Importance of HALP Score in Breast Cancer and its Diagnostic Value in Predicting Axillary Lymph Node Status
By Ali Duran1, Huseyin Pulat2, Ferhat Cay1, Ugur Topal3Affiliations
doi: 10.29271/jcpsp.2022.06.734ABSTRACT
Objective: To determine the diagnostic value of the haemoglobin, albumin, lymphocyte, and platelet (HALP) score in predicting axillary lymph node (ALN) involvement.
Study Design: Observational study.
Place and Duration of Study: Department of General Surgery, Balıkesir University and Mersin City Hospital, Turkey from January 2016–December 2021.
Methodology: Included in the study were 307 patients who underwent surgical treatment for breast cancer. HALP values were calculated by multiplying the haemoglobin, albumin, and lymphocyte values and dividing the resulting value by the platelet number. The patients were divided in two groups, being those with low HALP (Group 1) and high HALP (Group 2) scores. We examined the potential of the HALP score for the prediction of ALN involvement.
Result: Group 1 had 65 patients and the Group 2 had 242 patients. At the cut-off point, a HALP score of <29.01 predicted the presence of axillary involvement with a sensitivity of 84.33% and a specificity of 26.1%. The sentinel lymph node sampling rate was similar in both groups (12.3% vs. 16.9% p=0.365). The presence of positive lymph nodes in the axilla was higher in group 1 (67.7% vs. 53.3% p=0.038). There was no correlation between HALP score, and the metastatic lymph node and total lymph node count.
Conclusion: The use of HALP score alone for the prediction of axillary lymph node positivity in patients with breast cancer is not advised. In the present study, a low HALP score was associated with aggressive tumour activity, such as advanced tumour and axillary lymph node positivity.
Key Words: Breast cancer, Axillary lymph node involvement, Immunity, Nutrition.
INTRODUCTION
Breast cancer is the most common form of cancer among women and is the second leading cause of cancer-related death after lung cancer.1 Development in the diagnosis and treatment of breast cancer around the world, Turkey included, have contributed significantly to the survival of patients and disease-free survival.
The earliest and most common metastasis site in breast cancer patients are the axillary lymph nodes (ALN), which is an important indicator of progression and clinical staging, and the status of the axillary nodes plays an important role also in the determination of a clinical treatment plan.2
Axillary dissection is the standard approach for patients with pathologically positive nodes. Sentinel lymph node biopsy (SLNB) is performed in patients with negative lymph nodes on palpation or imaging, although both axillary lymph node dissection (ALND) and SLNB are associated with such side effects as infection, lymphedema, pain, infection, and hematoma.3
The determination of the presence of metastasis in the axilla from preoperative data in breast cancer has been the subject of many studies, and several have presented formulas and nomogram programs for the calculation of the relationship between identifiable parameters and axillary metastases.4-6 Clinics make use of different non-invasive imaging methods, including ultrasound, mammography, Magnetic resonance imaging (MRI), and positron emission tomography/computed tomography (PET/CT) for the examination of the state of the axilla based on its morphological and functional abnormalities, although the rate of false negatives when used alone is considered too high. Ultrasound-guided fine-needle aspiration may reveal preoperative lymph node metastasis, although if a negative result is obtained, sampling is still required to confirm the state of the axilla.7,8 There is, therefore, an urgent need for a non-invasive and less expensive approach to the prediction of the status of the axilla in breast cancer patients.
Inflammation and nutrition are important in the formation and progression of cancer.9,10 In recent studies, a new immune nutrition index called HALP has been developed that is calculated from haemoglobin, albumin, lymphocyte and platelet values. The condition of the patient's immune system and nutritional status, its prognostic importance, and its relationship with the postoperative period, have been shown in many types of cancer.11-13 The aim of the present study was to assess the diagnostic value of the HALP score for the prediction of ALN metastasis in breast cancer.
