: Event-free survival; pCR: Pathological complete response; MPR: Major pathological response; ORR: Objective response rate; DCR: Disease control rate; DFS: Disease free survival; PFS: Progression free survival; OS: Overall survival. |
|||||||||
Table II: Detailed treatment-related adverse effects of neoadjuvant immunotherapy in NSCLC patients.
|
Types of common TRAEs |
Any-grade AEs |
Grade 3–4 AEs |
||||||
|
Study |
N |
Rate |
95% CI |
Study |
N |
Rate |
95% CI |
|
|
Total |
12 |
542 |
0.60 |
0.39-0.80 |
17 |
708 |
0.19 |
0.08-0.31 |
|
Blood and lymphatic system disorders |
9 |
375 |
0.22 |
0.11-0.33 |
6 |
268 |
0.14 |
0.05-0.23 |
|
Skin reaction |
9 |
316 |
0.37 |
0.16-0.57 |
4 |
142 |
0.04 |
-0.01-0.10 |
|
Diarrhea/colitis |
8 |
217 |
0.18 |
0.10-0.26 |
5 |
88 |
0.05 |
0.00-0.10 |
|
Asthenia |
8 |
278 |
0.36 |
0.23-0.50 |
3 |
141 |
0.02 |
-0.00-0.04 |
|
Dyspnea |
7 |
279 |
0.03 |
0.01-0.05 |
4 |
167 |
0.02 |
0.00-0.04 |
|
Nausea/Vomiting |
7 |
261 |
0.27 |
0.12-0.42 |
2 |
95 |
0.02 |
-0.01-0.04 |
|
Pneumonitis |
6 |
188 |
0.04 |
0.01-0.07 |
4 |
165 |
0.03 |
0.00-0.06 |
|
Liver function test abnormality |
6 |
207 |
0.12 |
0.08-0.16 |
4 |
153 |
0.03 |
0.00-0.05 |
|
Hyperthyroidism |
5 |
109 |
0.07 |
0.02-0.12 |
1 |
15 |
0.13 |
-0.04-0.31 |
|
Lung infection |
2 |
63 |
0.05 |
-0.01-0.10 |
1 |
40 |
0.03 |
-0.02-0.07 |
|
Cardiac disorders |
3 |
130 |
0.07 |
0.03-0.11 |
|
|
|
|
Table III: p-values and Begg’s tests before and after adjustment.
|
Groups |
p-value (unadjusted) |
p-value (adjusted) |
Begg's test (unadjusted) |
Begg's test (adjusted) |
|
pCR |
0.115 |
0.124 |
1.57 |
1.54 |
|
MPR |
0.520 |
0.553 |
-0.64 |
0.59 |
|
operation rate |
0.002 |
0.002 |
-3.09 |
3.05 |
|
R0 resection rate |
0.118 |
0.144 |
-1.56 |
1.46 |
|
ORR |
0.471 |
0.499 |
-0.72 |
0.68 |
|
DCR |
0.186 |
0.213 |
-1.32 |
1.25 |
|
pCR: Pathological complete response; MPR: Major pathological response; ORR: Objective response rate; DCR: Disease control rate. |
||||
|
Group |
pCR |
MPR |
||||||
|
No. of studies |
Rate (95% CI) |
P heterogeneity between groups |
I2 (%) |
No. of studies |
Rate (95% CI) |
P heterogeneity between groups |
I2 (%) |
|
|
Total |
18 |
0.25 (0.16-0.36) |
- |
87.12 |
15 |
0.39 (0.25-0.53) |
- |
90.88 |
|
Area |
|
|
|
|
|
|
|
|
|
North America |
7 |
0.15 (0.07-0.26) |
0.10 |
72.10 |
5 |
0.31 (0.16-0.49) |
0.20 |
84.96 |
|
Europe |
4 |
0.40 (0.13-0.71) |
92.87 |
4 |
0.35 (0.02-0.79) |
96.76 |
||
|
Asia |
7 |
0.29 (0.18-0.41) |
66.25 |
6 |
0.50 (0.38-0.63) |
50.50 |
