Factors Affecting the Efficacy of Regorafenib in Metastatic Colorectal Cancer: Is Tumour Sidedness Important?
By Seher Nazli Kazaz1, Ali Caner Ozdover1, Nergis Usta2, Atila Yildirim1, Evren Fidan1, Feyyaz Ozdemir1Affiliations
doi: 10.29271/jcpsp.2023.06.659ABSTRACT
Objective: To investigate the outcomes of regorafenib treatment in refractory metastatic colorectal cancer (mCRC) patients by primary tumour sidedness, the effects of previously targeted therapies, RAS status and inflammatory markers.
Study Design: Observational study.
Place and Duration of the Study: Department of Medical Oncology, Karadeniz Technical University, Faculty of Medicine, Trabzon, Turkey, between January 2012 and September 2020.
Methodology: Clinical data of 102 mCRC patients treated with regorafenib were compared according to the right and left colon subgroups, in terms of factors affecting outcomes of regorafenib treatment. Kaplan-Meier method was used to identify factors associated with the overall survival.
Results: Disease control rate (DCR) with regorafenib were similar in both right and left-sided colon tumours (60% vs. 61%, respectively, p>0.99). The median overall survival (OS) was 6.6 months in patients with right-sided colon cancers and 10.1 months in patients with left-sided colon cancers, but it was not significant (p=0.238). When evaluating by RAS status, there was an increase in favour of the right-sided mCRC in progression-free survival and OS, without statistical significance. In multivariate analysis, the patients with metastatic sites <3 and the number of prior systemic therapies ≤3 line had significantly higher survival.
Conclusion: The tumour burden affected the response to regorafenib in subsequent treatments and regorafenib was also effective in heavily treated mCRC patients. There was no difference in PFS and OS in terms of tumour sidedness by regorafenib treatment.
Key Words: Colorectal cancer, Regorafenib, Tumour sidedness.
INTRODUCTION
Colorectal cancer (CRC) is the 4th most common cancer and ranks 3rd in cancer-related deaths worldwide despite screening methods.1 About 20% of colorectal cancer patients are diagnosed in the metastatic stage.2 With advances in treatment in the last two decades, the overall survival rate for metastatic CRC (mCRC) has exceeded 30 months.3,4 Many patients can receive at least 2nd line therapy. For 1st line therapy, tumour response rates are 30-60%, while for 2nd line therapy they drop to 5-10%.5 Treatment selection is based on patients characteristics, tumour sidedness, molecular tests including RAS mutation and BRAF mutation and Eastern Cooperative Oncology Group-Performance Status (ECOG-PS).
Standard treatment options in 1st and 2nd line therapy for mCRC patients include combinations of targeted therapies [eg. anti vascular endothelial growth factor (VEGF) and anti-epidermal growth factor receptor (EGFR) agents for RAS wild-type patients] with fluoropyrimidine, oxaliplatin or irinotecan-based chemotherapy.
Another important consideration in deciding treatment in the primary and secondary setting is primary tumour location. Evidence suggests that right-sided (RS) colon cancer patients have the worst outcomes. Although the optimal treatment sequence is still under debate, in a meta-analysis of three main trials, the survival benefit in RS RAS wild-type colon cancer was significantly higher with an EGFR inhibitor than with bevacizumab and patients with RS colon tumours benefited from bevacizumab with a longer survival trend that did not reach statistical significance.6
Many patients have good performance status after the progression of two lines of therapy and most of them need further treatment. Therefore, treatment options for mCRC beyond 2nd line treatment are important. Currently, regorafenib and trifluridine / tipiracil are recommended for 3rd line treatment of mCRC. In Turkey, of these two agents, only regorafenib is eligible. Rechallenge is another option, but only for patients in whom chemotherapy was discontinued for reasons other than progression.7 The ongoing FIRE -4 trial is evaluating a rechallenge with an anti-EGFR in the third-line setting.8
Regorafenib has a broad kinase inhibition profile with its antiangiogenic, anti-immunosuppressive, antiproliferative and antimetastatic properties. In several phase III studies, regorafenib provided significant progression-free survival (PFS) advantage and disease stabilisation in mCRC patients. Nevertheless, the response rates and tumour regression rates were low in these trials. These results seem to be more indicative of a consistent rate of stable disease.9,10 On the other hand, it has been reported that the sequence of targeted therapies may affect outcomes. In retrospective analyzes, anti-EGFR therapy improved PFS significantly better in patients who had not previously received bevacizumab than in patients who had received prior bevacizumab.11 A Phase II study with regorafenib before cetuximab demonstrated a longer overall survival (OS) than the current standard sequence.12 This can be explained by the fact that previous anti-VEGF treatment increased VEGF-A and hypoxia/HIF signalling and genome instability, resulting in adaptive changes in tumour cells in the microenvironment and resistance of some mutant genotypes to anti-EGFR treatment.13
This study aimed to determine the factors that affect the efficacy of regorafenib, such as tumour sidedness, RAS status, sequence of previously targeted therapies and inflammatory markers in mCRC patients.
