Impact of Urinary pH on the Efficacy of a Postoperative Single Instillation of Mitomycin-C
By Cagdas Senel1, Ahmet Asfuroglu2, Ibrahim Can Aykanat3, Melih Balci2, Yilmaz Aslan2, Altug Tuncel2Affiliations
doi: 10.29271/jcpsp.2022.06.768ABSTRACT
Objective: To assess the effect of the urinary pH value on the efficacy of a postoperative single instillation of mitomycin-C.
Study Design: A descriptive study.
Place and Duration of Study: Department of Urology, Ankara Numune Training and Research Hospital, Ankara, Turkey from 2011 to 2016.
Methodology: Patients newly diagnosed with low-risk non-muscle invasive bladder cancer and given a postoperative single instillation of mitomycin-C were retrospectively reviewed. The demographic data and pre-instillation urinary pH values of the patients were recorded. All patients included in the study (n=117) were followed up for five years. The primary outcome was the time to the first recurrence. The patients were divided into two groups: Group 1 consisted of 87 patients with no recurrence and Group 2 comprised 30 patients that had recurrence during the follow-up.
Results: The mean pre-instillation urinary pH value was significantly lower in Group 2 than in Group 1 (5.89 vs. 5.37, p <0.001). The receiver operating characteristic analysis revealed that the cut-off value of urinary pH in predicting recurrence was 5.25. The patients with a urinary pH value of 5.25 or greater had significantly higher recurrence-free survival rates.
Conclusions: The patients with higher urinary pH before a single instillation of mitomycin-C had better recurrence-free survival.
Key Words: Bladder cancer, Mitomycin-C, Single instillation, Urinary pH.
INTRODUCTION
Bladder cancer (BC) accounts for 3% of all newly diagnosed cancer cases and 2.1% of all cancer-related deaths.1 Approximately 75% of BC cases present with non-muscle invasive bladder cancer (NMIBC) confined to the mucosa (stage Ta, carcinoma in situ) or submucosa (stage T1).2
Although the complete transurethral resection of bladder tumor (TUR-BT) is the main treatment for NMIBC, patients with NMIBC have over 40% recurrence rates.3,4
A postoperative immediate single instillation (SI) of chemotherapeutic agents prevents the implantation of floating tumor cells into the bladder urothelium following TUR-BT and destructs residual tumor cells at the resection site and/or on small unnoticed tumors.2
It is known that the SI of chemotherapeutic agents results in lower recurrence rates among patients with NMIBC.5,6 For this purpose, several agents can be used as SI, including mitomycin-C (MMC), epirubicin, and pirarubicin that have been demonstrated to be beneficial.6 Although the SI of intravesical chemotherapeutic agents has been reported to prolong recurrence-free survival (RFS) irrespective of the drug used,7 MMC is one of the most commonly used intravesical chemotherapeutic agents for SI8-10 and has proven its efficacy in reducing recurrence rates. Currently, increased drug concentration, decreased urine production, alkaline urine, and two-hour application time are widely accepted recommendations for an optimized regimen of intravesical MMC treatment.9
The aim of this study was to determine the impact of the urinary pH value on the recurrence rates of patients with low-risk NMIBC, who were given an immediate postoperative SI of intravesical MMC.
METHODOLOGY
The current study was conducted at the Department of Urology, Ankara Numune Training and Research Hospital, Ankara, Turkey from 2011 to 2016. The study was approved by the local Ethics Committee (Ethics Committee Ruling number: E1-21-1518) and conducted in accordance with the principles of the Declaration of Helsinki. As a result of the retrospective review of the clinic’s medical reports between 2011 and 2016, the authors identified 227 patients newly diagnosed with low-risk NMIBC (primary, solitary, TaG1, <3 cm tumor, and no carcinoma in situ) followed up for at least five years.
A total of 117 patients were included in the study after excluding 110 patients that were not administered MMC due to hematuria or suspicion of bladder perforation or inability to tolerate instillation for two hours. All the 117 patients were given 40 mg MMC diluted with 20 ml sterile saline via a urethral catheter indwelled during the operation within six hours following TUR-BT. The duration of instillation was two hours. All the histopathological specimens were examined with standard procedures. Tumor grade was determined according to the 1973 World Health Organisation grading system, and tumor stage was determined according to the 2009 TNM classification.
