Maternal Serum Cripto-1 Levels in Pregnancies Complicated with Placenta Previa and Placenta Accreta Spectrum (PAS)
By Zeynep Gedik Ozkose1, Suleyman Cemil Oglak2, Mustafa Behram3, Ozge Ozdemir4, Zuat Acar5, Ismail Ozdemir1Affiliations
doi: 10.29271/jcpsp.2022.12.1570ABSTRACT
Objective: To examine maternal serum Cripto-1 levels in placenta accreta spectrum (PAS) pregnancies and compare them with placenta previa (PP) cases and healthy pregnancies.
Study Design: A prospective case-control study.
Place and Duration of Study: Kanuni Sultan Suleyman Training and Research Hospital, Istanbul, Turkey, from April to September 2021.
Methodology: Sixty singleton pregnant patients with PP complicated with PAS were enrolled, 45 singleton pregnant women with a diagnosis of PP without PAS, and 48 healthy uncomplicated gestational age-matched singleton pregnant women. Cripto-1 levels were determined and evaluated.
Results: The median maternal serum concentrations of Cripto-1 were greater in pregnant women with PAS (3.11 ng/mL) than in the PP (2.52 ng/mL) and the control groups (2.01 ng/mL, p<0.001). Based on the Youden index, a 2.557 ng/mL cut-off value of maternal serum Cripto-1 level had a 76.7% sensitivity and 72.1% specificity to diagnose pregnancies complicated with PAS. A negative and statistically significant linear relationship was found between maternal serum Cripto-1 concentration and the gestational week at birth (r= -0.325, p<0.001). A positive and statistically significant linear relationship was found between maternal serum concentrations of Cripto-1 and maternal length of hospital stay after birth (r= 0.320, p<0.001).
Conclusion: Serum Cripto-1 levels were significantly increased levels in pregnant women suffering from PAS than in pregnant women with PP and uncomplicated healthy pregnancies. Higher expression of Cripto-1 might be a crucial factor in the pathogenesis of PAS.
Key Words: Abnormal placental implantation, placenta accreta spectrum, Cripto-1, Placenta previa.
INTRODUCTION
Placenta previa (PP) is described as the condition where the placenta is implanted into the lower uterine segment and directly overlies the cervical os partially or completely.1 This condition could be complicated by the placenta accreta spectrum (PAS) which is characterised by placental trophoblast invasion towards the myometrium beyond Nitabuch’s layer, as a consequence of a partial or total decidua basalis defect.2
PAS is one of the most hazardous pregnancy complications since excessive bleeding may end in disseminated intravascular coagulation, multisystem organ failure, need for intensive care unit (ICU) admission, hysterectomy, or even death.3 The incidence of PAS has increased over the last decades and is closely associated with the increasing number of cesarean sections and increased rates of PP.2 Damage to the endometrial and myometrial uterine lining during cesarean section or curettage might induce inflammation, which significantly increases the risk for PAS.4 Although the related factors for PAS are clearly identified, aetiology, pathogenesis, and underlying mechanisms remain to be elucidated.5
Besides the traditional theory of inflammatory injury, recent studies reported that the underlying molecular mechanisms of invasive placentation involve a combination of lack of decidua basalis, excessive extravillous trophoblastic invasion, and abnormal vascular remodelling.6 Recently released animal and human studies advanced that numerous regulatory molecules expressed by the trophoblast or decidua play functional roles in managing the trophoblast migration and invasion process, and placental angiogenesis. An imbalance of regulatory signalling between the maternal-fetal interface components can lead to an abnormally deep invasion, resulting in PAS.7
Various studies have shown the participation of various transforming growth factor-beta (TGF-β) superfamily proteins and their associated receptors in a large number of essential reproductive events, including uterine decidualization, and placentation.8 Cripto-1 is a signalling protein that functions as a co-receptor in the TGF-β signalling pathways.9 Cripto-1 has a potent angiogenic activity and enhanced the proliferation, differentiation, and invasion of endothelial cells, proposing that Cripto-1 can act as an oncogenic factor for endothelial cells and directly modulates angiogenesis in these cells, as does vascular endothelial growth factor (VEGF). Cripto-1 is overexpressed in numerous cancer types.10 Also, this protein is greatly expressed at the maternal-fetal interface and serves functional roles in early embryonic development and pregnancy maintenance.8 Several comparisons have been drawn between the PAS microenvironment and tumour behaviour and found that both conditions require the capability of cells to induce angiogenesis and activate invasion.6 Consistent with the participation of Cripto-1 in controlling angiogenesis, it was hypothesised that Cripto-1 may be highly expressed by the trophoblast cells in cases with PAS. The aim of this study was to determine maternal serum Cripto-1 levels in pregnancies complicated with PAS and compare them with PP cases and uncomplicated healthy controls.
METHODOLOGY
In this prospective case-control study, serum samples from 153 pregnant women were examined at Kanuni Sultan Süleyman Training and Research Hospital, from April to September 2021. The study group consisted of 60 cases presenting with a singleton pregnancy diagnosed between 28 and 34 weeks of pregnancy with a placenta previa complicated with PAS. PP without PAS group consisted of 45 singleton pregnant women who were admitted with a diagnosis of PP without PAS between 28 and 34 weeks of gestation. In all cases, the antenatal diagnosis of PP with and without PAS was confirmed intraoperatively. Blood samples from 48 healthy uncomplicated gestational age-matched singleton pregnant women were collected and served as controls. All participants provided signed informed consent. The study project has been approved by the Ethics Committee of the same hospital (2021.03.82).