METHODOLOGY
Patients treated with regorafenib for metastatic colon and rectum cancer who were treated at Karadeniz Technical University, Faculty of Medicine, Trabzon, Turkiye, from 2012 to 2020 were screened. Patients whose data could not be reached were excluded from the study. As a result, 102 patients who met these criteria were included in the study between January 2012 and September 2020. All patients had received fluoropyrimidine, irinotecan, and oxaliplatin together with targeted agents such as cetuximab/panitimumab and/or bevacizumab in their previous treatment. Clinical, pathological and molecular characteristics were analyzed retrospectively. Age, gender, disease status, primary tumour site, histological grade, primary tumour surgery, Kirsten-ras (KRAS) mutation status, metastatic site(s), administered chemotherapy and biological agents and survival data were collected from patient hospital records retrospectively.
RS colon cancer starts from the cecum and includes up to the distal two-thirds of the transverse colon, left-sided (LS) colon cancer starts from the proximal third of the transverse colon and includes up to the rectum.7,11
Among these 102 patients, 25 had RS, 77 had LS colon cancer. The frequency of patient characteristics was evaluated according to the right and left colon cancer groups, and disease control rates (DCR) were evaluated among the tumour sidedness. Prognostic factors for PFS and OS were evaluated between the two groups. Regorafenib was administered orally 80-160 mg daily, for the first three weeks of each every 4 week cycle.
Data were analysed with SPSS version 25.0. Normal distribution variables in descriptive statistics were expressed as mean ± standard deviation, and non-parametric distribution was expressed as median (min-max). The distribution of variables was evaluated with the Kolmogorov-Smirnov test and Shapiro-Wilk test. An independent t-test (normal distribution) and Mann-Whitney U test (non-parametric) were used to compare continuous variables. Pearson’s and Fisher’s Exact Chi-square testing was used for categorical comparisons. The hazard ratio (HR) and 95% confidence intervals (CI) for exitus and alive associated risk factors were estimated in OS time dependent-Cox proportional hazard regression models and also age, gender, surgery, etc. risk parameters were evaluated the effects on survival with the Cox regression model, p<0.05 was considered significant. Treatment outcomes were DCR, PFS and OS. Response assessment was considered as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) according to the RECIST criteria (version 1.1).10 DCR was defined as the sum of all patients with CR, PR, and SD. Time from the regorafenib initiation date to the date of disease progression or any cause of death was defined as PFS. The Kaplan-Meier method was performed using log-rank analysis to estimate PFS and OS. A comparison of platelets counts, neutrophil counts, lymphocyte counts, carcinoembryonic antigen (CEA), platelet/lymphocyte ratio (PLR), neutrophil/lymphocyte ratio (NLR) and bilirubin levels before and after the use of regorafenib was performed. Wilcoxon test was used for those with symmetrical distribution of the differences between paired measurements of dependent variables and sign test for those without. For repeated measurements, the Wilcoxon test was used for those with normal distribution, and the sign test for those without normal distribution.
RESULTS
The patient characteristics based on the primary tumour sidedness are shown in Table I. In total, 102 patients were included in this study, 66 (65%) were male and 36 (35%) were female. Among these patients, 25 had right-sided , and 77 had left-sided colon cancer. The mean age was 58±10 years in RS mCRC patients and 60±10 years in LS mCRC patients. When evaluating the frequency of RS and LS colon cancer according to gender; RS colon cancer was observed high in women, and LS colon cancer was observed high in men (p=0.017). RAS mutation status was available in 73 patients (71%). RAS wild-type was higher in LS tumours than in RS tumours (p=0.034). MSI status was available in fewer patients and was not statistically evaluated. In RS tumours, the number of metastatic sites are statistically higher than the LS mCRCs (p=0.023). Most of the patients received ≤3 lines of prior systemic therapies in both arms. The majority of patients with both LS and RS colon cancer in first-line therapy received bevacizumab as targeted therapy.