Written informed consent was obtained from all the patients before surgery and the intravesical instillation of the chemotherapeutic agent. The patients’ demographic data, urinary pH values, and risk factors (smoking and occupational exposure) were recorded.
Urine pH measurements were performed using the Cobas® 6500 urine analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Three indicators, namely methyl red, bromothymol blue, and phenolphthalein were used to measure the urine pH level. All the patients were followed up with cystoscopy and urinary cytology in the third month after TUR-BT, and if their findings were negative, the evaluations were undertaken again in the ninth month, followed by annually for at least five years. The patients with macroscopic hematuria also underwent cystoscopy in addition to this follow-up schedule.
The authors’ primary outcome was the time of the first recurrence. According to the follow-up data, the sample was divided into two groups: The patients with no tumor recurrence were defined as Group 1 (n = 87) while those that developed tumor recurrence as Group 2 (n = 30).
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) v. 16.0 for Windows (SPSS Inc. Chicago, IL, USA). All numeric variables that we tested were normally distributed. Therefore, the t-test was used to compare the numeric variables between the groups, and the chi-square test was used to compare categorical variables. Numeric variables were presented as mean ± standard deviation while categorical variables were as number (% percentage). The receiver operating characteristic (ROC) analysis was performed to determine the cut-off value of urinary pH to predict recurrence. The Kaplan-Meier survival analysis was used to determine the RFS rates and the log-rank test was used for comparisons. A p-value of <0.05 was considered as statistically significant.
RESULTS
The mean age of the patients was 60.6 ± 12.6 years. Of the 117 patients, 11.1% (n = 13) were female and 88.9% (n = 104) were male. Risk factors, including smoking and occupational exposure, were not statistically different between the groups. The mean pre-instillation urinary pH value was 5.76 ± 0.75. The patients in Group 1 had a significantly higher mean urinary pH value than those in Group 2 (5.89 vs. 5.37, p <0.001). The baseline demographics and urinary pH values of the groups are summarised in Table I.
Table I: Comparison of the demographic data and urinary pH values between the study groups.
|
Variables |
Group 1 (n = 87) |
Group 2 (n = 30) |
p-value |
|
Age (years) |
59.7 ± 13.7 |
63.2 ± 8.7 |
0.112* |
|
Gender, n (%) Male Female |
78 (89.7) 9 (10.3) |
26 (86.7) 4 (13.3) |
0.655** |
|
Urinary pH |
5.89 ± 0.79 |
5.37 ± 0.41 |
<0.001* |
|
Occupational exposure, n (%) |
16 (18.4) |
10 (33.3) |
0.091** |
|
History of smoking, n (%) |
75 (86.2) |
24 (80) |
0.418** |
|
Smoking (pack years) |
37 ± 19.9 |
42 ± 19.9 |
0.285* |
|
ASA score, n (%) I II III |
2 (2.3) 65 (74.7) 20 (23) |
- 27 (90) 3 (10) |
|
|
ASA: American society of anesthesiologists. Continuous variables are presented as mean ± standard deviation, and nominal values are presented as numbers with percentages. Bold font indicates statistical significance * T-test. ** Chi-square test. |
|||
Table II: Comparison of the demographic data and tumor recurrence according to the cut-off value of urinary pH.
|
Variables |
Urinary pH <5.25 (n = 36) |
Urinary pH ≥5.25 (n = 81) |
p-value |
|
Age (years) |
62.9 ± 12 |
59.6 ± 12.9 |
0.192* |
|
Gender, n (%) Male Female |
31 (86.1) 5 (13.9) |
73 (90.1) 8 (9.9) |
0.531** |
|
Recurrence, n (%) Yes No |
14 (38.9) 22 (61.1) |
16 (19.8) 65 (80.2) |
0.029** |
|
Occupational exposure, n (%) Yes No |
8 (22.2) 28 (77.8) |
18 (22.2) 63 (77.8) |
1** |
|
Smoking history, n (%) Yes No |
31 (86.1) 5 (13.9) |
68 (84) 13 (16) |
0.765** |
|
Variables are presented as mean ± standard deviation or numbers and percentages bold font indicates statistical significance. * T-test. ** Chi-square test. |
|||
The ROC analysis revealed that the best cut-off value for urinary pH for the prediction of recurrence was 5.25 (95% confidence interval [CI]: 0.597-0.793, p = 0.001). According to the cut-off value of 5.25, in Group 1 22 of 87 patients had a urinary pH value of <5.25 while 14 of 30 had in Group 2. Table II presents the demographics and recurrence rates of the patients according to this cut-off value.