Cases with multiple pregnancies, congenital fetal malformations, gestational hypertensive disorders, ruptured amniotic membranes, stillbirths, infections, co-existing diseases, including hepatic and renal diseases, and previous cancer diagnosis were excluded. All patients who were admitted during the active labour phase were also excluded.
In all cases, the ultrasound (US) examinations and the diagnosis of abnormal placentation were obtained during the prenatal period by experienced maternal-fetal medicine specialists utilising transabdominal and transvaginal US scans. PP was described as the placental tissue overlying the internal cervical os partially or totally.2 Prenatal PAS disorder diagnosis was according to the following US findings; abnormal placental lacunae, loss of the clear zone, uterovesical hypervascularity, and bladder wall interruption.11
In patients who underwent a hysterectomy, PAS was diagnosed by the histopathological evaluation conducted by a pathologist blinded to the US and surgery outcomes. Placental invasion depth was described as the trophoblastic invasion degree towards the myometrium. All patients were classified based on the maximum placental invasion depth detected since diverse placental invasion degrees might co-exist in the same uterus.12 In cases that did not experience hysterectomy, the existence of PAS was diagnosed based on the FIGO clinical grading system.13
Based on the clinical protocol, pregnant women with persisted PP without PAS in the third trimester were scheduled to undergo a cesarean delivery between 360/7 and 376/7 weeks of gestation. PP patients with suspected PAS disorder were scheduled to undergo a cesarean delivery between 340/7 and 360/7 weeks of pregnancy.14 The gestational age was calculated by the patient-reported last menstrual period, confirmed by the first-trimester US examination.15
The authors retrieved peripheral maternal blood samples by venipuncture. For the PAS group and PP without PAS group patients, blood collection was conducted at the admission time before blood transfusion or steroid administration. At the blood sample collection time, the gestational ages of the cases were matched, and the three groups were comparable regarding gestational age.
Serum concentrations of Cripto-1 were measured using an ELISA kit. Each assay was calibrated using a Cripto-1 standard curve using the manufacturer’s instructions. The variations’ intra- and interassay coefficients were <8% and <10% respectively. The detection limit of the Cripto-1 ELISA kit was 0.22 ng/mL and samples were checked twice.
Kolmogorov-Smirnov and Shapiro-Wilk tests were utilised to test normality. The group factors that may correlate with the outcomes were analysed independently by either One-Way ANOVA or Kruskal Wallis tests. The discrepancies in percentages between groups were compared by utilising Chi-Square or Fisher’s Exact test. Descriptive statistics were utilised to summarise the data and presented it as a count with the percentage of column total for categorical variables, mean ± standard deviation for normally distributed continuous variables, and median (minimum-maximum) for skewed continuous variables. Spearman's correlation coefficient was utilised to investigate the monotonic relationships between two independent variables. To define the diagnostic power of outcome variable Cripto-1, Receiver operating characteristic analysis (ROC) was utilised. An area of 0.50 implies that the variable adds no information. The area under the ROC curve and 95% confidence intervals (CIs) were calculated. In addition, the cut-off point that can be used to distinguish MAP patients by using the variable Cripto-1 was calculated by using the Youden index and summarised with the relevant sensitivity and specificity points. All statistical analyses were done by using IBM SPSS Statistics for Windows, version 25.0. (Armonk, NY: IBM Corp.) and a p-value less than 0.05 is considered statistically significant.
RESULTS
Throughout the course of the study, 49 pregnant women were identified with placenta previa without PAS, 67 pregnant women with placenta previa complicated with PAS, and 48 randomly selected healthy pregnant controls. After the utilisation of the exclusion criteria and excluding patients who were lost to follow-up, 45 patients remained in the placenta previa group, and 60 patients in the PAS group.