Table I: Patient characteristics according to tumor sidedness (N=102).
|
Characteristics |
Total mCRC |
Right sided mCRC |
Left sided mCRC |
p-value |
|
Age, years (mean±SD) |
59±10 |
58±10 |
60 ±10 |
0.568 |
|
<65 years, n (%) |
63 (62) |
19 (76) |
44 (57) |
0.104 |
|
>65 years, n (%) |
39 (38) |
6 (24) |
33 (43) |
|
|
Sex, n (%) |
|
|
|
|
|
Male Female |
66 (65) |
11 (44) 14 (56) |
55 (71) |
0.017 |
|
Surgery on primary tumour, n (%) |
|
|
|
<0.99 |
|
Yes |
53 (52) |
13 (52) |
40 (52) |
|
|
No |
49 (48) |
12 (48) |
37 (48) |
|
|
Disease status, n (%) |
|
|
|
|
|
Metastatic |
61 (60) 41 (40) |
13 (52) |
48 (62) |
0.482 |
|
RAS status, n (%) |
|
|
|
|
|
Wild Mutant |
42 (41) |
5 (20) 9 (36) |
37 (48) |
0.034 |
|
Unknown |
29 (29) |
11 (44) |
18 (23) |
|
|
Number of Metastatic site(s), n (%) |
|
|
|
|
|
<3 |
49 (48) |
7 (28) 18 (72) |
42 (55) |
0.023 |
|
Prior systemic therapies, n (%) |
|
|
|
|
|
<3 |
91 (89) 11 (11) |
24 (96) 1 (4) |
67 (87) |
0.208 |
|
1. Line targeted therapy, n (%) |
|
|
|
|
|
Anti-VEGF |
68 (67) |
15 (60) 2 (8) |
53 (69) 8 (10) |
0.512 |
|
None |
24 (23) |
8 (32) |
16 (21) |
|
|
2. Line targeted therapy, n (%) |
|
|
|
|
|
Anti-VEGF |
31 (30) 14 (14) |
10 (40) 5 (20) |
21 (27) 9 (12) |
0.178 |
|
None |
57 (56) |
10 (40) |
47 (61) |
|
|
CRC; colorectal cancer. Left side colon cancer: splenic flexure to proximal third of the transverse colon. Right side colon cancer: cecum to distal two-thirds of the transverse colon. p-value is calculated by Pearson's chi square and Fisher's exact chi square test for categorical variables and Mann-Whitney U test for continuous data. Variable age is analyzed by t-test. |
||||
Table II: Univariate/Multivariate analysis of different prognostic factors for progression free survival in patients treated with regorafenib.
|
Parameters |
PFS |
Univariate Analyses Adjusted HR (95% CI) |
p-value |
Multivarivariate Analyses Adjusted HR (95% CI) |
p-value
|
|
Age, years |
|
|
|
|
|
|
<65 years >65 years |
6.407 5.881 |
4.910-7.903 2.223-9.539 |
0.925 |
|
|
|
Sex, n (%) |
|
|
|
|
|
|
Male Female |
5.355 |
3.786-6.925 |
0.395 |
|
|
|
Surgery on primary tumor, n (%) |
|
|
|
|
|
|
Yes |
5.651 |
2.604-8.698 |
0.452 |
|
|
|
RAS status, n (%) |
|
|
|
|
|
|
Wild Mutant |
6.637 |
4.133-9.140 |
0.354 |
|
|
|
Number of metastatic site(s), n (%) |
|
|
|
|
|
|
<3 >3 |
7.162 4.665 |
5.945-8.379 2.456-6.875 |
0.038 |
1.644 (1.094-2.468) |
0.017 |
|
Prior systemic therapies, n (%) |
|
|
|
|
|
|
<3 >3 |
5.749 |
4.074-7.425 |
0.152 |
0.533 |
0.071 |
|
Tumor sideness, n (%) |
|
|
|
|
|
|
Right colon Left colon |
5.454 |
1.647-9.261 |
0.877 |
|
|
|
1. Line targeted therapy, n (%) |
|
|
|
|
|
|
Anti-VEGF |
6.407 |
5.013-7.800 |
0.916 |
|
|
|
None |
5.355 |
1.664-9.046 |
|
|
|
|
2. Line targeted therapy, n (%) |
|
|
|
|
|
|
Anti-VEGF Anti-EGFR None |
3.318 |
0.021-6.615 |
0.383 |
|
|
|
The multivariate analysis model was made with number of metastatic site and prior systemic therapies. Omnibus tests of model coefficients: -2 log likelihood, 738.202, chi-square, 7.783, p: 0.020. |
|||||
Table III: Univariate/Multivariate analysis of different prognostic factors for overall survival in patients treated with regorafenib.
|
Parameters |
OS |
Univariate Analyses mCRC-OS (months) Median [min-max] