Frequency and Antibiotics Sensitivity Pattern of Culture-Positive Salmonella Typhi in Children
By Mubashir Ahmad, Nownhall Shah, Muhammad Asif SiddiquiAffiliations
doi: 10.29271/jcpsp.2023.03.303ABSTRACT
Objective: To calculate the frequency of positive blood culture in clinically diagnosed cases of enteric fever and antibiotic sensitivity patterns in culture-positive cases of S.typhi
Study Design: Observational Study.
Place and Duration of the Study: Department of Paediatrics Medicine, Services Hospital Lahore, from November 15th 2020 to May 15th 2021.
Methodology: A total of 246 patients, fulfilling the definition of a suspected case of enteric fever were enrolled. Blood cultures were drawn on the spot. Antimicrobial sensitivity for 8 antimicrobial agents-Ampicillin, amoxicillin, chloramphenicol, cefixime, ceftriaxone, cefotaxime, ciprofloxacin, meropenem, and Azithromycin, were performed. A p-value of <0.05 was considered statistically significant.
Results: Blood cultures were positive in 62 (25.2%), patients out of which 34 (54.9%) were females and 28 (45.1%) were males, of which, 58 were S. typhi and 4 were S. Paratyphi A or B. Cefixime was sensitive in 27.4% of patients and intermediate sensitivity was found in 3.2% of cases and 69.4% of cases were resistant, ceftriaxone was sensitive in 38.7% of cases and Azithromycin was sensitive in 96.7% of cases, whereas meropenem showed 100% sensitivity. Chloramphenicol and Ciprofloxacin were resistant in 80.6% and 27.3% of the cases respectively. Among isolates, 32.3% (20) were categorised as sensitive enteric fever; 64.5% (40) as MDR, and 3.2% (2) as XDR enteric fever.
Conclusion: MDR and XDR enteric fever are a major concern. For such cases, Azithromycin remains the best oral antibiotic with a sensitivity of up to 96.7%. Meropenem was sensitive in 100% of cases and was the only antibiotic with no documented resistance in this study.
Key Words: Enteric fever, Salmonella, Antibiotic sensitivity, Blood culture, MDR, XDR, Azithromycin, Meropenem.
INTRODUCTION
Enteric fever is a bacterial infection affecting countries all around the world and is caused by Salmonella serotype typhi and parathypi.1 It is a major cause of morbidity and mortality, especially in developing countries. If not treated properly, it can lead to hypotension, stupor, hepatitis, encephalitis, gut perforation, and even death.2
Annually there are 11-21 million typhoid fever cases reported worldwide, with the death toll being around 128000 to 161000.3 In the subcontinent, the incidence of enteric fever is higher in Pakistan (451.7 per 100,000 persons/year) as compared to India (214.2 per 100,000 persons/year).4 Enteric fever had an estimated worldwide case fatality rate of 0.95% in 2017, with the case fatality rate being highest in the paediatric population, old age, and in those living in resource-poor countries.5
Antimicrobial resistance is one of the biggest challenges that modern medicine has to face. More than 35000 people died due to antibiotic resistance in USA.6
The treatment of enteric fever based on culture and sensitivity varies from country to country with the emergence of MDR and XDR enteric fever in developing countries due to the misuse of antibiotics.7 A study conducted in Karachi, Pakistan reported a higher incidence of enteric fever due to the ingestion of contaminated food and unnecessary antibiotic usage.7
Previously, ampicillin, trimethoprim-sulfamethoxazole, and chloramphenicol were used to treat enteric fever. Resistance to these first-line antibiotics (MDR) was first seen in the early 1980s. Multidrug resistance (MDR) enteric fever has now become prevalent in most of South Asia, with figures reaching 13% in India and 44% in Pakistan.8 This led to the widespread use of Fluoroquinolones to treat enteric fever, however, resistance to Fluoroquinolones also emerged, and as per SEAP (surveillance of enteric fever in Asia Project), the resistance of S.typhi to fluoroquinolones in Pakistan was reported to be 90%.9 After resistance to Quinolones emerged, clinicians started using third-generation Cephalosporins (ceftriaxone and cefixime) and until recently, resistance against them was rare.10 However, in 2016, during the outbreak of enteric fever in Karachi and Interior Sindh in Pakistan, strains resistant to third-generation Cephalosporins has emerged.11
Currently, Carbapems (meropenem, imipenem) and Azithro-mycin remain the most widely used agents to treat the XDR enteric fever and so far, resistance to these two agents is rare. Currently, there is a lack of local data from Punjab province, regarding the antibiotic sensitivity pattern of enteric fever.