The overall RFS rates in years 1, 3, and 5 were 87.2%, 77.1%, and 67.3%, respectively. The mean recurrence time was 22 ± 14.9 months in Group 2. The RFS rates at year 1, 3, and 5 were 88.9%, 82.2% and 66.5%, respectively in patients with a urinary pH value of 5.25 or greater and 83.3%, 65.8% and 53.8%, respectively in those with a urinary pH value of <5.25 (p = 0.023). Figure 1 shows the RFS rates of the patients at a urinary pH cut-off value of 5.25.
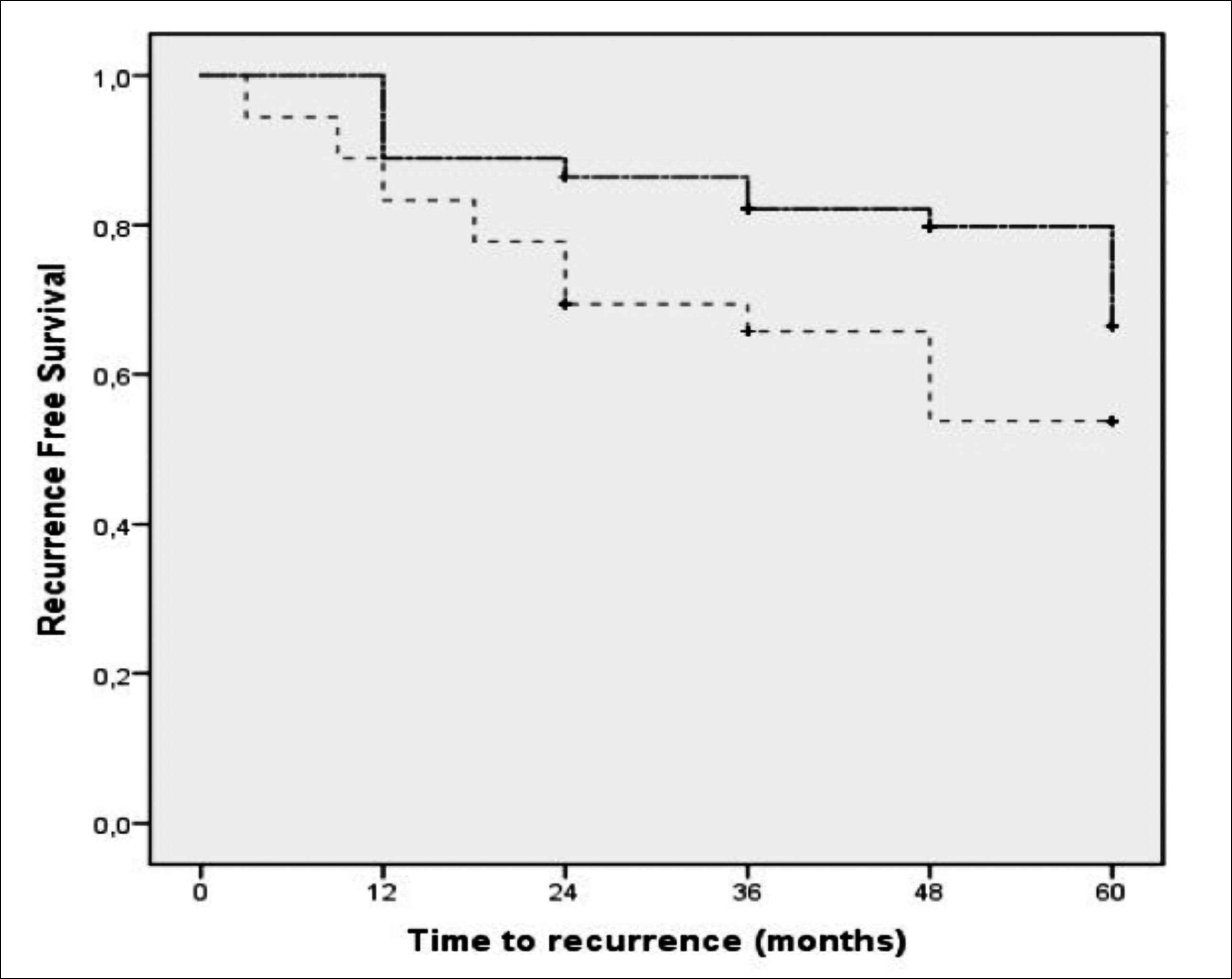 Figure 1: Recurrence-free survival rates at the urinary pH cut-off value of 5.25. The continuous line indicates urinary pH ≥5.25, dotted line indicates urinary pH <5.25.