||
|
Arms |
|
|
|
|
|
|
|
|
|
Single |
16 |
0.24 (0.14-0.36) |
0.63 |
88.17 |
12 |
0.43 (0.27-0.61) |
0.26 |
92.61 |
|
Dual |
2 |
0.32 (0.07-0.63) |
. |
3 |
0.26 (0.08-0.51) |
81.83 |
||
|
Intervention |
|
|
|
|
|
|
|
|
|
IO |
8 |
0.15 (0.05-0.29) |
0.08 |
87.4 |
8 |
0.18 (0.07-0.32) |
0.00 |
83.14 |
|
IO+IO |
2 |
0.33 (0.17-0.52) |
. |
1 |
0.52 (0.30-0.74) |
- |
||
|
IO+CT/RT |
9 |
0.35 (0.24-0.47) |
75.86 |
8 |
0.60 (0.49-0.71) |
63.46 |
||
|
Immune target |
|
|
|
|
|
|
|
|
|
PD-1 |
10 |
0.26 (0.14-0.40) |
0.69 |
84.23 |
9 |
0.44 (0.28-0.61) |
0.42 |
83.01 |
|
PD-L1 |
6 |
0.25 (0.08-0.46) |
92.12 |
6 |
0.31 (0.11-0.55) |
9396 |
||
|
CTLA-4 |
1 |
0.15 (0.02-0.45) |
- |
|
- |
- |
||
|
PD-1+CTLA-4 |
2 |
0.33 (0.17-0.52) |
. |
1 |
0.52 (0.30-0.74) |
- |
||
|
NA: Not available; IO: Immuno-oncology drugs; CT: Chemotherapy; RT: Radiotherapy. |
||||||||
Table V: Subgroup analysis of pathological response rate (pCR and MPR) of neoadjuvant immunotherapy in lung cancer patients.
|
Group |
ORR |
DCR |
||||||
|
No. of studies |
Rate (95% CI) |
P heterogeneity between groups |
I2 (%) |
No. of studies |
Rate (95% CI) |
P heterogeneity between groups |
I2 (%) |
|
|
Total |
17 |
0.44 (0.21-0.68) |
- |
97.34 |
15 |
0.88 (0.75-0.98) |
- |
92.93 |
|
Area |
|
|
|
|
|
|
|
|
|
North America |
7 |
0.35 (0.04-0.75) |
0.24 |
97.10 |
5 |
0.90 (0.81-0.96) |
0.17 |
30.43 |
|
Europe |
5 |
0.29 (0.04-0.65) |
96.14 |
5 |
0.78 (0.34-1.00) |
97.55 |
||
|
Asia |
6 |
0.67 (0.36-0.92) |
94.61 |
5 |
0.97 (0.90-1.00) |
72.08 |
||
|
Arms |
|
|
|
|
|
|
|
|
|
Single |
15 |
0.45 (0.19-0.71) |
0.839 |
97.73 |
14 |
0.88 (0.73-0.98) |
0.97 |
93.86 |
|
Dual |
2 |
0.39 (0.05-0.81) |
. |
1 |
0.89 (0.77-0.97) |
. |
||
|
Intervention |
|
|
|
|
|
|
|
|
|
IO |
8 |
0.19 (0.02-0.45) |
0.00 |
95.53 |
7 |
0.79 (0.45-1.00) |
0.00 |
95.73 |
|
IO+IO |
2 |
0.16 (0.04-0.33) |
. |
2 |
0.78 (0.61-0.92) |
. |
||
|
IO+CT/RT |
8 |
0.77 (0.64-0.87) |
80.48 |
7 |
0.97 (0.92-1.00) |
72.73 |
||
|
Immune target |
|
|
|
|
|
|
|
|
|
PD-1 |
9 |
0.67 (0.42-0.87) |
0.00 |
93.88 |
8 |
0.98 (0.95-1.00) |
0.00 |
35.75 |
|
PD-L1 |
6 |
0.19 (0.00-0.53) |
97.44 |
5 |
0.68 (0.27-0.97) |
96.78 |
||
|
CTLA-4 |
1 |
0.62 (0.32-0.86) |
- |
1 |
0.92 (0.64-1.00) |
- |
||
|
PD-1+CTLA-4 |
2 |
0.16 (0.04-0.33) |
. |
2 |
0.78 (0.61-0.92) |
. |
||
|
ORR: Objective response rate; DCR: Disease control rate. |
||||||||
Table VI: Subgroup analysis of radiological response rate (ORR and DCR) of neoadjuvant immunotherapy in lung cancer patients.