Salmonella is a dynamic and evolving organism, and overuse of certain agents may lead to the development of resistance over time. Thus, clinicians must be aware of the latest antibiotic sensitivity pattern to effectively alter their prescribing habits to avoid the development of antimicrobial resistance and to avoid overuse of antibiotics of last resort (Carbapenems and Azithromycin). The Data obtained from this study will be helpful in this regard and it will also help in the formulation of standard guidelines for empirical treatment of enteric fever in this region and will also help to prevent the overuse and misuse of antibiotics. The objective of this study was to calculate the frequency of positive blood culture in clinically diagnosed cases of enteric fever and antibiotic sensitivity patterns in culture-positive cases of Salmonella.
METHODOLOGY
This observational study was conducted using the non-probability consecutive sampling technique in the Department of Paediatrics Medicine, Services Hospital, Lahore, from 15th November 2020 to 15th May 2021. Sample size was calculated as 246 by keeping confidence interval (CI) of 95%, 4.5%, margin of error and 13% expected frequency of culture positive cases.12
Clinically diagnosed case of enteric fever was defined as a fever of 38 degree centigrade or above for 3 or more days along with GIT symptoms like nausea, vomiting, diarrhoea, constipation or prostration, and typhidot IgG or IgM positive.13 MDR enteric fever was defined as a suspected/probable case that is laboratory confirmed by isolation of S. typhi from blood, or/and stool, bone marrow, urine, or any specimen that is resistant to 3 or more first-line drugs including quinolones. First-line drugs are chloramphenicol, ampicillin, and trimethoprim/sulfamethoxazole [TMP/SMX].13 XDR enteric fever was the one caused by S. typhi or paratyphi A, B, or C strains which are resistant to all antibiotics recommended for treatment.13 Culture-positive patients were those having S. typhi or paratyphi growth (1 or more colonies), obtained from blood culture after a maximum of 7 days of incubation.
A total of 246 patients fulfilling the definition of clinically diagnosed enteric fever were included in the study after receiving informed consent from parents/guardians.
The inclusion criteria were patients of either gender, under 14 years of age fulfilling criteria of a clinically diagnosed case of Salmonella. Exclusion criteria were patients taking antibiotics before presenting to hospital within 7 days.
Demographic and socioeconomic data were collected on a pre-formed questionnaire and blood cultures were drawn on the spot and sent to the pathology department of Services hospital, Lahore. Blood was inoculated into 40-45ml brain-heart infusion broth using BactT/alert virtuo automated blood culture machine [bioMérieux, Inc)]. Incubation was done at 37 degrees. Salmonella isolates were tested for antimicrobial susceptibility by the Kirby-Bauer disk diffusion method on Mueller-Hinton agar with standard antimicrobial disks. Antimicrobial susceptibility for 8 antimicrobial agents-ampicillin, amoxicillin chloramphenicol, cefixime, ceftriaxone, cefotaxime, ciprofloxacin, meropenem, and Azithromycin were performed. The diameter of the zone of inhibition caused by each antimicrobial disc on the Salmonella isolates was measured in mm and compared with the standard chart recommended by The European Committee on Antimicrobial Susceptibility Testing,14 to classify the organism as either sensitive, resistant, or having intermediate sensitivity to the particular antibiotic. Culture-positive cases and sensitivity patterns were recorded as per the operational definition.
Data were entered and analysed using SPSS version 21. Numerical variables such as (age and number of culture-positive patients) were presented as mean ± standard deviation (SD), and categorical variables (gender, type of isolate, antimicrobial sensitivity pattern, and culture positivity) were analysed as frequency and percentage. Gender, age, and duration of fever were stratified. For categorical variables, the chi-square test was applied. A p-value of <0.05 was considered statistically significant.