Figure 1: Recurrence-free survival rates at the urinary pH cut-off value of 5.25. The continuous line indicates urinary pH ≥5.25, dotted line indicates urinary pH <5.25.
DISCUSSION
Although most BC cases are diagnosed with NMIBC, a significant proportion of patients with NMIBC suffer tumor recurrence.4 European Organisation for Research and Treatment of Cancer (EORTC) risk calculator for BC reveals that the probability of recurrence for low-risk NMIBC is 15% for the first year and 31% for the first five years.11 To reduce tumor recurrence, SI has been applied for years with proven efficacy. In 2013, Perlis et al. reported that SI prolonged recurrence free interval by 38% and reduced early recurrence by 12%.5 Another meta-analysis including 13 studies and 2,384 patients showed that SI reduced the recurrence risk by 35% and the five-year recurrence rate from 58.8% to 44.8%. In addition, the authors of the study reported that patients with an EORTC recurrence score of ≥5 did not benefit from instillation.6 Therefore, the current European Association of Urology (EAU) guidelines2 recommend SI following TUR-BT in patients with low-risk NMIBC (primary, solitary, TaG1, <3 cm tumor, and no carcinoma in situ) to reduce the recurrence risk. Another study reported the RFS rates at 1, 2, and 5 years to be 73%, 62%, and 51%, respectively among the NMIBC cases given SI irrespective of their risk group.12 In a study by Bosschieter et al., it was found that the recurrence risk was 31% at three years and 43% at five years in patients with low-risk NMIBC that were given SI.13 Present results showed that the RFS rates of patients with low-risk NMIBC were 87.2%, 77.1% and 67.3% at year 1, 3 and 5, respectively.
Optimising intravesical instillation to further reduce the recurrence risk has been the main goal.9 In the literature, studies on the optimisation of intravesical instillation have mostly focused on MMC. In order to maximise the benefit of intravesical MMC instillation, several factors including the duration of exposure of the bladder to the chemotherapeutic agent, optimal drug dose, and fluid intake have been investigated.14 Previous trials compared the effect of 0.5-hour and one-hour exposure times on recurrence and showed lower recurrence rates in a longer exposure.15,16 Although there is no study comparing one- and two-hour instillation durations, the current EAU guidelines recommend that the duration of instillation should be one to two hours.2 In order to increase drug concentration, it is recommended to reduce fluid intake and residual urine volume before and during instillation.2,17 Previous studies favored 40 mg MMC in 20 ml saline as the optimal concentration.17,18 In the current study, the authors administered 40 mg MMC in 20 ml saline for two hours to the patients after eight hours of fasting.