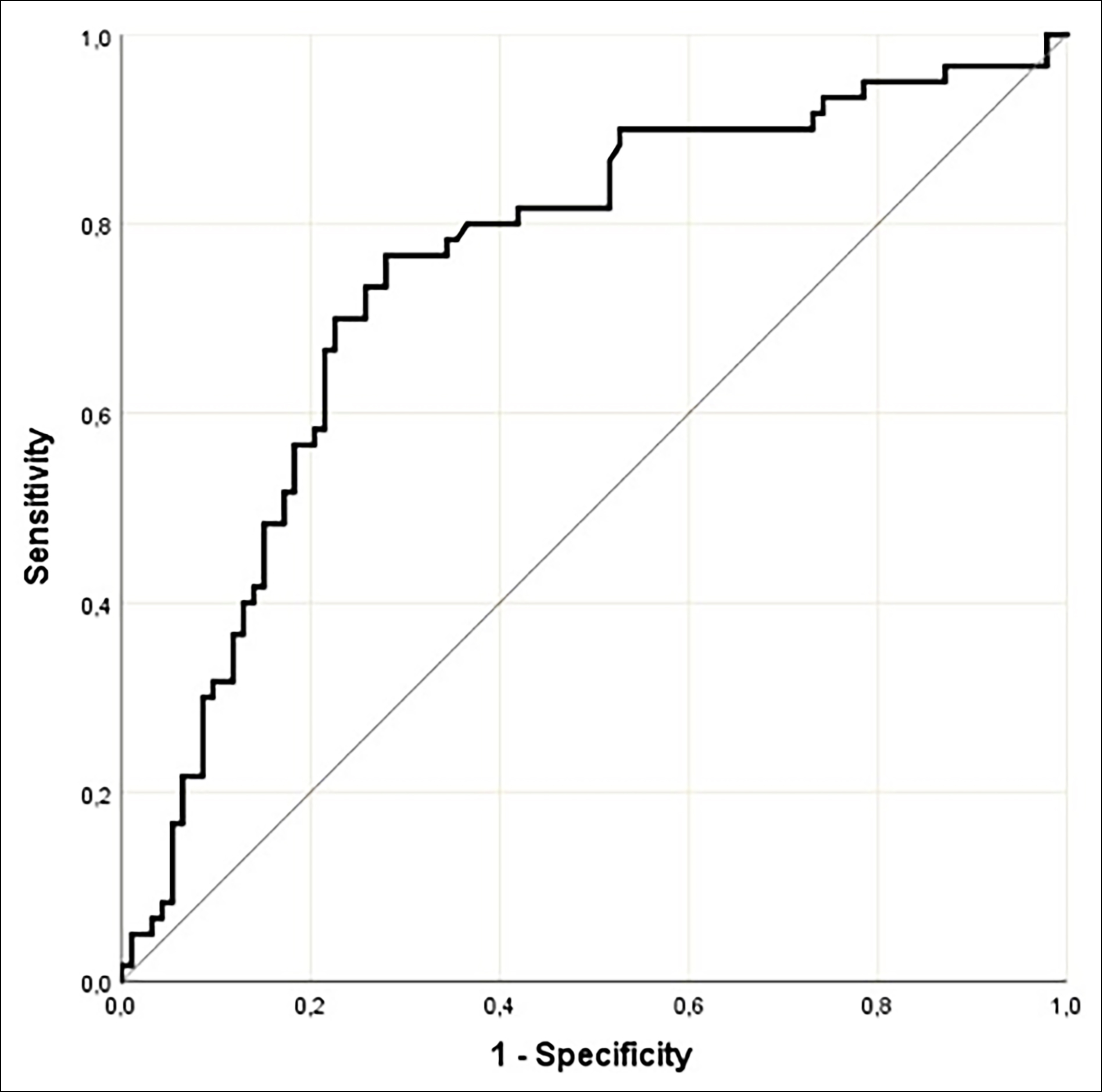 Figure 1: ROC curve for maternal serum cripto-1 concentrations in pregnant women with PAS.
Figure 1: ROC curve for maternal serum cripto-1 concentrations in pregnant women with PAS.
The demographic and clinical characteristics and maternal outcomes of the study cohort are presented in Table I. The three groups were comparable concerning the gestational week at maternal blood sample collection (p=0.874). The median serum concentration of maternal Cripto-1 was significantly higher in the PAS group (3.11 ng/mL, mean= 4.59 ± 4.25 ng/mL) and the placenta previa group (2.52 ng/mL, mean= 3.59 ± 3.27 ng/mL) compared to the control group (2.01 ng/mL, mean= 2.32 ± 1.32 ng/mL, p<0.001). Analysis of pregnancies complicated with PAS compared to pregnancies with placenta previa revealed that the mean maternal serum Cripto-1 concentration was significantly higher in the PAS group (p<0.001). Following the analysis of the area under the ROC curve, the authors revealed that maternal serum Cripto-1 level could be deemed a significant variable for diagnosing PAS (AUC= 0.751 ± 0.041, 95% CI= 0.675-0.817, <0.001, Figure 1). Based on the Youden index, a 2.557 ng/mL cut-off value of maternal serum Cripto-1 level diagnosed pregnancies complicated with PAS with 76.7% sensitivity and 72.1% specificity. A negative and statistically significant linear relationship was observed between maternal serum Cripto-1 concentration and the gestational week at birth (r= -0.325, p<0.001, Figure 2). A positive and statistically significant linear relationship was also detected between maternal serum concentrations of Cripto-1 and maternal length of hospital stay after birth (r= 0.320, p<0.001, Figure 3). The neonatal outcomes of the participants are summarised in Table II. Gestational week at birth and the mean birth weight was significantly lower, and the preterm birth rate was significantly higher in the placenta previa group compared to the control group (p<0.001).