METHODOLOGY
Patients who had been diagnosed with breast cancer and had undergone surgery from January 2016 to December 2021 were included in this observational study. Permission for the study was granted by the Balıkesir University Faculty of Medicine Ethics Committee, dated and numbered 08.09.2021-2021/187. Patient data were obtained from the hospital automation system, the online database of the Ministry of Health (e-pulse), and the hospital archive files. Patients with missing follow-up data and male patients were excluded from the study.
The HALP index score is calculated using the formula: haemoglobin (g/L) × albumin (g/L) × lymphocytes (/L) / platelets (/L). Patient blood samples were taken at the time of admission for surgery, and the tumour types were determined according to World Health Organization criteria. Breast cancer was staged according to the 6th edition of the Cancer Staging Manual published by the American Cancer Joint Committee on Cancer.14 Treatment decisions were made by a multidisciplinary team of surgeons, oncologists and radiation oncologists specialized in breast cancer. Neoadjuvant therapy was administered to patients with locally advanced tumours. The choice of total mastectomy or breast-conserving surgery was made based on tumour size, the multifocality and multicentricity of the tumour, and patient preference. The expression of the oestrogen receptor (ER) and progesterone receptor (PR) was determined through immunohistochemistry. Human epidermal growth factor receptor 2 (HER2) expression was determined based on immunohistochemistry or fluorescent in situ hybridization (FISH), depending on the case.
Cut-off values garnered from Receiver operating characteristic (ROC) curves were analysed. The patients who were included in the study were divided into two groups as Group 1 (low-HALP score) and Group 2 (high-HALP score), depending on the defined cut-off value. The demographic characteristics of the patients, hemogram findings, carbohydrate antigen 15-3 (CA15-3) levels, menstrual pattern, menopausal status, history of oral contraceptive use, number of children, breastfeeding status, family history of cancer, pathological diagnosis, neoadjuvant treatment status, sentinel lymph node sampling, T stage, the number of metastatic and total lymph nodes, ER (%), PR (%), CERBB2, Ki-67 (%), and the surgical and oncological treatment applied to the patients in the postoperative period were compared. In addition, the HALP score at the determined cut-off value predicting an axillary positive lymph node was examined.
IBM SPSS Statistics (Version 23.0. Armonk, NY: IBM Corp.) was used for the statistical analysis of the data. Categorical variables were expressed as numbers and percentages, while continuous data with abnormal distribution were expressed as mean and standard deviation, and data without normal distribution were expressed as minimum-maximum values. A Shapiro-Wilk test was used to determine whether the data were normally distributed, and a Chi-square test and Fisher's exact test were applied for the comparison of categorical variables. An Independent Student's t-test was used for the comparison of groups with normal distribution, and a Mann-Whitney U-test for the comparison of groups without normal distribution. The sensitivity and specificity of the HALP score were calculated based on the axillary lymph node positivity of the patients. The cut-off value was determined from the area under the ROC curve. A Spearman correlation test was used to determine the relationship between lymph node count and HALP score. Statistical significance was accepted as <0.05 in all tests.
RESULTS
A ROC curve was drawn to establish a cut-off HALP score and yielded an area under the curve of 52.5% (p=0.457). At the cut-off point, a HALP score of <29.01 predicted the presence of axillary involvement with a sensitivity of 84.33% and specificity of 26.1%. The ROC analysis is presented in Figure 1.