RESULTS
A total of 246 patients were included in the study. The mean age of children in this study was 6.15±3.341 years, ranging between 1 and 13 years. Among them, 111 (45.1%) were males and 135 (54.9%) were females.
The mean duration of fever was 6.90 ± 2.57 days. Duration of fever ranges between 3-14 days. Out of a total of 246 patients, blood culture was positive for Salmonella in 62 (25.2%) patients, and blood culture was negative in 184 (74.8%) patients (Figure 1).
Out of 62 patients for which blood culture was positive, 34 (54.9%) were females and 28 (55.1%) were males.
Out of 62 positive blood cultures, 58 were S. typhi, whereas 4 were S.paratyphi A or B (3 were S. paratyphi A and 1 was S. paratyphi B). Among the isolates sensitive typhoid cases were 20 (32.3%); MDR cases were 40 (64.5%), while XDR cases were only 2 (3.2%). The antibiotic sensitivity pattern is shown in Table I. As far as gender distribution was concerned, 24 cases of MDR were detected out of a total of 34 culture-positive female patients and 16 MDR cases were detected in male patients out of a total of 28 culture-positive cases, 1 XDR case was detected in both genders each (p=0.540, chi-square value = 1.231).
Table I: Antibiotic sensitivity pattern of Salmonella isolates.
|
Antibiotic |
Sensitive |
Intermediate sensitivity |
Resistant |
Total |
|
Ampicillin |
14(22.6%) |
0 |
48(77.4%) |
62 |
|
Chloramphenicol |
10(16.1%) |
2(3.2%) |
50(80.6%) |
62 |
|
Cefixime |
17(27.4%) |
2(3.2%) |
43(69.4%) |
62 |
|
Ceftriaxone |
24(38.7%) |
21(33.9%) |
17(27.4%) |
62 |
|
Trimethoprim / Sulfamethoxazole |
17(27.4%) |
1(1.6%) |
44(71%) |
62 |
|
Amoxicillin |
42(67.7%) |
3(4.8%) |
17(27.3%) |
62 |
|
Ciprofloxacin |
20(32.3%) |
40.3(25.8%) |
17(27.3%) |
62 |
|
Azithromycin |
60(96.7%) |
N/A |
2(3.3%) |
62 |
|
Meropenem |
62(100%) |
0 |
0 |
62 |
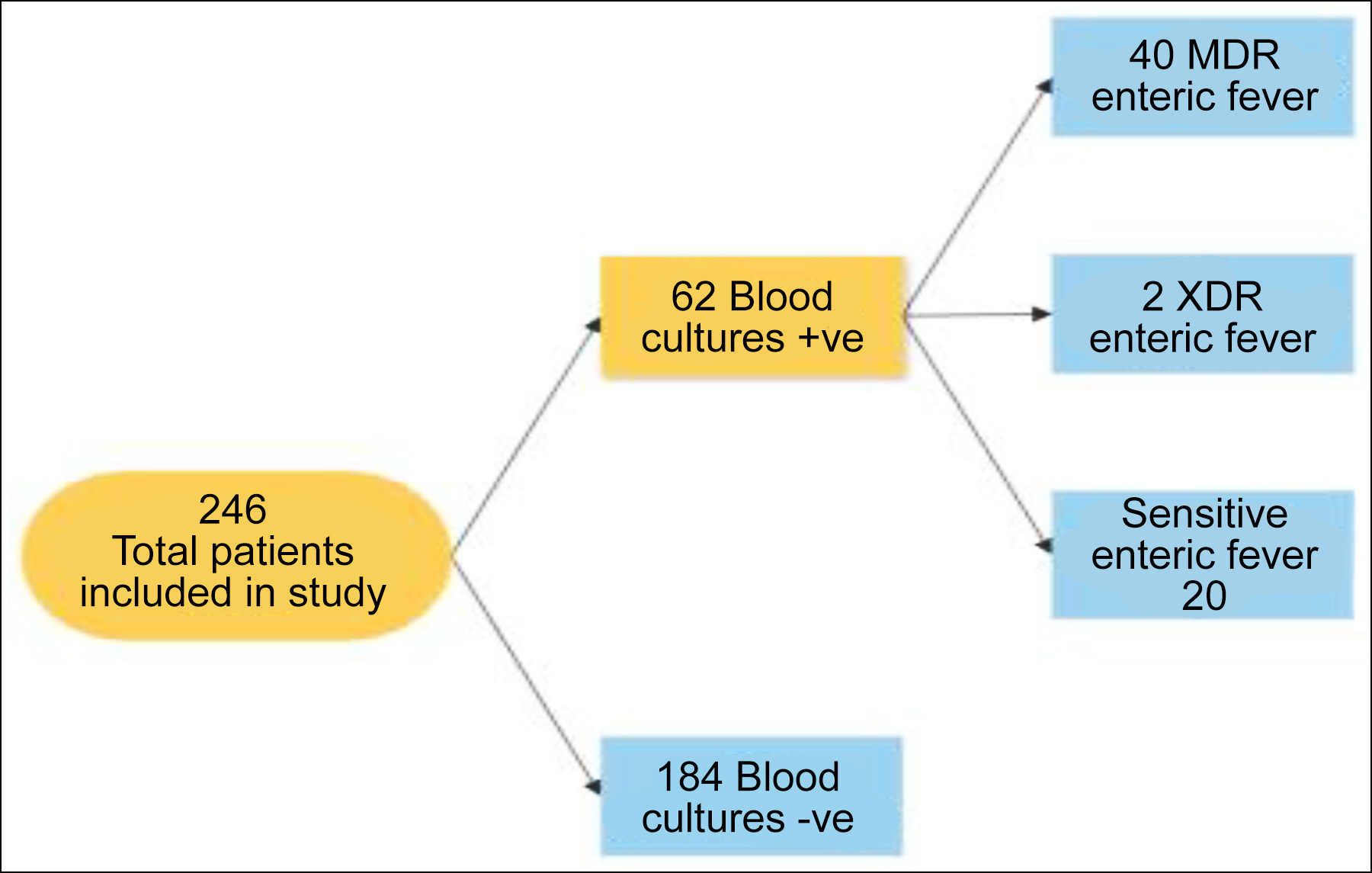 Figure 1: Study flow diagram.
Figure 1: Study flow diagram.
DISCUSSION