|
Group |
Any-grade TRAEs |
G3-5 TRAEs |
||||||
|
No. of studies |
Rate (95% CI) |
P heterogeneity between groups |
I2 (%) |
No. of studies |
Rate (95% CI) |
P heterogeneity between groups |
I2 (%) |
|
|
Total |
9 |
0.57 (0.38-0.76) |
- |
91.82 |
16 |
0.15 (0.06-0.28) |
- |
93.07 |
|
Area |
|
|
|
|
|
|
|
|
|
North America |
3 |
0.64 (0.57-0.71) |
0.87 |
. |
6 |
0.14 (0.07-0.21) |
0.31 |
42.66 |
|
Europe |
4 |
0.52 (0.09-0.93) |
96.83 |
5 |
0.21 (0.00-0.68) |
97.86 |
||
|
Asia |
2 |
0.61(0.50-0.72) |
. |
5 |
0.08 (0.03-0.14) |
37.84 |
||
|
Arms |
|
|
|
|
|
|
|
|
|
Single |
- |
- |
- |
- |
14 |
0.17 (0.05-0.33) |
0.54 |
94.65 |
|
Dual |
- |
- |
- |
2 |
0.11 (0.05-0.20) |
0.00 |
||
|
Intervention |
|
|
|
|
|
|
|
|
|
IO |
5 |
0.45 (0.20-0.70) |
0.23 |
93.69 |
9 |
0.08 (0.03-0.16) |
0.35 |
70.09 |
|
IO+IO |
3 |
0.67 (0.30-0.93) |
. |
2 |
0.15 (0.03-0.31) |
. |
||
|
IO+CT/RT |
1 |
0.76 (0.50-0.96) |
- |
7 |
0.24 (0.02-0.57) |
96.55 |
||
|
Immune target |
|
|
|
|
|
|
|
|
|
PD-1 |
4 |
0.66 (0.38-0.89) |
0.81 |
90.24 |
7 |
0.11 (0.04-0.20) |
0.07 |
68.01 |
|
PD-L1 |
3 |
0.45 (0.10-0.83) |
. |
7 |
0.15 (0.00-0.43) |
96.66 |
||
|
CTLA-4 |
1 |
0.54 (0.25-0.81) |
- |
1 |
0.46 (0.19-0.75) |
- |
||
|
PD-1+CTLA-4 |
1 |
0.67 (0.30-0.93) |
- |
2 |
0.15 (0.03-0.31) |
. |
||
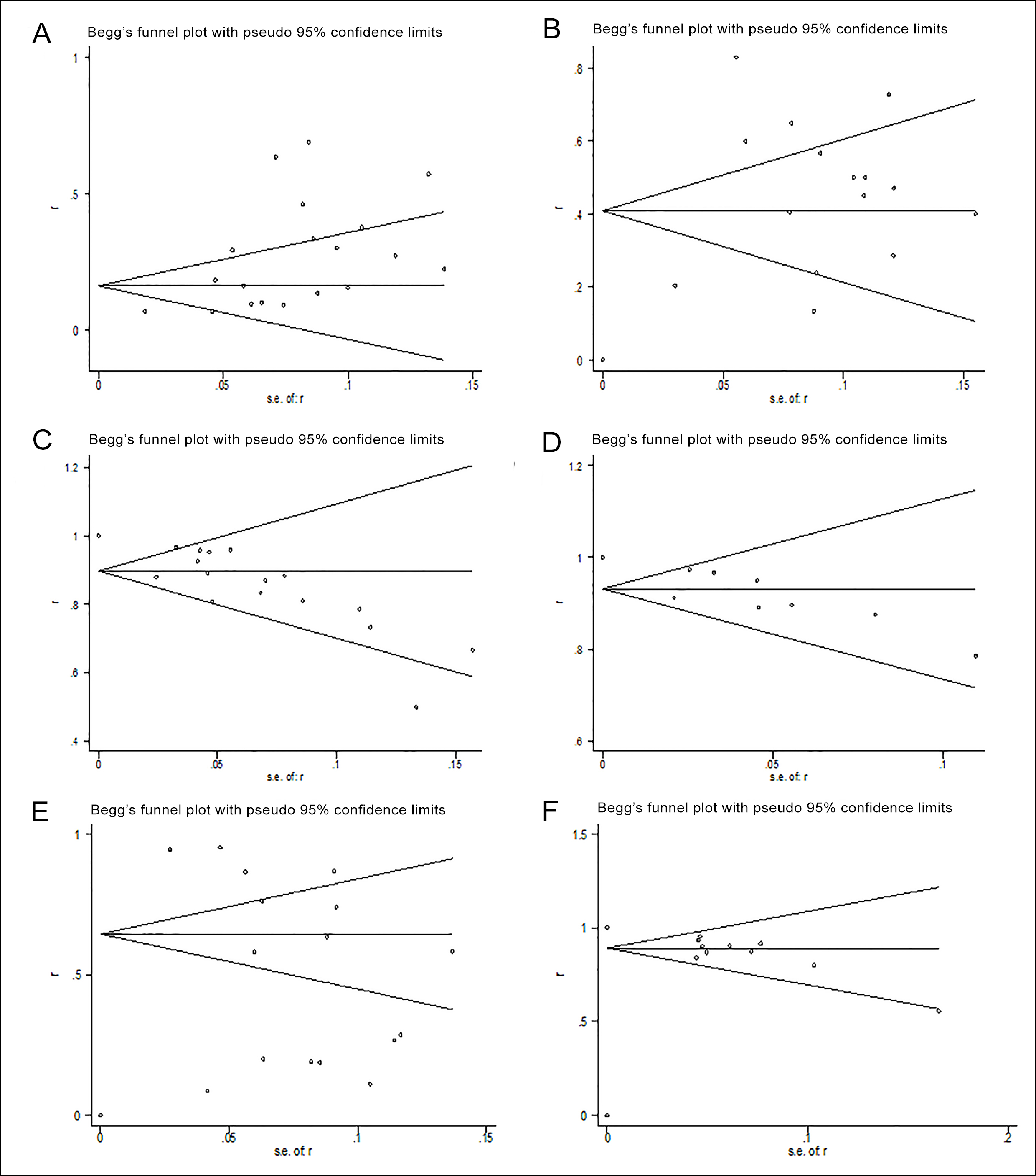 Fig S1: A quantitative assessment of publication bias.
Fig S1: A quantitative assessment of publication bias.
Moreover, a significant percentage of data was not available so that it was hardly able to perform a more complete subgroup analysis. Thus, high-quality pf phase III trials with ICIs in the neoadjuvant setting are eagerly needed.
CONCLUSION
Promising clinical results indicated that neoadjuvant administration of ICIs is effective and safe. With more exciting data observed, it may further change clinical practice for early nonmetastatic NSCLC.
Appendix 1: Search strategies.|