Urine pH and urinary alkalinisation are other discussed issues in the optimisation of intravesical MMC treatment.9,14 In 1985, Jauhiainen et al. assessed the effect of pH on the antitumor activity of six cytostatics, including MMC in the cell line, which were incubated at different pH values (5 to 7.4). The authors reported that MMC had lower activity at pH 5.19 Dalton et al. showed that the concentration of MMC in urine in vitro incubation was decreased by 44% at pH 5 and by <4% at pH 7.20 However, in another study, it was stated that MMC activity was not affected by pH in human bladder tumor explants.18 In a prospective study by Seo et al., the effect of oral sodium bicarbonate administration on the concentration of the active form of MMC was evaluated. The authors reported that although sodium bicarbonate therapy significantly increased the urine pH values, changes in the concentration of the active form of MMC were not statistically significantly different (285.3 vs. 305.3 ug/ml, p = 0.605).21
In 2001, a randomised phase III trial evaluated the efficacy of optimised intravesical MMC instillation in patients with NMIBC. A total of 230 patients were divided into two groups as optimized (n = 119) and standard (n = 111) treatment. In the optimised-treatment group, the patients were given a 40 mg dose of MMC, and the MMC concentration was manipulated by decreasing the urine volume and stabilised by urinary alkalinisation. The patients in the standard-treatment group received 20 mg MMC without any manipulation or alkalinisation. The patients in the optimised-treatment group were reported to have a significantly higher RFS rate (41% vs. 24.6%) and a longer median time to recurrence (29.1 vs. 11.8 months) at five years compared to the standard-treatment group. The authors concluded that optimisation of intravesical MMC treatment was significantly associated with improved efficacy and recommended the use of sodium bicarbonate to alkalinise the urine.17 Maeda et al. investigated the effect of urine pH on weekly MMC maintenance treatment. In that study, 124 patients were divided into two groups according to urinary pH using the cut-off value of 5.5 (pH <5.5 and pH ≥5.5). The authors reported that the patients with urinary a pH of ≥5.5 had improved three- and five-year RFS rates than those with a urinary pH value of <5.5 (64.2% and 52.9% vs. 41.9%, and 38.4%, respectively). The authors concluded that monitoring and modifying urinary pH could improve the efficacy of MMC instillation.22 Ersoy et al. evaluated the association between urinary pH and the efficacy of SI with MMC in patients with low-risk BC. They divided the patients were three groups as standard (n = 11), optimised (n = 15), and control (n = 23). The patients in the standard group were instilled MMC within the first six hours following TUR-BT. In the optimised group, the patients were given 1.3 g of sodium bicarbonate for urinary alkalinisation before the MMC instillation while those in the control group were given no treatment. The mean pH value was 6.4 in the optimised group after alkalinisation and 5.88 for both the standard and control groups. The one-year RFS rates were reported as 86.7%, 100%, and 91.3%, and the three-year RFS rates as 79.4%, 100%, and 82.6% for the optimized, standard, and control groups, respectively, and there was no difference between the groups. The authors stated that their preliminary results could not show the efficacy of urinary alkalinisation; therefore, they terminated the study.23 According to the present results, the patients with recurrence had a lower mean urinary pH value.
In the international literature, several studies have investigated the effect of urinary pH on the risk of BC and recurrence rates. Alguacil et al. evaluated the effect of urine pH on the risk of developing BC among patients with urothelial cell carcinoma (n = 712) and controls (n = 611). The authors found that acidic urinary pH (≤6) was associated with an increased risk of BC (odds ratio = 1.5, 95% CI: 1.2-1.9).24 A study by Stone et al. assessed the effect of urinary pH on tumor recurrence rates in NMIBC by dividing the patients into two groups according to their pH values being ≤ 6 or >6. It was reported that there was no association between urinary pH and recurrence rates.25
Although previous studies provide controversial findings concerning the effect of urinary pH on the risk of BC, recurrence rates, and MMC concentration or the efficacy of SI with MMC, our results indicated that at acidic urinary pH values (<5.25), the incidence of tumor recurrence was higher in patients with low-risk NMIBC that were given SI of MMC. In addition, most of the clinical studies in the literature have focused on NMIBC with various stages including Ta and T1 tumors which might influence the results or have studied on a smaller sample size. On the other hand, this study has certain limitations. Firstly, due to the retrospective design of the study, the authors were unable to record some demographic parameters of the patients, including comorbidities. Secondly, the study cohort did not include patients that were given medication to alkalinize urine; therefore, the researchers were not able to determine the effect of alkalinisation on these results. Lastly, the authors did not include the patients that were not given SI of MMC as a control group.
CONCLUSION
Patients who developed recurrence had lower mean urinary pH values. In addition, the urinary pH value being 5.25 or greater was associated with an increased RFS rate in patients that had received SI of MMC for the treatment of low-risk NMIBC. In patients with this urinary pH value, either urinary alkalinisation before SI with MMC or a closer follow-up may be beneficial. However, these results need to be confirmed by prospective studies with larger sample sizes.
ETHICAL APPROVAL:
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the local ethics committee (Ethics Committee Ruling No. E1-21-1518. Ankara City Hospital). Written informed consent was obtained from all the patients.
PATIENTS’ CONSENT:
Informed consent was obtained from all patients before the beginning of the study.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTIONS:
CS: Conception and design, data analysis and interpretation, drafting, critical revision, statistical analysis, and final approval.
AA: Data acquisition, data analysis and interpretation, statistical analysis, and final approval.
ICA: Data acquisition and final approval.
MB: Conception and design, drafting the work, critical revision, and final approval.
YA: Drafting, statistical analysis, and final approval.
AT: Conception and design, critical revision, and final approval.
REFERENCES
- Richters A, Aben KKH, Kiemeney L. The global burden of urinary bladder cancer: An update. World J Urol 2019; 38(8):1895-904. doi: 10.1007/s00345-019-02984-4.
- Babjuk M, Burger M, Compérat E, Gontero P, Liedberg F, Masson-Lecomte A, et al. EAU guidelines on non-muscle-invasive bladder cancer (TaT1 and CIS). Arnhem: EAU; 2021. Available from: https://uroweb.org/guideline/non- muscle-invasive-bladder-cancer [cited 20 Jan 2021].
- Kobayashi H, Kikuchi E, Mikami S, Maeda T, Tanaka N, Miyajima A, et al. Long term follow-up in patients with initially diagnosed low grade Ta non-muscle invasive bladder tumors: Tumor recurrence and worsening progression. BMC Urol 2014; 14:5. doi: 10.1186/1471- 2490-14-5.
- Vedder MM, Márquez M, de Bekker-Grob EW, Calle ML, Dyrskjøt L, Kogevinas M, et al. Risk prediction scores for recurrence and progression of non-muscle invasive bladder cancer: An international validation in primary tumours. PLoS One 2014; 9(6):e96849. doi: 10.1371/ journal.pone.0096849.
- Perlis N, Zlotta AR, Beyene J, Finelli A, Fleshner NE, Kulkarni GS. Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: An updated meta-analysis on 2548 patients and quality-of-evidence review. Eur Urol 2013; 64(3):421-30. doi: 10.1016/j. eururo.2013.06.009.
- Sylvester RJ, Oosterlinck W, Holmang S, Sydes MR, Birtle A, Gudjonsson S, et al. Systematic review and individual patient data meta-analysis of randomised trials comparing a single immediate instillation of chemotherapy after transurethral resection with transurethral resection alone in patients with stage pTa-pT1 urothelial carcinoma of the bladder: Which patients benefit from the instillation? Eur Urol 2016; 69(2): 231-44. doi: 10.1016/j.eururo.2015.05.050.
- Kang M, Jeong CW, Kwak C, Kim HH, Ku JH. Single, immediate postoperative instillation of chemotherapy in non-muscle invasive bladder cancer: A systematic review and network meta-analysis of randomised clinical trials using different drugs. Oncotarget 2016; 7(29):45479-88. doi: 10.18632/oncotarget.9991.
- Zamboni S, Baumeister P, Mattei A, Mordasini L, Antonelli A, Simeone C, et al. Single postoperative instillation for non-muscle invasive bladder cancer: Are there still any indication? Transl Androl Urol 2019; 8(1):76-84. doi: 10.21037/tau.2018.08.20.
- Zargar H, Aning J, Ischia J, So A, Black P. Optimizing intravesical mitomyc in C therapy in non-muscle-invasive bladder cancer. Nat Rev Urol 2014; 11(4):220-30. doi: 10.1038/nrurol.2014.52.
- Huncharek M, Geschwind JF, Witherspoon B, McGarry R, Adcock D. Intravesical chemotherapy prophylaxis in primary superficial bladder cancer: A meta-analysis of 3703 patients from 11 randomised trials. J Clin Epidemiol 2000; 53(7):676-80. doi: 10.1016/s0895-4356(99)00 203-6.
- Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006; 49(3):466-77. doi: 10.1016/j.eururo.2005. 12.031.