Out of the total of 246 patients, blood culture was positive for Salmonella in 62 (25.2%) patients. This is comparable to a large hospital-based study conducted in Karachi, Pakistan which showed a culture positive rate of 22%.15 Data obtained from population-based studies conducted in Vietnam, Delhi, and Egypt paints a different picture with blood culture positivity rate in febrile cases reported to be around 8.5%, 5%, and 4.2%, respectively.16
In one systemic review and meta-analysis, it was seen that out of 10,355 confirmed enteric cases, 2,719 (26.3%) had complications. In this study, the mean duration of fever was 6.90±2.571 days. Duration of fever ranges between 3-14 days, whereas the mean duration of fever in a study conducted in India was 9 days.17
This study showed the incidence of MDR typhoid to be 64.5%, whereas the incidence of XDR typhoid was 3.2%. Enteric fever caused by resistant strains is an ever-increasing problem in developing and resource-limited countries like Pakistan. It is well documented in the literature to be endemic in South-East Asia including Pakistan, Nepal, Bangladesh, and India.18 In recent years, resistance to first-line antibiotics like amoxicillin, chloramphenicol, and TMP/SMX is a major concern.
The rising cases of MDR and XDR typhoid can be blamed on the injudicious use of over-the-counter available medication and also to some extent over prescription of medication by non-qualified medical practitioners.
Research data shows that the prevalence of MDR in Asian countries is 23%. A study shows that the prevalence of MDR increased from 34.2% to 48.5% from 2001 to 2006.19
A weekly epidemiological report by the National Institute of Health (NIH) Islamabad, indicated that a total of 14,360 XDR enteric fever has been reported in Karachi from January 2017 to June 2021. Whereas in the rest of Sindh, a total of 5741 cases of XDR enteric were reported in the same period, with district Hyderabad being the district having the greatest number of cases, 69.5%.11
XDR enteric prevalence is not exactly given in the literature as it is a relatively newer variant and its first outbreak was reported in November 2016 in Karachi, Sindh, Pakistan.20 The current study shows a prevalence of XDR enteric fever at 3.2% in Lahore, Pakistan. This study showed that ciprofloxacin was resistant in 27.3% of the cases.