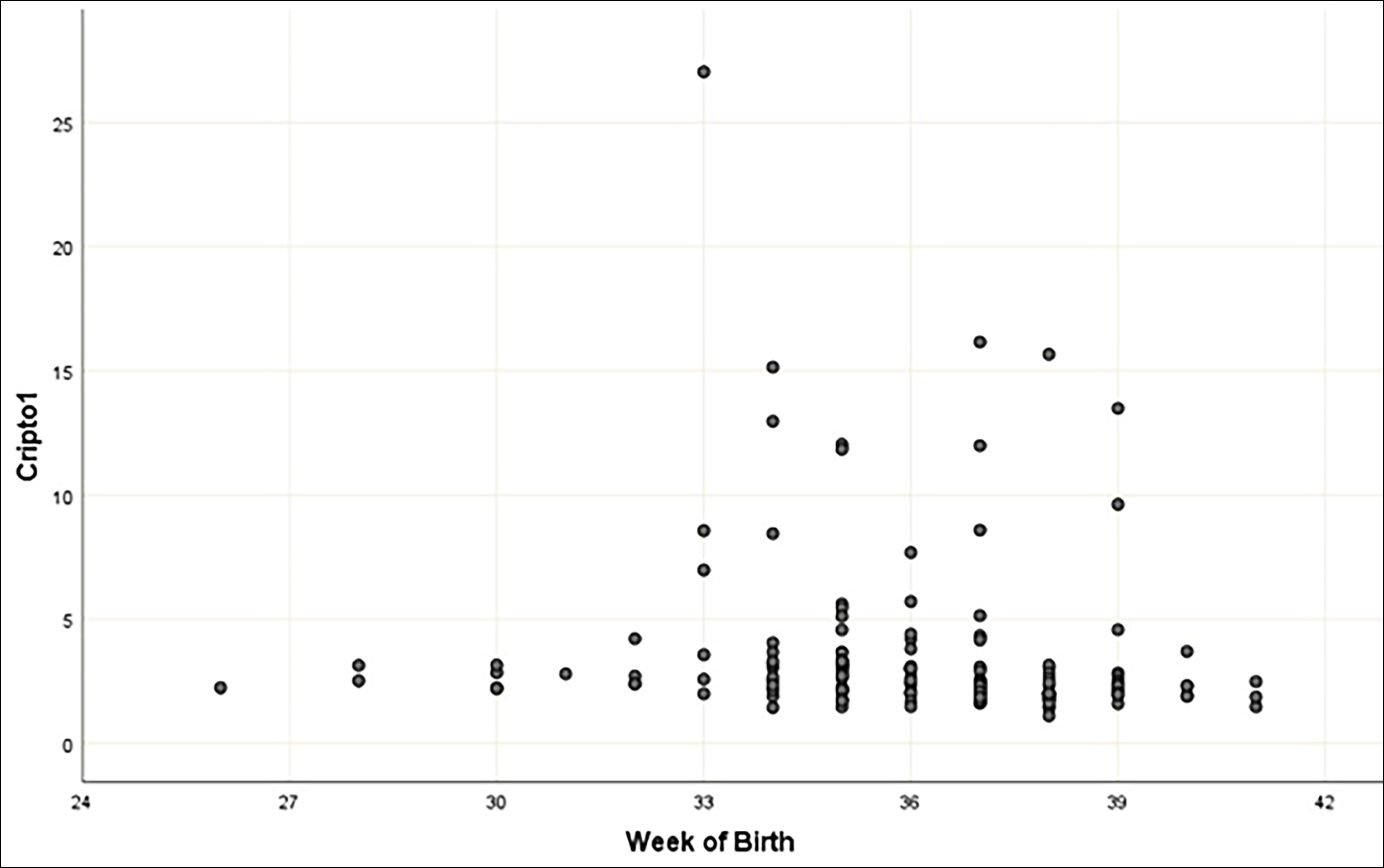 Figure 2: The relationship between maternal serum cripto-1 concentrations and the gestational week at birth according to Spearman's correlation coefficient.
Figure 2: The relationship between maternal serum cripto-1 concentrations and the gestational week at birth according to Spearman's correlation coefficient.
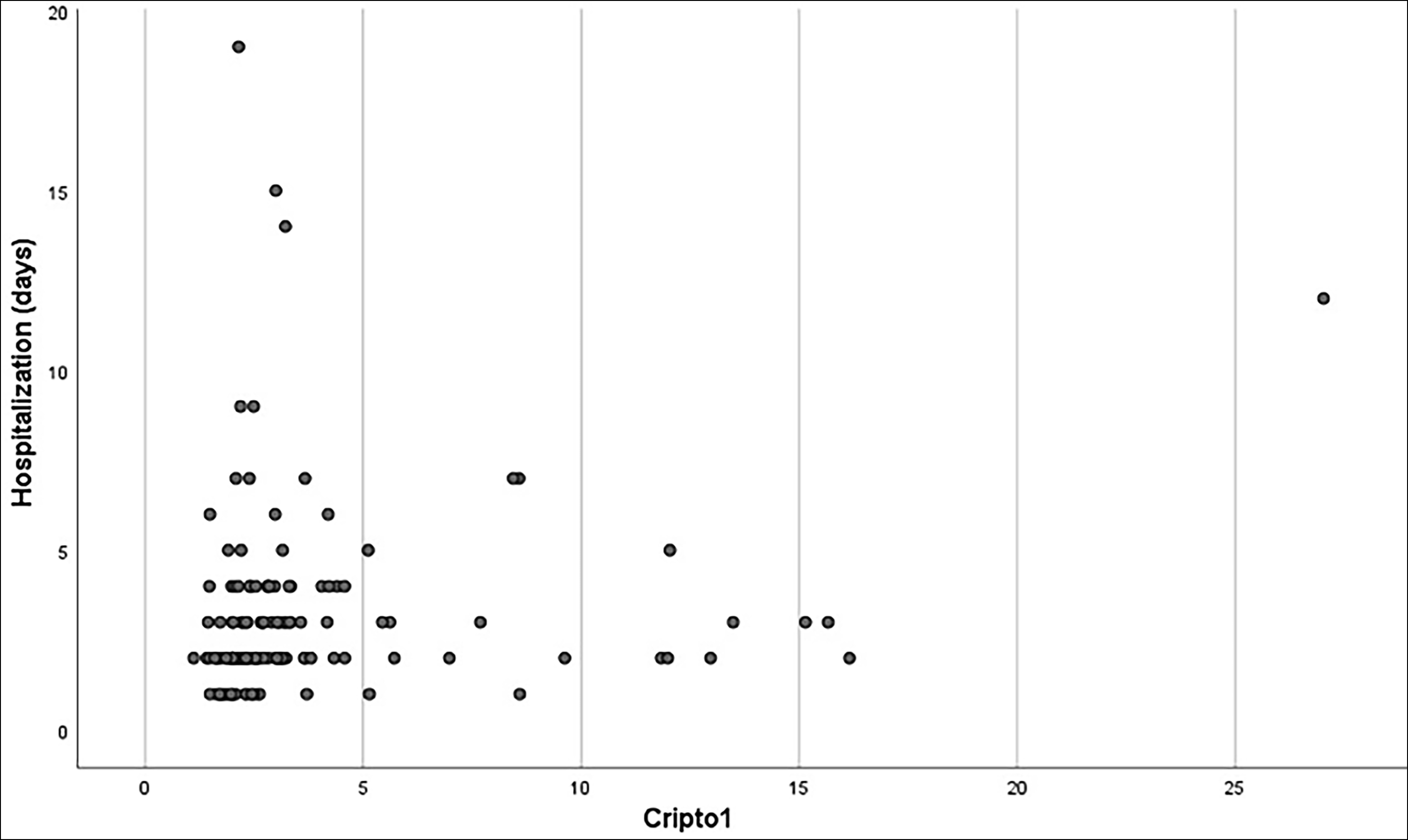 Figure 3: The relationship between maternal serum cripto-1 concentrations and length of hospital stay after birth according to spearman's correlation coefficient.
Figure 3: The relationship between maternal serum cripto-1 concentrations and length of hospital stay after birth according to spearman's correlation coefficient.
DISCUSSION
In the current study, maternal serum Cripto-1 concentrations of pregnant women were assessed in connection with the existence or non-existence of PAS. This study clarified that Cripto-1 showed significantly increased levels in the serum of pregnant women complicated by PAS than pregnant women with PP and individuals with uncomplicated healthy pregnancies. A negative and statistically significant linear relationship was also found between maternal serum Cripto-1 concentration and the gestational week at birth; and a positive and statistically significant linear relationship between maternal serum concentration of Cripto-1 and maternal length of hospital stay after birth.
Table I: Demographic and clinical characteristics and maternal outcomes of the participants.|
|
Control group (n=48) |
Placenta previa group (n=45) |
PAS group (n=60) |
p-value |
|
Maternal age, years |
28.39±6.65a |
31.28±5.73b |
32.78±5.03b |
0.001 |
|
Advanced maternal age (>35 years), n (%) |
10 (20.8%)a |
11 (24.4%)a |
25 (41.7%)b |
0.040 |
|
Gravidity |
3 (1-10)a |
3 (1-12)a |
3 (1-10)b |
<0.001 |
|
Parity |
1 (0-6)a |
1 (0-6)a |
1 (0-6)b |
<0.001 |
|
Woman with the presence of previous miscarriage, n (%) |
17 (35.4%) |
10 (22.2%) |
26 (43.3%) |
0.079 |
|
Number of previous cesarean section |
0 (0-3)a |
0 (0-3)a |
2 (0-6)b |
<0.001 |
|
Smoking, n (%) |
2 (4.2%) |
1 (2.2%) |
1 (1.7%) |
0.707 |
|
Anaemia during pregnancy, n (%) |
17 (35.4%) |
22 (48.9%) |
24 (40.0%) |
0.407 |
|
Gestational week at blood sample collection |
29.47 ± 2.66 |
29.12 ± 2.88 |
29.71 ± 3.07 |
0.874 |
|
Cripto-1, ng/mL |
2.01 (1.11-9.62)a |
2.52 (1.48-16.15)b |
3.11(1.44-27.04)c |
<0.001 |
|
Emergency cesarean delivery, n (%) |
3 (6.3%) |
9 (20.0%) |
8 (13.3%) |
0.144 |
|
Intraoperative massive haemorrhage, n (%) |
0 (0%)a |
7 (15.6%)b |
21 (35.0%) |
<0.001 |
|
Intravenous iron infusion, erythrocyte or blood product transfusion, n (%) |
|
|
|
|
|
Intravenous iron infusion |
0 (0%)a |
7 (15.6%)b |
21 (35.0%)b |
<0.001 |
|
Erythrocyte transfusion |
0 (0%)a |
16 (35.6%)b |
24 (40.0%)b |
<0.001 |
|
Blood product transfusion |
0 (0%)a |
22 (48.9%)b |
40 (66.7%)b |
<0.001 |
|
Maternal intensive care unit admission, n (%) |
0 (0%)a |
0 (0%)a |
4 (6.7%)b |
0.041 |
|
Maternal length of hospital stay, days |
2.54 ± 0.54a |
4.22 ± 2.74b |
5.08 ± 2.81c |
<0.001 |
|
Additional surgical procedures, n (%) |
|
|
|
|
|
Internal iliac artery ligation |
0 (0%)a |
4 (8.9%)a |
21 (35.0%)b |
<0.001 |
|
Uterine lower segment resection |
0 (0%)a |
4 (8.9%)a |
16-26.70b |
<0.001 |
|
Hysterectomy |
0 (0%)a |
3 (6.7%)a |
31 (51.7%)b |
<0.001 |
|
Descritive statistics summarised as; mean ± standard deviation (One Way ANOVA test) and median (minimum-maximum) (Kruskal Wallis test) for scales, n (%) for categoricals (Chi-square or Fisher’s Exact test). Subscripts such as a,b and c used to show differences for means and medians. |
||||
Table II: Neonatal outcomes of the study cohort.
|
|
Control group (n=48) |
Placenta previa group (n=45) |
PAS group (n=60) |
p-value |
|
Gestational week at birth |
38.29 ± 1.44a |
35.45 ± 2.86b |
34.63 ± 1.87c |
<0.001 |
|
Preterm delivery, n (%) |
6 (12.5%)a |
24 (54.5%)b |
52 (86.7%)c |
<0.001 |
|
Fetal gender (male), n (%) |
23 (47.9%) |
21 (46.7%) |
34 (56.7%) |
0.524 |
|
Birth weight, g |
3222.29±586.07a |
2675.33±586.23b |
2565.66±413.71b |
<0.001 |
|
1-min Apgar score |
7 (5-8)a |
7 (2-8)b |
7 (5-8)b |
0.014 |
|
1-min Apgar score <7, n (%) |
15 (31.3%)a |
22 (48.9%)a,b |
35 (58.3%)b |
0.019 |
|
5-min Apgar score |
9(7-9)a |
9(5-9)b |
9(7-9)b |
<0.001 |
|
5-min Apgar score <7, n (%) |
0 (0%)a |
7 (15.6%)b |
5 (8.3%)a,b |
0.020 |
|
Umbilical cord blood pH value |
7.3 (7.2-7.3) |
7.3 (6.7-7.5) |
7.3 (7.2-7.3) |
0.213 |
|
Neonatal intensive care unit admission, n (%) |
7 (14.6%)a |
22 (48.9%)b |
28 (46.7%)b |
<0.001 |
|
Neonatal intensive care unit admission, days |
10 (7-12) |
10 (6-30) |
10 (7-12) |
0.622 |
|
Descritive statistics summarised as; mean ± standard deviation (One Way ANOVA test) and median (minimum-maximum) (Kruskal Wallis test) for scales, n (%) for categoricals (Chi-square or Fisher’s Exact test). Subscripts such as a,b and c used to show differences for means and medians. |
||||
Due to its subtle ultrasound characteristics, PAS remains undiagnosed before delivery in approximately 50-66% of cases, despite the high specificity and sensitivity of US examination.16 Magnetic resonance imaging (MRI) has yet to evidently show a significant improvement in management or pregnancy outcomes. MRI is a high-cost procedure that is infrequently applicable in most low- and medium-income countries. Irrespective of the imaging procedure utilised, prenatal diagnosis of PAS cases remains subjective since the accurate diagnosis depends on the experience of the operator.13 Thus, better detection of PAS cases through the utilisation of biomarkers might aid clinicians additionally to US examination in detecting these cases in the early weeks of gestation and might generate more appropriate planning of scheduled cesarean delivery with an experienced surgery team, which could end in a more favourable outcome for the pregnant woman.16 Several potential biomarkers were examined in pregnant women with PAS, including VEGF, and high mobility group box protein-1.4,17 However, a highly sensitive serum biomarker for abnormal placental invasion remains unknown at the current time.6 Previous evidence indicated that Cripto-1 overexpression can stimulate tumour formation and Cripto-1 is overexpressed in 50-80% of distinct cancer types.10 The anti-Cripto-1 antibody inhibited 50% of the Cripto-1-stimulated neovessel formation, proposing that Cripto-1 may involve in neovascularisation throughout cancer formation.18 In addition to conducting an essential role in cellular transformation, Cripto-1 is essential for embryo attachment, germ layer differentiation, and organ development.9 Cripto-1 is mainly secreted by extravillous trophoblast (EVT) cells. In a healthy pregnancy, Cripto-1 is highly expressed during the early weeks of gestation, while this expression becomes lower in the latter weeks.19,20 During the latter weeks of gestation, EVT cells convert into a non-proliferative form and exhibit a low apoptotic index, suggesting that Cripto-1 contributes to the invasion and migration processes of these cells.21
Bandeira et al. examined the Cripto-1 expression in healthy and creta placentas in the term placental bed utilising immunohistochemistry. They found that the intensity of Cripto-1-reactive cells and Cripto-1 expression was significantly higher in the placenta creta group than in the control group. Also, Cripto-1 expression was significantly correlated with the depth of invasion as Cripto-1 was more abundant in increta and percreta placentas than in the accreta placentas, suggesting the critical importance of EVT cells in the overall degree of placental invasiveness.22 In a recent study by Jiang et al., reverse transcription-PCR was utilised to quantify the Cripto-1 mRNA expression in the placenta while Western blotting was conducted to analyse the placental Cripto-1 protein expression. They found that the PAS group had higher expression levels of Cripto-1 compared with the control group. Furthermore, the amount of expression differed by anatomic sites as expression levels of Cripto-1 at the invasion zone is significantly higher than that in the non-creta zone, indicating the increase of EVT invasion and excessive placental invasion. In the PAS group, the expression levels of Cripto-1 in placental tissues were significantly higher in pregnancies with placental increta than those with placenta accreta. They suggest that the Cripto-1 might disrupt the regulatory mechanisms of epithelial-to-mesenchymal transition (EMT) in EVT cells and stimulate abnormal signal pathways to increase the intensity and duration of EVT invasion.23 In this study, it was shown that the median maternal serum concentrations of Cripto-1 were greater in pregnant women with PAS (3.11 ng/mL) than in the PP group (2.52 ng/mL) and in the control group (2.01 ng/mL, p<0.001). Also, the authors calculated the diagnostic power of the Cripto-1 by using ROC analysis and found it as 0.751 ± 0.041 (95% CI= 0.675-0.817, p <0.001). The Youden index was used to find the cut-off point for Cripto-1 and demonstrated that PAS is classified correctly for maternal serum Cripto-1 values of 2.557 ng/mL and higher with a sensitivity of 76.7% and a specificity of 72.1%. Therefore, that Cripto-1 is a novel identification circulating biomarker in pregnancies complicated with PAS.
