Hearing Loss After the First Low Dose Administration of Cisplatin
By Atila Yildirim, Evren Fidan, Feyyaz OzdemirAffiliations
doi: 10.29271/jcpsp.2022.09.1216ABSTRACT
Cisplatin is commonly used antineoplastic drug that is effective against different types of tumours. Nephrotoxicity, neurotoxicity, and ototoxicity constitute the main dose-limiting side effects of the drug. We present a case of sensorineural hearing loss after the first low dose of cisplatin. Five days after receiving an intravenous 30 mg/day at 120 minutes, the patient presented with serious bilateral sensorineural hearing loss. Cisplatin-induced hearing impairment is generally dose-related and cumulative; however, the toxicity can occur after the first dose without a cumulative high-dose and can be irreversible.
Key Words: Cisplatin, Hearing Loss, Ototoxicity.
INTRODUCTION
Cisplatin is a chemotherapy agent commonly used in the treatment of various malignant neoplasms. Nephrotoxicity, neurotoxicity, and ototoxicity constitute the main dose-limiting side effects of the drug.1 Cisplatin-induced hearing loss is generally characterised by the bilateral, progressive, and irreversible sensorineural hearing loss. Hearing impairment is generally dose-related and cumulative.2 When the cumulative dose of cisplatin exceeds 300 mg, the risk of hearing loss increases proportionally.3 However, in previous studies, it has been shown that hearing loss can occur even after low-dose cisplatin application.4 Ototoxicity can occur within hours and days after cisplatin application5 and it also affects the quality of life.6 The cellular and molecular mechanisms by which cisplatin causes ototoxicity are not fully understood. Ototoxic damage may be related to the formation of reactive oxygen species (ROS) and the consumption of antioxidant enzymes. Especially, the outer hair cells at the basal fold and the inner hair cells of the cochlea are damaged.7 Risk factors for cisplatin-induced hearing loss include: anemia, hypoalbuminemia, being too young or too old, having renal failure, diuretic use, and pre-existing auditory dysfunction.8 Herein, we report sensorineural hearing loss due to the cisplatin after the first low dose.
CASE REPORT
A 29-year male patient who did not have any systemic disease presented to the urology clinic with testicular mass. The patient underwent left inguinal orchiectomy. The pathology confirmed the diagnosis of a germ cell tumour (GCT) and the patient was directed to our medical oncology clinic. The patient's ECOG performance scale was "0". He was not taking any other drugs known to cause ototoxicity. Glomerular filtration rate (GFR) was normal as all the laboratory findings. There was no contraindication of chemotherapy. Before the treatment, the patient was not tested for hearing impairment but during physical examination, there was no evidence of hearing loss. Chemotherapy was started with the protocol of BEP (Bleomycin-Etoposide-Cisplatin). The dose of cisplatin received by the patient was 30 mg/day for five days, and it was given in 250 cc of 0.9% isotonic NaCl over 120 minutes. However, hearing loss was observed in the patient on the fifth day after the end of the first cycle of chemotherapy. The patient was examined in the Department of Otolaryngology because of hearing loss. On audiological examination of the patient, in Pure Tone Audiometry (PTA) right ear PTA was 42dB, and the left ear PTA was 42 dB (Figure 1). In the speech discrimination test, the right ear speech discrimination score (SDS) was 44% and the left ear SDS was 40%. According to the audiological evaluation results, findings were consistent with the moderate high-frequency sensorineural type hearing loss in both ears of the patient. As there was no history of other risk factors for causing hearing loss, it was thought that the patient's hearing loss was linked to the cisplatin and cisplatin in the patient’s chemotherapy protocol was discontinued. One month later, the right ear PTA was 58 dB, and the left ear PTA was 56 dB (Figure 2). The audiometric study showed an increase in the patient’s hearing loss.
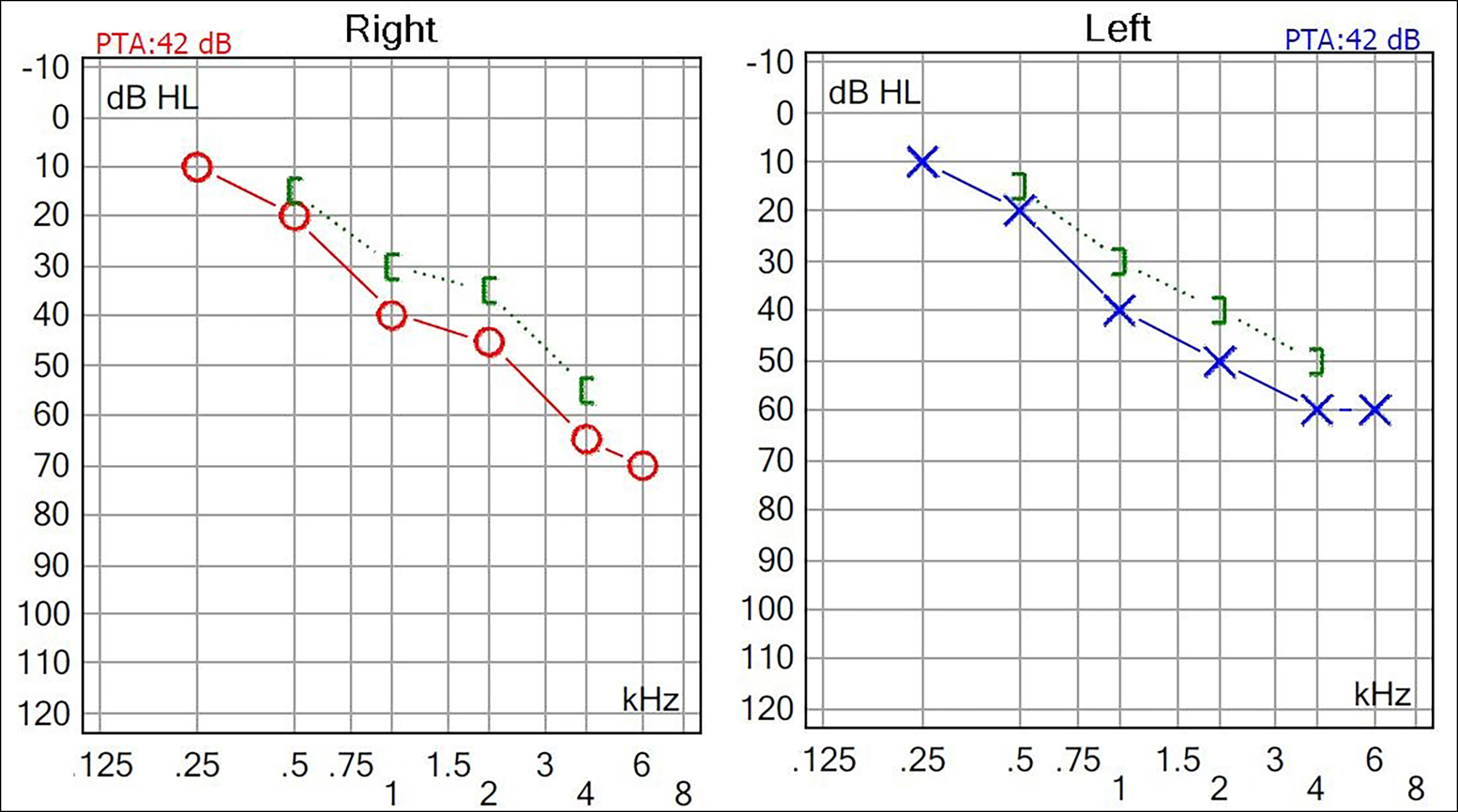 Figure 1: Audiogram peformed after the fifth day of cisplatin therapy. Right ear pure tone audiometry (PTA): 42 dB, left ear PTA: 42 dB. The x-axis shows the frequency (kHz), the y-axis shows the hearing level in decibels (dBHL: decibel hearing level).
Figure 1: Audiogram peformed after the fifth day of cisplatin therapy. Right ear pure tone audiometry (PTA): 42 dB, left ear PTA: 42 dB. The x-axis shows the frequency (kHz), the y-axis shows the hearing level in decibels (dBHL: decibel hearing level).
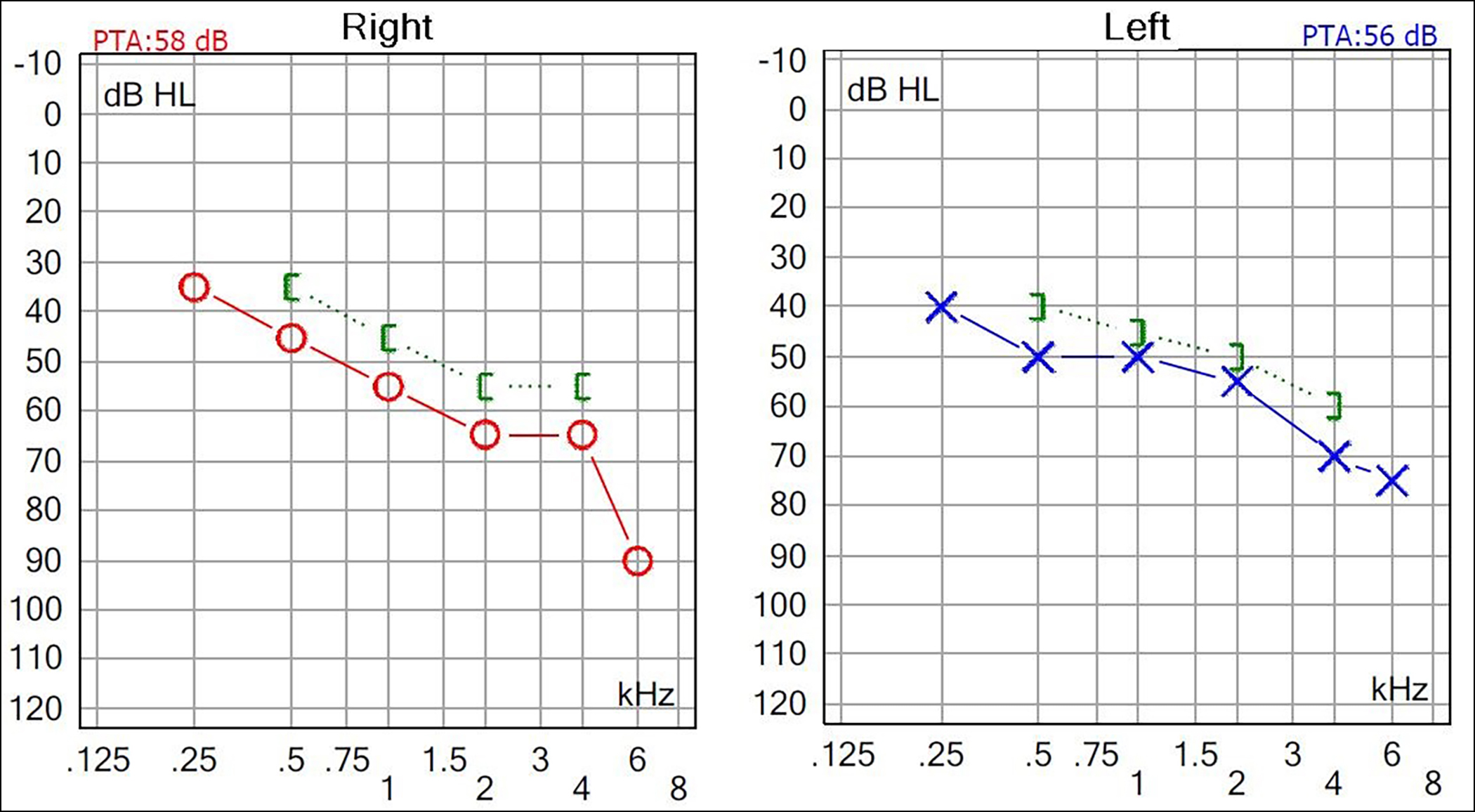 Figure 2: One month after the first audiogram. Right ear PTA: 58 dB, left ear PTA:56 dB. Further progression was observed in the patient's hearing loss.
Figure 2: One month after the first audiogram. Right ear PTA: 58 dB, left ear PTA:56 dB. Further progression was observed in the patient's hearing loss.
DISCUSSION
Cisplatin is a commonly used antineoplastic drug that is effective against a variety of tumours. However, the clinical application of this drug is limited due to serious and sometimes irreversible toxicity, including neurotoxicity, gastrointestinal toxicity, nephrotoxicity, myelosuppression, and ototoxicity. These toxicities are generally dependent on the doses. Cisplatin is the most known ototoxic drug, and when patients receive 50 mg/m² cisplatin in a single dose, an average of 33% of ototoxicity was reported.9 In the literature, the incidence of ototoxicity due to cisplatin is 67% on average.10 Tinnitus and high-frequency hearing loss may occur in the patients. This side effect seen in patients is associated with both high-single and high-cumulative doses. In a study by Bokemeyer et al., cisplatin caused 20% permanent symptomatic ototoxicity in patients receiving chemotherapy for testicular cancer. This rate increased to 60% when the cumulative dose exceeded 600 mg/m2.2 The dose of cisplatin taken by our patient was 30 mg/day for five days. However, findings compatible with moderate sensorineural hearing loss were obtained in both ears of the patient. Although, in general, cisplatin-related ototoxicity is not reversible, Aguilar-Markulis et al. reported that the patients with cisplatin-induced ototoxicity were completely normalised in 2% and partial recovery was seen in 26%.11 On audiological examination of the patient after one month, significant progress in his hearing loss was detected and seemed likely irreversible. Particularly, at-risk patients include those with pre-existing auditory dysfunction (e.g. elderly individuals, patients with head and neck cancer), pediatric population, history of radiotherapy, poor nutrition, low serum albumin, anemia, and receiving potential nephrotoxic drug treatment (e.g. aminoglycosides, loop diuretics).12 The patient was 29 years old and had a good oral intake, his serum albumin was normal, and there was no anemia, no ototoxic drug use, and no history of cranial radiotherapy. To reduce the toxicities associated with cisplatin and to increase treatment success, it is important to do adequate hydration, electrolyte replacement, strong antiemetic therapy, audiometry at various time intervals, and early administration of cytokines such as granulocyte colony-stimulating factor (G-CSF). We performed adequate hydration and checked for electrolyte imbalance in the patient, but none was found.
In conclusion, we present this case to draw attention to the fact that cisplatin-related ototoxicity can occur after the first dose without a cumulative high dose and can be irreversible. It is important to inform the patients about the possible side effects of chemotherapy and to take appropriate precautions before chemotherapy.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
AY, EF: Designing and writing.
FO: Designing and editing.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2010; 2(11):2490-518. doi: 10.3390/toxins2112490.
- Bokemeyer C, Berger CC, Hartmann JT, Kollmannsberger C, Schmoll HJ, Kuczyk MA, et al. Analysis of risk factors for cisplatin induced ototoxicity in patients with testicular cancer. Br J Cancer 1998; 77(8):1355-1362. doi: 10.1038/bjc.1998.226.
- Konrad-Martin D1, James KE, Gordon JS, Reavis KM, Phillips DS, Bratt GW, et al. Evaluation of audiometric threshold shift criteria for ototoxicity monitoring. J Am Acad Audiol 2010; 21(5):301-4. doi: 10.3766/jaaa.21.5.3.
- Nagy JL, Adelstein DJ, Newman CW, Rybicki LA, Rice TW, Lavertu P. Cisplatin ototoxicity: The importance of baseline audiometry. Am J Clin Oncol 1999; 22(3):305-8. doi: 10.1097/00000421-199906000-00020.
- Rybak LP. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr Opin Otolaryngol Head Neck Surg 2007; 15(5):364-9. doi: 10.1097/MOO.0b013e3282eee452.
- Haugnes HS, Stenklev NC, Brydoy M, Dahl O, Wilsgaard T, Laukli E, et al. Hearing loss before and after cisplatin-based chemotherapy in testicular cancer survivors: A longitudinal study. Acta Oncol 2018; 57(8):1075-83. doi: 10.1080/ 0284186X.2018.1433323.
- Hazlitt RA, Min J, Zuo J. Progress in the development of preventative drugs for cisplatin-ınduced hearing loss. J Med Chem 2018; 61(13):5512-24. doi: 10.1021/acs.jmed chem.7b01653.
- Schacht J, Talaska AE, Rybak L. Cisplatin and amino-glycoside antibiotics: Hearing loss and its prevention. Anat Rec 2012; 295(11):1837-50. doi: 10.1002/ar.22578.
- Kaufman Arenberg I. Dizziness and balance disorders: An ınterdisciplinary Approval. New York, NY, Kugler, 1993.
- Eiamprapai P1, Yamamoto N, Hiraumi H, Ogino-Nishimura E, Kitamura M, Hirano S, et al. Effect of cisplatin on distortion product otoacoustic emissions in Japanese patients. Laryngoscope 2012; 122(6):1392-6. doi: 10.1002/lary.23336.
- Aguilar-Markulis NV, Beckley S, Priore R, Mettlin C. Auditory toxicity effects of long-term cis-dichloro-diammineplatinum II therapy in genitourinary cancer patients. J Surg Oncol 1981; 16(2):111-23. doi: 10. 1002/jso.2930160203.
- Markman M. Toxicities of the platinum antineoplastic agents. Expert Opin Drug Saf 2003; 2(6):597-607. doi: 10.1517/14740338.2.6.597.