PubMed 537 #1 (((((((((((((((immun*) OR (ipilimumab)) OR (CTLA-4)) OR (pd-1)) OR (Nivolumab)) OR (pembrolizumab)) OR (sintilimab)) OR (camrelizumab)) OR (Cemiplimab)) OR (toripalimab)) OR (tislelizumab)) OR (PD-L1)) OR (Atezolizumab)) OR (durvalumab)) OR (avelumab)) OR (checkpoint inhibitors) #2 (((((lung adenocarcinoma) OR (squamous cell lung carcinoma)) OR (large cell lung cancer)) OR (lung carcinoid)) OR (NSCLC) ) OR (non small cell lung cancer[MeSH Terms]) #3 Neoadjuvant #4 #1 AND #2 AND #3 ClinicalTrials.gov 18 Condition or disease: Non Small Cell Lung Cancer Study type: Interventional studies (clinical trials) Interventional/Treatment: Neoadjuvant immunotherapy Embase 4021 #1 ('lung'/exp OR lung) AND ('adenocarcinoma'/exp OR adenocarcinoma) OR (squamous AND ('cell'/exp OR cell) AND ('lung'/exp OR lung) AND ('carcinoma'/exp OR carcinoma)) OR (large AND ('cell'/exp OR cell) AND ('lung'/exp OR lung) AND ('cancer'/exp OR cancer)) OR (('lung'/exp OR lung) AND ('carcinoid'/exp OR carcinoid)) OR nsclc OR 'non small cell lung cancer'/exp OR 'non small cell lung cancer' #2 immun* OR ipilimumab OR 'ctla 4' OR 'pd 1' OR nivolumab OR pembrolizumab OR sintilimab OR camrelizumab OR cemiplimab OR toripalimab OR tislelizumab OR 'pd l1' OR atezolizumab OR durvalumab OR avelumab OR (checkpoint AND inhibitors) #3 adjuvant #4 #1 AND #2 AND #3 Cochrane Library 182 #1 (((((((((((((((immun*) OR (ipilimumab)) OR (CTLA-4)) OR (pd-1)) OR (Nivolumab)) OR (pembrolizumab)) OR (sintilimab)) OR (camrelizumab)) OR (Cemiplimab)) OR (toripalimab)) OR (tislelizumab)) OR (PD-L1)) OR (Atezolizumab)) OR (durvalumab)) OR (avelumab)) OR (checkpoint inhibitors) #2 (((((lung adenocarcinoma) OR (squamous cell lung carcinoma)) OR (large cell lung cancer)) OR (lung carcinoid)) OR (NSCLC) ) OR (non small cell lung cancer) #3 Neoadjuvant #4 #1 AND #2 AND #3 Web of Science 231 #1 (((((((((((((((immun*) OR (ipilimumab)) OR (CTLA-4)) OR (pd-1)) OR (Nivolumab)) OR (pembrolizumab)) OR (sintilimab)) OR (camrelizumab)) OR (Cemiplimab)) OR (toripalimab)) OR (tislelizumab)) OR (PD-L1)) OR (Atezolizumab)) OR (durvalumab)) OR (avelumab)) OR (checkpoint inhibitors) #2 (((((lung adenocarcinoma) OR (squamous cell lung carcinoma)) OR (large cell lung cancer)) OR (lung carcinoid)) OR (NSCLC) ) OR (non small cell lung cancer) #3 Neoadjuvant #4 #1 AND #2 AND #3 |
Appendix 2: Quality assessment of the included studies Newcastle-Ottawa Scale for assessing the quality of studies in meta-analysis.
|
Study |
Selection |
Comparability |
Outcome/ exposure |
Overall Rating (more stars= lower risk of bias) |
|
Yang (2017) |
★★ |
★ |
★★★ |
★★★★★★ |
|
Provencio (2020) |
★★ |
- |
★★ |
★★★★ |
|
Lee (2021) |
★★ |
- |
★★ |
★★★★ |
|
Bar (2019) |
★★ |
- |
★★ |
★★★★ |
|
Ready (2019) |
★★ |
- |
★★ |
★★★★ |
|
Altorki (2019) |
★★★ |
★★ |
★★ |
★★★★★★★ |
|
Cascone (2021) |
★★★ |
★★ |
★★ |
★★★★★★★ |
|
Gao (2020) |
★★ |
- |
★★★ |
★★★★★ |
|
Besse (2020) |
★★ |
- |
★★ |
★★★★ |
|
Bott (2019) |
★★ |
- |
★★★ |
★★★★★ |
|
Reuss (2020) |
★★ |
- |
★★★ |
★★★★★ |
|
Lei (2020) |
★★★ |
★★ |
★★ |
★★★★★★★ |
|
Rothschild (2020) |
★★ |
- |
★★★ |
★★★★★ |
|
Shu (2020) |
★★ |
- |
★★★ |
★★★★★ |
|
Tfayli (2020) |
★★ |
- |
★★ |
★★★★ |
|
Wislez (2020) |
★★ |
- |
★★ |
★★★★ |
|
Duan (2021) |
★★ |
- |
★★★ |
★★★★★ |
|
Eichhorn (2021) |
★★ |
- |
★★★ |
★★★★★ |
|
Hong (2021) |
★★ |
- |
★★ |
★★★★ |
|
Shen (2021) |
★★ |
- |
★★★ |
★★★★★ |
|
Wang (2021) |
★★ |
- |
★★★ |
★★★★★ |
GRANT SUPPORT OR OTHER SOURCES OF FUNDING:
This study was supported by Capital’s Funds for Health Improvement and Research (No. 2018-2-4065), National Natural Science Foundation of China (No. 81873396), and China-Japan Friendship Hospital (No. 2018-HX-26).
ETHICAL APPROVAL:
The relevant research data was obtained from reliable published sources and free of ethical issues.