The study identified chloramphenicol to be resistant in up to 80.6% of cases, which is very high and confirms that chloramphenicol is not the right choice for treatment in this part of the world. Although the narrative of recycling older conventional medicines is ongoing all over the world and many studies are resonating with this narrative.20 This high resistance is in contrast to another study conducted in Karachi Pakistan, which showed isolates to be sensitive to chloramphenicol in 41% of cases.20 This discrepancy may be due to different regional strains of S. typhi and different prescribing behaviours of doctors.
In this study, isolates were 38.7% sensitive to ceftriaxone, which is in contrast to another study from central Asia which demonstrated up to 100% sensitivity to Ceftriaxone.21
Cefixime and amoxicillin sensitivity was found in 27.4% and 67.4% cases, respectively; this demonstrates very high prevalence of resistance in routinely used oral antibiotics in children. Azithromycin is one of the most effective oral medicines for uncomplicated enteric fever. It remains the only highly effective oral antibiotic for the treatment of enteric fever and isolates in this study showed sensitivity in up to 96.7% of the cases and resistance was shown in only 3.3% of the isolates. Meropenem was sensitive in all the cases and remains the only antibiotic with no resistance seen so far.
Limitations of this study include a small sample size and cross-sectional nature of this study. This study was limited to one centre only. More extensive multicentric studies, as well as population-based studies are needed before general guidelines for antibiotics prescription can be made.
CONCLUSION
Emergence of MDR and XDR strains of enteric fever is on the rise with very high resistance against the two most commonly used antibiotics (Ceftriaxone and Cefixime). Prescribing practices need to be modified and the unjustified use of antibiotics must be discouraged. The authors also recommend access to clean water and improving sanitation and hygiene conditions of the population.
FUNDING:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
DISCLOSURE:
It is a dissertation based study.
ETHICAL APPROVAL:
This observational study was reviewed and approved by the Institutional Review Board of Services Institute of Medical Sciences/Services Hospital Lahore (IRB-2021-913-SIMS).
PATIENTS’ CONSENT:
Informed consents were taken from all participants included in the study.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
MA: Conceptualisation, design, literature review, methodology, project administration, data curation, visualisation, and writing of the original draft.
NS: Data curation, statistical analysis, supervision, writing the original draft, and methodology.
AS: Data curation, literature review, writing original draft, and methodology.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Das JK, Hasan R, Zafar A, Ahmed I, Ikram A, Nizamuddin S, et al. Trends, associations, and antimicrobial resistance of Salmonella typhi and paratyphi in Pakistan. Am J Trop Med Hyg 2018; 99(3_Suppl):48–54. doi: 10.4269/ajtmh.18- 0145.
- Stanaway JD, Reiner RC, Blacker BF, Goldberg EM, Khalil IA, Troeger CE, et al. The global burden of typhoid and paratyphoid fevers: A systematic analysis for the global burden of disease study 2017. Lancet Infect Dis 2019; 19(4):369–81. doi: 10.1016/S1473-3099(18)30685-6.
- Rauniyar GP, Bhattacharya S, Chapagain K, Shah GS, Khanal B. Typhoid fever among admitted pediatric patients in a tertiary care center: A descriptive cross-sectional study. J Nepal Med Assoc 2021; 59(241):871. doi: 10.31729/jnma. 6044.
- Islam K, Mahmud R, Chowdhury MK, Hossain FS, Biswas PK, Sarker S. Recent sensitivity pattern of Salmonella Typhi in a private hospital. J Med 2018; 19(1):15-7.
- MogasaleV, MaskeryB, Ochiai RL, Lee JS, Mogasale VV, Ramani E, et al. Burden of typhoid fever in low-income and middle-income countries: A systematic, literature-based update with risk-factor adjustment. Lancet Glob Health 2014; 2(10):e570-e80. doi: 10.1016/S2214-109X(14) 70301-8.