The excessive chorionic villi invasion into the deeper layers of the uterine wall in the deficiency or absence of decidualisation frequently coexists with significant and idiosyncratic placental neovascularisation. Accordingly, increased neovessel formations commonly lengthen the radial, arcuate, and parametrial arteries, enlarging the diameters of the vessels, which becomes hardly capable of homeostatic reaction following placental removal or abruption.24 Therefore, pregnancies complicated with PAS were significantly associated with an increased risk of catastrophic bleeding during the antenatal period or cesarean delivery. Maternal haemorrhage is related to an increased risk of maternal morbidity, including the need for additional surgical procedures, blood transfusion, postpartum haemorrhage, and longer hospital length of stay (LOS).3,25 In this study, there was a negative and statistically significant linear relationship between maternal serum Cripto-1 concentrations and the gestational week at birth, and a positive and statistically significant linear relationship between maternal serum concentration of Cripto-1 and maternal length of hospital stay after birth.
The primary strength of this investigation is that, as far as is known, this is the first research until now that has evaluated circulating levels of Cripto-1 in pregnant women with PAS and compared them with PP cases and healthy pregnant women with normal placental implantation. This study’s primary limitation was the absence of proving the enhanced expression of placental Cripto-1 by the histopathological investigation of the placentas after delivery.
CONCLUSION
Maternal serum Cripto-1 concentrations were significantly increased in pregnant women with PAS than in pregnancies with PP and healthy pregnancies with normal placental implantation. Also, a significant negative correlation between maternal serum concentrations of Cripto-1 and the gestational week at birth, and a significant positive correlation between maternal serum Cripto-1 levels and maternal length of hospital stay after birth were detected. Higher expression of Cripto-1 might be a crucial factor in the pathogenesis of PAS.
ETHICAL APPROVAL:
The study project has been approved by the ethics committee of the Kanuni Sultan Suleyman Training and Research Hospital (Approval No. 2021.03.82).
PATIENTS’ CONSENT:
All participants provided signed informed consent.
COMPETING INTEREST:
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors have no conflicts of interest to declare that are relevant to the content of this article.
AUTHORS’ CONTRIBUTION:
ZGO: Surgical and medical practices, concept, design, data collection or processing, analysis or interpretation, writing.
SCO: Concept, design, analysis or interpretation, literature search, writing, and critical review.
MB: Concept, design, and data interpretation.
OO: Concept and literature search.
ZA: Design, analysis or interpretation.
IO: Surgical and medical practices, analysis or interpretation.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Oglak SC, Olmez F, Tunç S. Evaluation of antepartum factors for predicting the risk of emergency cesarean delivery in pregnancies complicated with placenta previa. Ochsner J 2022; 22(2):146-53. doi: 10.31486/toj.21.0138.
- Kollmann M, Gaulhofer J, Lang U, Klaritsch P. Placenta praevia: Incidence, risk factors and outcome. J Matern Fetal Neonatal Med 2016; 29(9):1395-8. doi: 10.3109/14767058. 2015.1049152.
- Silver RM, Branch DW. Placenta accreta spectrum. N Engl J Med 2018; 378(16):1529-1536. doi: doi: 10.1056/NEJMcp1709324.
- Xie H, Qiao P, Lu Y, Li Y, Tang Y, Huang Y, Bao Y, Ying H. Increased expression of high mobility group box protein 1 and vascular endothelial growth factor in placenta previa. Mol Med Rep 2017; 16(6):9051-9. doi: 10.3892/mmr.2017. 7682.
- Liu X, Wang Y, Wu Y, Zeng J, Yuan X, Tong C, Qi H. What we know about placenta accreta spectrum (PAS). Eur J Obstet Gynecol Reprod Biol 2021; 259:81-9. doi: 10.1016/j.ejogrb.2021.02.001.
- Bartels HC, Postle JD, Downey P, Brennan DJ. Placenta accreta spectrum: A review of pathology, molecular biology, and biomarkers. Dis Markers 2018; 2018:1507674. doi: 10.1155/2018/1507674.
- Chantraine F, Blacher S, Berndt S, Palacios-Jaraquemada J, Sarioglu N, Nisolle M, et al. Abnormal vascular architecture at the placental-maternal interface in placenta increta. Am J Obstet Gynecol 2012; 207(3):188.e1-9. doi: 10.1016/j.ajog.2012.06.083.