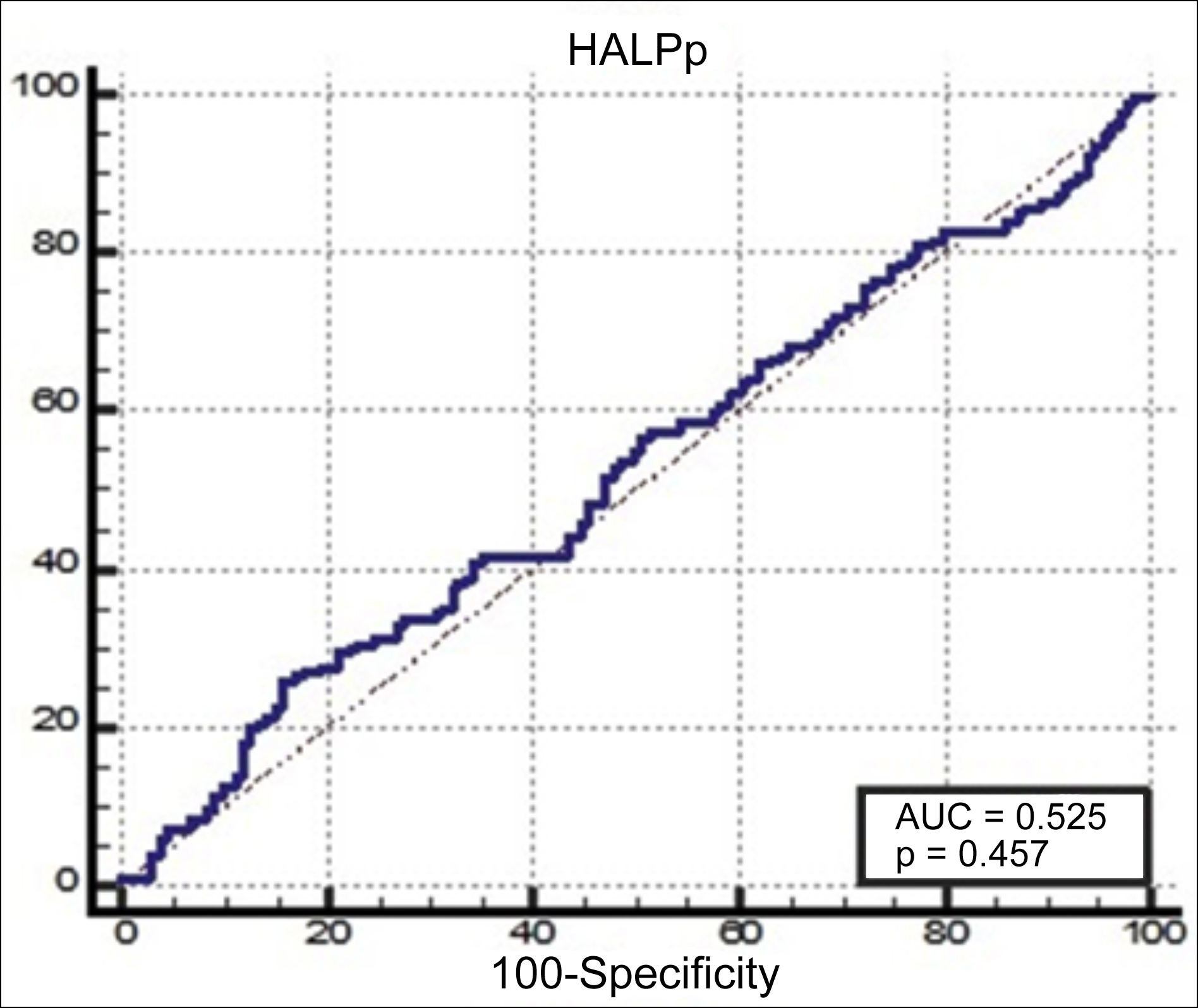 Figure 1: Receiver operating characteristic curve analysis of the HALP score for lymph positive.
Figure 1: Receiver operating characteristic curve analysis of the HALP score for lymph positive.
Group 1 included 65 patients and Group 2 included 242 patients with a mean age of 56.3 and 58.5 (p=0.204), respectively. Menopausal status (p=0.517). Smoking was more common in Group 1 (72.3% vs 59.5% p=0.039), and the Hgb value (12 vs. 13.2 p<0.001), Lymphocyte count (1.51 vs. 2.18 p<0.001) and albumin value (4.37 vs. 4.42 p=0.016) were lower in Group 1 (Table I).
Table I: Demographic data and clinical background.
|
|
Group 1 Low |
Group 2 High |
p-value |
|
65 (%) |
242 (%) |
||
|
Age |
56.3±12.6 |
58.5±12.5 |
0.204a |
|
OCU (months) |
6 (0-36) |
5 (0-36) |
0.625b |
|
Menstrual regularity |
|||
|
Regular |
14 (60.9) |
39 (49.4) |
0.331c |
|
Irregular |
9 (39.1) |
40 (50.6) |
|
|
Menopause status |
|||
|
Negative |
24 (36.9) |
79 (32.6) |
0.517 c |
|
Positive |
41 (63.1) |
163 (67.4) |
|
|
Cigarette smoking |
47 (72.3) |
144 (59.5) |
0.039*. c |
|
Childbirth |
56 (86.2) |
199 (82.2) |
0.454 c |
|
Number of children |
3 (1-6) |
3 (1-6) |
0.387b |
|
Presence of breastfeeding |
56 (86.2) |
199 (82.2) |
0.454 c |
|
Breastfeeding status (years) |
4 (1-10) |
4 (1-12) |
0.534b |
|
Family history of malignancy |
18 (27.7) |
64 (26.4) |
0.840 c |
|
Haemoglobin (gr/dl) |
12 (2.3-14.5) |
13.2 (9.2-15.9) |
<0.001b.** |
|
Platelet counts (mm3 /L) |
319 (58-724) |
270.5 (24-651) |
<0.001b.** |
|
Neutrophils counts (mm3 /L) |
4.27 (0.07-375) |
4.39 (1.6-10.06) |
0.999b |
|
Lymphocytes counts (mm3 /L) |
1.51 (0-4.49) |
2.18 (1.19-4.61) |
<0.001b.** |
|
Monocytes counts (mm3 /L) |
0.34 (0.09-1.98) |
0.36 (0.08-1.62) |
0.086b |
|
Albumin (gr/dl) |
4.37 (0.28-4.8) |
4.42 (2.5-17) |
0.016b.* |
|
ESR (mm/h) |
29 (6-100) |
25 (2-68) |
0.022b.* |
|
CA15.3 (U/ml) |
13.1 (2.4-1917.8) |
13.55 (1.8-314.6) |
0.641b |
|
OCU oral contraceptive use ESR Erythrocyte sedimentation rate *p<0.05, **p<0.001, a: Independent Student t-test=mean±SD, b Mann-Whitney U-test: Med (Min-Maks), c Chi-Square Test and Fisher's Exact Test = n (%). |
|||
Table II: Tumour characteristics.
|
|
Group 1 Low |
Group 2 High |
p-value |
|
65 (%) |
242 (%) |
||
|
Biopsy |
|||
|
Excision |
3 (4.6) |
18 (7.4) |
0.630 c |
|
Incisional |
- |
1 (0.4) |
|
|
Tru-cut |
62 (95.4) |
223 (92.1) |
|
|
Clinical stage |
|||
|
Early |
30 (46.2) |
149 (61.6) |
0.025*.c |
|
Local. Advanced |
35 (53.8) |
93 (38.4) |
|
|
Neoadjuvant therapy |
13 (20) |
39 (16.1) |
0.459 c |
|
Tumour diameter in radiology |
3 (2-18) |
3 (1-15) |
0.182b |
|
Surgical intervention performed |
|||
|
Mastectomy |
- |
4 (1.7) |
NA |
|
Mastectomy+SLNB |
2 (3.1) |
19 (7.9) |
|
|
Mastectomy+SLNB+axillary dissection |
3 (4.6) |
11 (4.5) |
|
|
BCS |
- |
3 (1.2) |
|
|
BCS+axillary dissection |
1 (1.5) |
13 (5.3) |
|
|
BCS+SLNB |
2 (3.1) |
9 (3.7) |
|
|
BCS+SLNB+axillary dissection |
1 (1.5) |
2 (0.8) |
|
|
MRM |
56 (86.2) |
181 (74.8) |
|
|
SLNB: Sentinel lymph node biopsy, BCS: Breast-conserving surgery, MRM: Modified radical mastectomy *p<0.05, **p<0.001, b Mann-Whitney U-test: Med (Min-Maks), c Chi-square Test and Fisher's Exact Test = n (%). |
|||
The patients in Group 1 were more frequently at a locally advanced stage (53.8% vs. 38.4% p=0.025). The recorded radiological tumour diameters and biopsy types were similar between the groups. The most common surgical procedure was Modified radical mastectomy in both groups (86.2% vs. 74.8.6 NA), as shown in Table II.