- About antibiotic resistance. Centers for disease control and prevention. [cited 2022 december22]. Available from https://www.cdc.gov/drugresistance/about.html
- Maharjan A, Dhungel B, Bastola A, Thapa Shrestha U, Adhikari N, Banjara MR, et al. Antimicrobial susceptibility pattern of Salmonella spp. isolated from enteric fever patients in Nepal. Infect Dis Rep 2021; 13(2):388-400. doi: 10.3390/idr13020037.
- Sultan BA, Langhani SK, Shahzaib I, Irshad Z, Perveen S, Tariq S. Isolation of extensively drug resistant Salmonella typhi. In blood culture from tertiary care University Hospital. Infec Dis J Pak 2020; 29(1).
- Parry CM, Ribeiro I, Walia K,Rupali P, Baker S, Basnyat B . Multidrug resistant enteric fever in South Asia: Unmet medical needs and opportunities. BMJ 2019; 364: k5322. doi: 10.1136/bmj.k5322.
- Klemm EJ, Shakoor S, Page AJ, Qamar FN, Judge K, Saeed DK, et al. Emergence of an extensively drug-resistant salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. mBio 2018; 9(1):e00 105-18. 10.1128/mBio.00105-18.
- Federal Disease surveillance and response unit field epidemiology Report. [cited 2022Aug22]. Available from: www.nih.org.pk/wp-content/uploads/2021/06/25-FELTP-Pakistan-Weekly-Epidemiological-Report-June-13-19-2021-.pdf.
- Khan M. A plausible explanation for male dominance in typhoid ileal perforation. Clin Experimental Gastroenterol 2012; 213. doi: 10.2147/CEG.S36569.
- National Institute of Health, Islamabad, Advisory for treatment and prevention of enteric fever. National Institute of Health, Islamabad. [cited 2022 Dec26]. Available from: nih.org.pk/wp-content/uploads/2020/07/Advisory-for-Prevention-and-Treatment-of-Typhoid-Fever-including-XDR-Typhoid.pdf.
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 12.0, 2022" [Internet]. EUCAST. [cited 2022Dec26]. Available from: www.eucast.org/ clinical_breakpoints.
- Yousafzai MT, Irfan S, Thobani RS, Kazi AM, Hotwani A, Memon AM, et al. Burden of culture confirmed enteric fever cases in Karachi, Pakistan: Surveillance for enteric fever in Asia project (SEAP), 2016-2019. Clin Infect Dis 2020; 71 (Supplement_3):S214-21. doi: 10.1093/cid/ciaa1308.
- Browne AJ, Kashef Hamadani BH, Kumaran EA, Rao P, Longbottom J, Harriss E, et al. Drug-resistant enteric fever worldwide, 1990 to 2018: A systematic review and meta-analysis. BMC Med 2020; 18(1):1-22. doi: 10.1186/s12916-019- 1443-1.
- Ray B, Raha A. Typhoid and enteric fevers in intensive care unit. Indian J Crit Care Med 2021; 25(Suppl 2):S144-9. doi: 10.5005/jp-journals-10071-23842.
- Amicizia D, Micale RT, Pennati BM, Zangrillo F, Iovine M, Lecini E, et al. Burden of typhoid fever and cholera: Similarities and differences. Prevention strategies for European travelers to endemic/epidemic areas. J Preventive Med Hygiene 2019; 60(4):E271. doi: 10.15167/2421-4248/jpmh2019. 60.4.1333.
- Hasan R, Zafar A, Abbas Z, Mahraj V, Malik F, Zaidi A. Antibiotic resistance among Salmonella enterica serovars Typhi and paratyphi A in Pakistan (2001-2006). J Infect Dev Ctries 2008; 2(4):289-94. doi: 10.3855/jidc.224.
- Saleem K, Zafar S, Rashid A. Antimicrobial sensitivity patterns of enteric fever in Pakistan: A comparison of years 2009 and 2019. J R Coll Physicians Edinb 2021; 51(2): 129-32. doi: 10.4997/JRCPE.2021.206.
- Rahman BA, Wasfy MO, Maksoud MA, Hanna N, Dueger E, House B. Multi-drug resistance and reduced susceptibility to ciprofloxacin among Salmonella enterica serovar Typhi isolates from the Middle East and Central Asia. New Microbes New Infect 2014; 2(4):88. doi: 10.1002/nmi2.46.