- Shafiei S, Farah O, Dufort D. Maternal cripto is required for proper uterine decidualisation and peri-implantation uterine remodeling. Biol Reprod 2021; 104(5):1045-57. doi: 10. 1093/biolre/ioab020.
- Bianco C, Rangel MC, Castro NP, Nagaoka T, Rollman K, Gonzales M, et al. Role of Cripto-1 in stem cell maintenance and malignant progression. Am J Pathol 2010; 177(2): 532-40.
- Bianco C, Strizzi L, Ebert A, Chang C, Rehman A, Normanno N, et al. Role of human cripto-1 in tumor angiogenesis. J Natl Cancer Inst 2005; 97(2):132-41. doi: 10.2353/ajpath. 2010.100102.
- Collins SL, Ashcroft A, Braun T, Calda P, Langhoff-Roos J, Morel O, et al. European working group on abnormally invasive placenta (EW-AIP). Proposal for standardised ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol 2016; 47(3):271-5. doi: 10.1002/uog. 14952.
- Cali G, Forlani F, Lees C, Timor-Tritsch I, Palacios-Jaraquemada J, Dall'Asta A, et al. Prenatal ultrasound staging system for placenta accreta spectrum disorders. Ultrasound Obstet Gynecol 2019; 53(6):752-760. doi: 10.1002/uog. 20246.
- Jauniaux E, Bhide A, Kennedy A, Woodward P, Hubinont C, Collins S. FIGO placenta accreta diagnosis and management expert consensus panel. FIGO consensus guidelines on placenta accreta spectrum disorders: Prenatal diagnosis and screening. Int J Gynaecol Obstet 2018; 140(3):274-80. doi: 10.1002/ijgo.12408.
- Gedik Özköse Z, Oğlak SC, Ölmez F. The comparison of maternal and neonatal outcomes between planned and emergency cesarean deliveries in placenta previa patients without placenta accreata spectrum. Ginekol Pol 2022. doi: 10.5603/GP.a2021.0160.
- Oğlak SC, Bademkıran MH, Obut M. Predictor variables in the success of slow-release dinoprostone used for cervical ripening in intrauterine growth restriction pregnancies. J Gynecol Obstet Hum Reprod 2020; 49(6):101739. doi: 10. 1016/j.jogoh.2020.101739.
- Schwickert A, Chantraine F, Ehrlich L, Henrich W, Muallem MZ, Nonnenmacher A, et al. Maternal serum VEGF predicts abnormally ınvasive placenta better than NT-proBNP: A multicenter case-control study. Reprod Sci 2021; 28(2): 361-70. doi: 10.1007/s43032-020-00319-y.
- Wehrum MJ, Buhimschi IA, Salafia C, Thung S, Bahtiyar MO, Werner EF, et al. Accreta complicating complete placenta previa is characterised by reduced systemic levels of vascular endothelial growth factor and by epithelial-to-mesenchymal transition of the invasive trophoblast. Am J Obstet Gynecol 2011; 204(5):411.e1-411.e11. doi: 10.1016/j.ajog. 2010.12.027.
- Adkins HB, Bianco C, Schiffer SG, Rayhorn P, Zafari M, Cheung AE, et al. Antibody blockade of the Cripto CFC domain suppresses tumor cell growth in vivo. J Clin Invest 2003; 112(4):575-87. doi: 10.1172/JCI17788.
- Gershon E, Hadas R, Elbaz M, Booker E, Muchnik M, Kleinjan-Elazary A, et al. Identification of trophectoderm-derived cripto as an essential mediator of embryo ımplantation. Endocrinology 2018; 159(4):1793-1807. doi: 10.1210/en. 2017-03039.
- Borbely AU, Sandri S, Fernandes IR, Prado KM, Cardoso EC, Correa-Silva S, et al. The term basal plate of the human placenta as a source of functional extravillous trophoblast cells. Reprod Biol Endocrinol 2014; 12:7. doi: 10.1186/ 1477-7827-12-7.
- Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr Rev 2005; 26(7): 877-97. doi: 10.1210/er.2005-0003.
- Bandeira CL, Urban Borbely A, Pulcineli Vieira Francisco R, Schultz R, Zugaib M, Bevilacqua E. Tumorigenic factor CRIPTO-1 is immunolocalised in extravillous cytotrophoblast in placenta creta. Biomed Res Int 2014; 2014:892856. doi: 10.1155/2014/892856.
- Jiang L, Wu X, Yan J, Chen R, Han Q, Zhang Q. Expression of cripto-1 in the placenta and its role in placenta accreta and placenta previa. Ginekol Pol 2019; 90(2):86-92. doi: 10. 5603/GP.2019.0015.
- Tseng JJ, Chou MM, Hsieh YT, Wen MC, Ho ES, Hsu SL. Differential expression of vascular endothelial growth factor, placenta growth factor and their receptors in placentae from pregnancies complicated by placenta accreta. Placenta 2006; 27(1):70-8. doi: 10.1016/j.placenta.2004. 12.011.
- Oglak SC, Obut M, Tahaoglu AE, Ugur Demirel N, Kahveci B, Bagli I. A prospective cohort study of shock index as a reliable marker to predict the patient’s need for blood transfusion due to postpartum hemorrhage. Pak J Med Sci 2021; 37(3):863-8. doi: 10.12669/pjms.37.3.3444.