The SLNB rate was similar between the groups (12.3% vs. 16.9% p=0.365). The presence of positive axillary lymph nodes was more common in Group 1 (67.7% vs. 53.3% p=0.038). The total number of lymph nodes dissected was higher in Group 1 (20 vs. 17.5 p=0.015). The most common pathological type in both groups was invasive ductal carcinoma (72.3% vs. 73.6% NA). The pathological stage and immunohistochemical receptor status of the tumour in the two groups were similar. Data are presented in Table III.
Table III: Pathological Outcomes.
|
|
Group 1 Low |
Group 2 High |
p-value |
|
65 (%) |
242 (%) |
||
|
SLNB |
8 (12.3) |
41 (16.9) |
0.365 c |
|
T Stage |
|||
|
T0 |
- |
3 (1.2) |
0.251 c |
|
T1 |
15 (23.1) |
67 (27.7) |
|
|
T2 |
35 (53.8) |
138 (57) |
|
|
T3 |
10 (15.4) |
18 (7.4) |
|
|
T4 |
5 (7.7) |
12 (5) |
|
|
Tis |
- |
4 (1.7) |
|
|
Presence of axillary lymph nodes |
44 (67.7) |
129 (53.3) |
0.038*.c |
|
CERB B2 category |
|||
|
0 |
52 (80) |
179 (74) |
0.230 c |
|
1 |
7 (10.8) |
17 (7) |
|
|
2 |
2 (3.1) |
10 (4.1) |
|
|
3 |
4 (6.2) |
36 (14.9) |
|
|
Pathologic stage |
|||
|
0 |
- |
5 (2.1) |
0.256 c |
|
1A |
8 (12.3) |
44 (18.2) |
|
|
2A |
15 (23.1) |
78 (32.2) |
|
|
2B |
16 (24.6) |
47 (19.4) |
|
|
3A |
13 (20) |
40 (16.5) |
|
|
3B |
4 (6.2) |
7 (2.9) |
|
|
3C |
9 (13.8) |
21 (8.7) |
|
|
The number of Metastatic lymph node |
4 (1-30) |
3 (1-34) |
0.513b |
|
Total number of lymph nodes |
20 (1-49) |
17.5 (1-50) |
0.015b.* |
|
ER (%) |
90 (0-100) |
80 (0-100) |
0.239b |
|
PR (%) |
30 (0-100) |
40 (0-100) |
0.297b |
|
Ki 67 (%) |
10 (1-80) |
10 (0-95) |
0.944b |
|
Pathological Diagnosis |
|
|
|
|
Adenoid cystic Carcinoma |
- |
1 (0.4) |
NAc |
|
Apocrin |
- |
1 (0.4) |
|
|
Ductal carcinoma in situ |
- |
4 (1.7) |
|
|
Invasive ductal carcinoma |
47 (72.3) |
178 (73.6) |
|
|
Invasive lobuler carcinoma |
5 (7.7) |
15 (6.2) |
|
|
Invasive lobuler carcinoma + paget disease |
- |
1 (0.4) |
|
|
Medullary carcinoma |
1 (1.5) |
11 (4.5) |
|
|
Metaplastic carcinoma |
1 (1.5) |
2 (0.8) |
|
|
Mixed carcinoma |
7 (10.8) |
16 (6.6) |
|
|
Mucinous carcinoma |
2 (3.1) |
9 (3.7) |
|
|
Mucinous carcinoma (colloid. clear) |
- |
1 (0.4) |
|
|
Mucinous carcinoma and neuroendocrine |
1 (1.5) |
- |
|
|
Neuro-endocrine |
- |
1 (0.4) |
|
|
Squamous cell carcinoma |
1 (1.5) |
1 (0.4) |
|
|
Solid papillary carcinoma |
- |
1 (0.4) |
|
|
Post-op treatment |
|||
|
Hormonal therapy |
5 (7.7) |
35 (14.5) |
0.072 c |
|
Hormonal therapy +Radiotherapy |
- |
2 (0.8) |
|
|
Chemoradiotherapy |
52 (80) |
152 (62.8) |
|
|
Chemotherapy |
8 (12.3) |
53 (21.9) |
|
|
SLNB Sentinel lymph node biopsy *p<0.05, **p<0.001b: Mann-Whitney U-test: Med (Min-Maks), c Chi-Square Test and Fisher's Exact Test = n (%). |
|||
No correlation was identified between HALP score, and metastatic lymph node and total lymph node counts (r=0.051; p=0.504 and r=0.056; p=0.333 respectively).
DISCUSSION
The present study, examining the accuracy of the HALP score, calculated in the preoperative period, on the prediction of axillary node positivity in breast cancer, calculated the sensitivity of the HALP score to be 84% and the specificity to be 26%. Axillary node positivity was found to be higher in the low HALP score group, in which smoking was more common and sedimentation was higher. An analysis of the advanced and early stage cancer types in the sample revealed a low HALP score to be associated with advanced-stage tumours.
Axillary lymph node involvement is the leading prognostic marker of disease-free and overall survival in breast cancer.15,16 Consequently, interventions of the axilla in the surgical treatment of breast cancer continues to be a leading area of interest in clinical and surgical oncology.
There are proven factors to be effective in the prediction of axillary node status. These include serum miRNA profile, tumour-infiltrating lymphocyte (TIL) within the tumour microenvironment and new markers such as CXCR4 level,6,17,18 as well as mammography, ultrasound, dynamic contrast (DCE)-MR, Doppler and ultrasound combined with computed tomography-based radiomic nomograms with varying sensitivity specificities.19-21 In addition, such immune composite indices as Neutrophil/lymphocyte ratio have also been shown to be associated with lymph node metastasis.22 There are a number of newly identified biomarkers that are still in the experimental stage and are currently not cost effective. Although radiology-based methods show relatively high sensitivity and specificity, they require the patient to receive additional radiation and contrast material, and accurate results cannot always be obtained with imaging.
The immune and nutritional statuses of patients can be evaluated from simple and reproducible parameters obtained from the blood. The predictive power of the HALP score has proven to be insufficient, although it provides a rational basis for the composite index in the present study, in which the role of the immune system in cancer progression is evaluated, as well as the relationship between nutritional status and cancer.
In the prognosis of colorectal cancers, a low HALP score has been found to be associated with a high mortality rate in literature. Furthermore, 5-year survival in these patients is low.23 In the study by Yalav et al., although no relationship was identified between HALP score and the prognosis of gastric cancer, it was found to be associated with positive lymph nodes and poor clinicopathological features.24 Arıkan et al. reported that in addition to the prognostic importance of the HALP score in pancreatic cancer, it was also able to predict the development of 73% (55.6–87.1) of pancreatic fistulas with a 62.11% (51.6-71.9) specificity (95%CI) and sensitivity (95%CI, AUC= 0.666, p=0.002).11 The evidence provided by the HALP score and the different tumours to which it is applied in studies are increasing, and its prognostic importance has become worthy of note, especially in gastrointestinal system and genitourinary cancers.11,13,23,25 This can be attributed to the fact that nutrition affects this organ system in particular, while its diagnostic and prognostic value in solid tumours, on the other hand, is limited, and has yet to be demonstrated.
The limited variety of operations and the retrospective design of the study are the main limitations of the present study. That said, the authors believe that the study contributes to literature as the first to evaluate the relationship between HALP score and breast cancer. Multicenter studies involving similar patient subgroups will need to be applied to contribute to filling this gap in literature. The available data are insufficient for an assessment of the diagnostic value of the HALP score.
CONCLUSION
HALP score – an immunonutritional composite index was shown to be unable to predict axillary lymph node positivity in breast cancer. This result may be attributed to the large cohort of patients who were managed differently in our study. In this study of a large patient cohort, a low HALP score was found to be associated with aggressive tumour activity, such as advanced tumour and axillary lymph node positivity.
ETHICAL APPROVAL:
The study was approved by Balıkesir University’s Non-Invasive Clinical Research Ethics Committee (approval No. 2021/187, date: 08.09.2021).
PATIENTS’ CONSENT:
Given the retrospective archive search approach in the study, informed consent was not obtained from the patients.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTIONS:
AD: Wrote the paper, data acquisition and analysis, interpretation, critical revision, drafting, final approval.
HP, FC: Conception and design, ınterpretation, final approval.
UT: Wrote the paper ,critical revision, final approval.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Estimated age-standardised incidence and mortality rates (World) in 2018, worldwide, both sexes, all ages, Globocan 2018. doi: 10.1053/j.gastro.2020.10.007
- He M, Zhang JX, Jiang YZ, Chen YL, Yang HY, Tang LC, et al. The lymph node ratio as an independent prognostic factor for node-positive triple-negative breast cancer. Oncotarget 2017; 8(27):44870-80. doi: 10.18632/oncotarget.17413.
- Wernicke AG, Goodman RL, Turner BC, Komarnicky LT, Curran WJ, Christos PJ, et al. A 10-year follow-up of treatment outcomes in patients with early stage breast cancer and clinically negative axillary nodes treated with tangential breast irradiation following sentinel lymph node dissection ornaxillary clearance. Breast Cancer Res Treat 2011; 125(3):893-902. doi: 10.1007/s10549-010-1167-6.
- Han L, Zhu Y, Liu Z, Yu T, He C, Jiang W, et al. Radiomic nomogram for prediction of axillary lymph node metastasis in breast cancer. Eur Radiol 2019; 29:3820-9. doi: 10.1007/s00330-018-5981-2.
- Ding J, Jiang L, Wu W. Predictive value of clinicopathological characteristics for sentinel lymph node metastasis in early breast cancer. Med Sci Monit 2017; 23:4102-8 . doi: 10.12659/msm.902795.
- Takada K, Kashiwagi S, Asano Y, Goto W, Kouhashi R, Yabumoto A, et al. Prediction of lymph node metastasis by tumour-infiltrating lymphocytes in T1 breast cancer. BMC Cancer 2020; 20(1):1-13. doi: 10.1186/s12885-020-07101-y.
- Van Nijnatten TJA, Ploume EH, Schipper RJ, Goorts B, Andriessen EH, Vanwetswinkel S, et al. Routine use of standard breast MRI compared to axillary ultrasound for differentiating between no, limited and advanced axillary nodal disease in newly diagnosed breast cancer patients. Eur J Radiol 2016; 85(12):2288-94. doi: 10.1016/j.ejrad.2016. 10.030.
- Tan H, Wu Y, Bao F, Zhou J, Wan J, Tian J, et al. Mammography-based radiomics nomogram: A potential biomarker to predict axillary lymph node metastasis in breast cancer. Br J Radiol 2020; 93(1111):20191019. doi: 10.1259/bjr. 20191019.
- McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009; 12(3):223-6. doi: 10.1097/MCO.0b013e328 32a7902.
- Mantzorou M, Koutelidakis A, Theocharis S, Gaginis C. Clinical value of nutritional status in cancer: What is its impact and how it affects disease progression and prognosis? Nutr Cancer 2017; 69(8):1151-76. .doi: 10.1080/01635581. 2017.1367947.
- Arıkan TB, Sozuer E, Topal U, Lale A, Yilmaz AZ. The value and prognostic significance of preoperative haemoglobin and albumin levels, and the lymphocyte and platelet count (HALP) scores in predicting pancreatic fistula in patients undergoing pancreaticoduodenectomy due to periampullary region tumours. Acta Med 2021; 37:1341.doi: 10.19193/0393-6384_2021_2_206.
- Yalav O, Topal U, Unal AG, Eray IC. Prognostic significance of preoperative haemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients undergoing curative resection for colorectal cancer. Ann Ital Chir 2021; 92:283-92.
- Kaya C, Caliskan S, Sungur M, Aydın C. HALP score and albumin levels in men with prostate cancer and benign prostate hyperplasia. Int J Clin Pract 2021; 75(3):e13766. doi: 10.1111/ijcp.13766.
- Byrd DR, Carducci MA, Compton CC, Fritz AG, Greene F. AJCC Cancer Staging Manual 2010; 7:97-100.
- Andersson Y, Bergkvist L, Frisell J, de Boniface J. Long-term breast cancer survival in relation to the metastatic tumour burden in axillary lymph nodes. Breast Can Res Treat 2018; 171(2):359-69. doi: 10.1007/s10549-018-4820-0.
- Kuijt GP, Voogd AC, van de Poll-Franse LV, Scheijmans LJ, van Beek MW, Roumen RM. The prognostic significance of axillary lymph-node micrometastases in breast cancer patients. Eur J Surg Oncol 2005; 31(5):500-5. doi: 10.1016/j. ejso.2005.01.001.
- Shiino S, Matsuzak J, Shimomura A, Kawauchi J, Takizawa S, Sakamoto H, et al. Serum miRNA–based prediction of axillary lymph node metastasis in breast cancer. Clin Can Res 2019; 25(6):1817-27. doi: 10.1158/1078-0432.CCR-18- 1414.
- Hiller D, Chu QD. CXCR4 and axillary lymph nodes: Review of a potential biomarker for breast cancer metastasis. Int J Breast Cancer 2011; 2011:420981.doi: 10.4061/2011/ 420981.
- Mao N, Dai Y, Lin F, Ma H, Duan S, Xie H, et al. Radiomics nomogram of DCE-MRI for the prediction of axillary lymph node metastasis in breast cancer. Front Oncol 2020; 10:2305. doi: 10.3389/fonc.2020.541849.
- Yang J, Wang T, Yang L, Wang Y, Li H, Zhou X, et al. Preoperative prediction of axillary lymph node metastasis in breast cancer using mammography-based radiomics method. Sci Rep 2019; 9(1):1-11. doi: 10.1038/s41598- 019-40831-z.
- Zhou J, Zhang B, Dong Y, Yu L, Gao T, Wang, Z. Value on the diagnosis of axillary lymph node metastasis in breast cancer by color Doppler ultrasound combined with computed tomography. Statistics 2020; 7:8.
- Ozyalvacli G, Yesil C, Kargi E, Kizildag B, Kilitci A, Yilmaz F. Diagnostic and prognostic importance of the neutrophil lymphocyte ratio in breast cancer. Asian Pacific J Can Prevention 2015; 15(23):10363-6. doi: 10.7314/apjcp. 2014.15.23.10363.
- Jiang H, Li H, Li A, Tang E, Xu D, Chen Y, et al. Preoperative combined haemoglobin, albumin, lymphocyte and platelet levels predict survival in patients with locally advanced colorectal cancer. Oncotarget 2016; 7(44):72076. doi: 10.18632/oncotarget.12271.
- Yalav O, Topal U, Ünal AG, Sarıtaş AG. Clinical value of hemoglobin and albumin levels and lymphocyte and platelet count (HALP) combination in predicting postoperative complications, lymph node positivity and prognosis in gastric cancer patients who underwent curative surgical resection. Cyprus J Med Sci 2020; 5(2):145-52 .doi: 10.5152/cjms.2020.1747.
- Peng D, Zhang CJ, Tang Q, Zhang L, Yang KW, Yu XT. Prognostic significance of the combination of preoperative haemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients with renal cell carcinoma after nephrectomy. BMC Urol 2018; 18(1):1-8. doi: 10. 1186/s12894-018-0333-8.