PATIENTS' CONSENT:
This study is a systematic review and meta-analysis that does not involve human specimens; therefore, doesn’t involve any related patients consents issues.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
CX: Proposed the hypothesis and idea for this work with all authors contributing to its development and analysis plan.
CX, HD, YC: Literature search and reviewed studies for inclusion.
YC, XL, SZ: Performed the data extraction and checking.
CX, HD, HC: Performed all meta-analyses and wrote the initial draft.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019; 144(8):1941-53. doi:10.1002/ijc.31937.
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11(1):39-51. doi:10. 1016/j.jtho. 2015.09.009.
- Nilssen Y, Brustugun OT, Møller B. Factors associated with emergency-related diagnosis, time to treatment and type of treatment in 5713 lung cancer patients. Eur J Public Health 2021; 31(5):967-974. doi:10.1093/eurpub/ckab071.
- Broderick SR. Adjuvant and neoadjuvant immunotherapy in non-small cell lung cancer. Thorac Surg Clin 2020; 30(2): 215-20 doi:10.1016/j.thorsurg.2020.01.001.
- Chuang JC, Liang Y, Wakelee HA. Neoadjuvant and adjuvant therapy for non-small cell lung cancer. Hematol Oncol Clin North Am 2017; 31(1):31-44. doi:10.1016/j.hoc.2016. 08.011.
- Heigener DF, Reck M. Immune Checkpoint inhibition in non-metastatic non-small cell lung cancer: Chance for cure? Drugs 2019; 79(18):1937-45 doi:10.1007/s40265-019- 01222-w.
- Blumenthal GM, Bunn PA, Chaft JE, McCoach CE, Perez EA, Scagliotti GV, et al. Current status and future perspectives on neoadjuvant therapy in lung cancer. J Thorac Oncol 2018; 13(12):1818-31. doi:10.1016/j.jtho.2018.09.017.
- Deslypere G, Gullentops D, Wauters E, Vansteenkiste J. Immunotherapy in non-metastatic non-small cell lung cancer: Can the benefits of stage IV therapy be translated into earlier stages? Ther Adv Med Oncol 2018; 10: 1758835918772810. doi:10.1177/1758835918772810.
- Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomised studies in meta-analyses. Eur J Epidemiol 2010; 25(9):603-5. doi:10. 1007/s10654-010-9491-z.
- Wang PX, Hong-Tianb LI, Liu JM. Meta-analysis of non-comparative binary outcomes and its solution by stata. J Evidence-Based Med 2012; 12(1):52-5. doi: 10.3969/j.issn.1671- 5144.2012.01.011.
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327 (7414):557-60. doi:10.1136/bmj.327.7414.557.
- Altorki N, Borczuk A, Saxena A, Port J, Stiles B, Lee B, et al. P2.04-92 neoadjuvant durvalumab with or without sub-ablative stereotactic radiotherapy (SBRT) in patients with resectable NSCLC (NCT02904954). J Thoracic Oncol 2019; 14(10):S746. doi:10.1016/j.jtho.2019.08.1597.
- Bar J, Urban D, Ofek E, Ackerstein A, Redinsky I, Golan N, et al. Neoadjuvant pembrolizumab (Pembro) for early stage non-small cell lung cancer (NSCLC): Updated report of a phase i study, MK3475-223. J Clin Oncol 2019; 37. doi:10.1200/JCO.2019.37.15_suppl.8534.
- Besse B, Adam J, Cozic N, Chaput-Gras N, Planchard D, Mezquita L, et al. Neoadjuvant atezolizumab (A) for resectable non-small cell lung cancer (NSCLC): Results from the phase II PRINCEPS trial. Ann Oncol 2020; 31:S794-S5. doi:10.1016/j.annonc.2020.08.1417.
- Bott MJ, Yang SC, Park BJ, Adusumilli PS, Rusch VW, Isbell JM, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2019; 158(1):269-76. doi:10.1016/j.jtcvs.2018.11.124.
- Cascone T, William WN, Weissferdt A, Leung CH, Lin HY, Pataer A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomised NEOSTAR trial. Nat Med 2021; 27(3):504-14. doi:10.1038/s41591-020-01224-2.