- Xylinas E, Kent M, Dabi Y, Rieken M, Kluth LA, Al Hussein Al Awamlh B, et al. Impact of age on outcomes of patients with non-muscle-invasive bladder cancer treated with immediate postoperative instillation of mitomycin C. Urol Oncol 2018; 36(3):89.e1-.e5. doi: 10.1016/j.urolonc. 2017.11.010.
- Bosschieter J, Nieuwenhuijzen JA, van Ginkel T, Vis AN, Witte B, Newling D, et al. Value of an immediate intravesical instillation of mitomycin C in patients with non-muscle-invasive bladder cancer: A prospective multicentre randomised study in 2243 patients. Eur Urol 2018; 73(2):226-32. doi: 10.1016/j.eururo.2017.06.038.
- Ragonese M, Racioppi M, Bassi PF, Di Gianfrancesco L, Lenci N, Filianoti A, et al. Mitomycin C: New strategies to improve efficacy of a well-known therapy. Urologia 2016; 83(Suppl 2):24-8. doi: 10.5301/uro.5000193.
- Giesbers AA, Van Helsdingen PJ, Kramer AE. Recurrence of superficial bladder carcinoma after intravesical instillation of mitomycin-C. Comparison of exposure times. Br J Urol 1989; 63(2):176-9. doi: 10.1111/j.1464- 410x.1989.tb05159.x.
- De Bruijn EA, Sleeboom HP, van Helsdingen PJ, van Oosterom AT, Tjaden UR, Maes RA. Pharmacodynamics and pharmacokinetics of intravesical mitomycin C upon different dwelling times. Int J Cancer 1992; 51(3):359-64. doi: 10.1002/ijc.2910510305.
- Au JL, Badalament RA, Wientjes MG, Young DC, Warner JA, Venema PL, et al. Methods to improve efficacy of intravesical mitomycin C: Results of a randomised phase III trial. J Natl Cancer Inst 2001; 93(8):597-604. doi: 10.1093/jnci/93.8.597.
- Wientjes MG, Badalament RA, Au JL. Use of pharmacologic data and computer simulations to design an efficacy trial of intravesical mitomycin C therapy for superficial bladder cancer. Cancer Chemother Pharmacol 1993; 32(4):255-62. doi: 10.1007/BF00686169.
- Jauhiainen K, Kangas L, Kapyla H, Alfthan O. Intravesical cytostatics: pH-dependence of antitumour activity. Urol Res 1985; 13(1):19-21. doi: 10.1007/BF00571751.
- Dalton JT, Wientjes MG, Badalament RA, Drago JR, Au JL. Pharmacokinetics of intravesical mitomycin C in superficial bladder cancer patients. Cancer Res 1991; 51(19):5144-52.
- Seo HK, Kim SH, Ahn KO, Lee SJ, Park WS, Kim S, et al. Recommended oral sodium bicarbonate administration for urine alkalinization did not affect the concentration of mitomycin-C in non-muscle invasive bladder cancer patients. Oncotarget 2017; 8(56):96117-25. doi: 10. 18632/oncotarget.21755.
- Maeda T, Kikuchi E, Matsumoto K, Miyajima A, Oya M. Urinary pH is highly associated with tumor recurrence during intravesical mitomycin C therapy for nonmuscle invasive bladder tumor. J Urol 2011; 185(3):802-6. doi: 10.1016/j.juro.2010.10.081.
- Ersoy H, Yaytokgil M, Karakoyunlu AN, Topaloglu H, Sagnak L, Ozok HU. Single early instillation of mitomycin C and urinary alkalinization in low-risk non-muscle-invasive bladder cancer: A preliminary study. Drug Des Devel Ther 2013; 7:1-6. doi: 10.2147/DDDT.S39541.
- Alguacil J, Kogevinas M, Silverman DT, Malats N, Real FX, García-Closas M, et al. Urinary pH, cigarette smoking and bladder cancer risk. Carcinogenesis 2011; 32(6):843-7. doi: 10.1093/carcin/bgr048.
- Stone BV, Ayangbesan A, Taylor BL, Golombos DM, Lewicki P, Al Hussein Al Awamlh B, et al. Urinary pH and the risk of recurrence in patients with non-muscle invasive bladder cancer. Can J Urol 2018; 25(4):9407-12.