- Duan H, Wang T, Luo Z, Tong L, Dong X, Zhang Y, et al. Neoadjuvant programmed cell death protein 1 inhibitors combined with chemotherapy in resectable non-small cell lung cancer: an open-label, multicenter, single-arm study. Transl Lung Cancer Res 2021; 10(2): 1020-8. doi:10. 21037/tlcr-21-130.
- Eichhorn F, Klotz LV, Kriegsmann M, Bischoff H, Schneider MA, Muley T, et al. Neoadjuvant anti-programmed death-1 immunotherapy by pembrolizumab in resectable non-small cell lung cancer: First clinical experience. Lung Cancer 2021; 153:150-7 doi:10.1016/j.lungcan.2021.01.018.
- Gao S, Li N, Gao S, Xue Q, Ying J, Wang S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 2020; 15(5): 816-26. doi:10.1016/j.jtho.2020.01.017.
- Hong MH, Ahn B, Kim HR, Lim SM, Lee S, Park SY, et al. FP03.02 Interim analysis of neoadjuvant chemoradiotherapy and durvalumab for potentially resectable stage III non-small cell lung cancer (NSCLC). J Thoracic Oncol 2021; 16(3):S194-S5. doi:10.1016/j.jtho.2021.01.084.
- Lee J, Chaft J, Nicholas A, Patterson A, Waqar S, Toloza E, et al. PS01.05 Surgical and clinical outcomes with neoadjuvant atezolizumab in resectable stage IB–IIIB NSCLC: LCMC3 trial primary analysis. J Thoracic Oncol 2021; 16(3):S59-S61. doi:10.1016/j.jtho.2021.01.320.
- Lei J, Yan X, Zhao J, Tian F, Lu Q, Jiang T. 62MO A randomised, controlled, multicenter phase II trial of camrelizumab combined with albumin-bound paclitaxel and cisplatin as neoadjuvant treatment in locally advanced NSCLC. Ann Oncol 2020; 31:S1441-S2. doi:10.1016/j.annonc.2020.10.550.
- Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Dómine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020; 21(11): 1413-22. doi:10.1016/S1470-2045(20) 30453-8.
- Ready N, Tong B, Clarke J, Gu L, Wigle D, Dragnev K, et al. P2.04-89 Neoadjuvant pembrolizumab in early stage non-small cell lung cancer (NSCLC): Toxicity, efficacy, and surgical outcomes. J Thoracic Oncol 2019; 14(10): S745 doi:10. 1016/j. jtho.2019.08.1594.
- Reuss JE, Anagnostou V, Cottrell TR, Smith KN, Verde F, Zahurak M, et al. Neoadjuvant nivolumab plus ipilimumab in resectable non-small cell lung cancer. J Immunother Cancer 2020; 8(2): e001282. doi:10.1136/jitc-2020-001282.
- Rothschild SI, Zippelius A, Eboulet EI, Savic Prince S, Betticher D, Bettini A, et al. SAKK 16/14: Anti-PD-L1 antibody durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA (N2) non-small cell lung cancer (NSCLC) – A multicenter single-arm phase II trial. Ann Oncol 2020; 31:S803-S4. doi:10.1016/j.annonc.2020.08.110.
- Shen D, Wang J, Wu J, Chen S, Li J, Liu J, et al. Neoadjuvant pembrolizumab with chemotherapy for the treatment of stage IIB-IIIB resectable lung squamous cell carcinoma. J Thorac Dis 2021; 13(3): 1760-8. doi:10.21037/jtd-21-103.
- Shu CA, Gainor JF, Awad MM, Chiuzan C, Grigg CM, Pabani A, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020; 21(6):786-95. doi:10.1016/S1470-2045(20) 30140-6.
- Tfayli A, Al Assaad M, Fakhri G, Akel R, Atwi H, Ghanem H, et al. Neoadjuvant chemotherapy and Avelumab in early stage resectable nonsmall cell lung cancer. Cancer Med 2020; 9(22):8406-11. doi:10.1002/cam4.3456.
- Wang J, Li J, Cai L, Chen S, Jiang Y. The safety and efficacy of neoadjuvant programmed death 1 inhibitor therapy with surgical resection in stage IIIA non-small cell lung cancer. Ann Transl Med 2021; 9(6):486. doi:10.21037/atm-21-670.
- Wislez M, Mazieres J, Lavole A, Zalcman G, Carre O, Egenod T, et al. Neoadjuvant durvalumab in resectable non-small cell lung cancer (NSCLC): Preliminary results from a multicenter study (IFCT-1601 IONESCO). Ann Oncol 2020; 31:S794 doi:10.1016/j.annonc.2020.08.1416.
- Yang C-FJ, McSherry F, Mayne NR, Wang X, Berry MF, Tong B, et al. Surgical outcomes after neoadjuvant chemotherapy and ipilimumab for non-small cell lung cancer. Ann Thorac Surg 2018; 105(3):924-9. doi:10.1016/j.athoracsur.2017. 09.030.
- Liu J, Blake SJ, Yong MCR, Harjunpää H, Ngiow SF, Takeda K, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 2016; 6(12):1382-99. doi:10.1158/2159- 8290.CD-16- 0577.
- O'Donnell JS, Hoefsmit EP, Smyth MJ, Blank CU, Teng MWL. The promise of neoadjuvant immunotherapy and surgery for cancer treatment. Clin Cancer Res 2019; 25(19):5743-51. doi:10.1158/1078-0432.CCR-18-2641.
- Kawaguchi K, Yokoi K, Niwa H, Ohde Y, Mori S, Okumura S, et al. A prospective, multi-institutional phase II study of induction chemoradiotherapy followed by surgery in patients with non-small cell lung cancer involving the chest wall (CJLSG0801). Lung Cancer 2017; 104:79-84. doi:10.1016/j. lungcan.2016. 12.011.
- Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Sci 2020; 367 (6477):eaax0182. doi:10.1126/science.aax0182.
- Chen Y, Peng X, Zhou Y, Xia K, Zhuang W. Comparing the benefits of chemoradiotherapy and chemotherapy for resectable stage III A/N2 non-small cell lung cancer: A meta-analysis. World J Surg Oncol 2018; 16(1):8. doi:10. 1186/ s12957-018-1313-x.
- Hu H, Zou S, Xu R, Ichiki Y, De Ruysscher D, Yu F, et al. Conversion therapy from N3 unresectable lung adenocarcinoma to radical surgery: A case report. Ann Transl Med 2019; 7(20): 590. doi:10.21037/atm.2019.09.113.
- Yang H, Yao F, Zhao Y, Zhao H. Clinical outcomes of surgery after induction treatment in patients with pathologically proven N2-positive stage III non-small cell lung cancer. J Thorac Dis 2015; 7(9): 1616-23. doi:10.3978/j.issn.2072- 1439.2015.09.07.
- Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: A proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol 2018; 29(8): 1853-60. doi:10.1093/annonc/mdy218.
- Hellmann MD, Chaft JE, William WN, Rusch V, Pisters KMW, Kalhor N, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014; 15(1): e42-e50. doi:10.1016/S1470-2045(13)70334-6.
- Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007; 25(28): 4414-22. doi:10.1200/JCO.2007. 10.6823.
- Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med 2018; 24(11): 1649-54. doi:10.1038/s41591-018-0197-1.
- Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 2019; 25(3): 477-86. doi:10.1038/s41591-018-0337-7.
- Rozeman EA, Sikorska K, Van De Wiel BA, Fanchi LF, Krijgsman O, Van Thienen HV, et al. 30 months relapse-free survival, overall survival, and long-term toxicity update of (neo)adjuvant ipilimumab (ipi) 1 nivolumab (nivo) in macroscopic stage III melanoma (OPACIN trial). Ann Oncol 2018; 29: x43. doi:10.1093/annonc/mdy487.
- Thomas M, Rube C, Hoffknecht P, Macha HN, Freitag L, Linder A, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: A randomised trial in stage III non-small-cell lung cancer. Lancet Oncol 2008; 9(7):636-48. doi:10.1016/S1470-2045(08)70156-6.
- Pelster MS, Amaria RN. Neoadjuvant immunotherapy for locally advanced melanoma. Curr Treat Options Oncol 2020; 21(2):10. doi:10.1007/s11864-020-0700-z.
- Kris MG, Faivre-Finn C, Kordbacheh T, Chaft J, Luo J, Tsao A, et al. Making checkpoint inhibitors part of treatment of patients with locally advanced lung cancers: The time is now. Am Soc Clin Oncol Educ Book 2020; 40:1-12. doi.10. 1200/EDBK_280807.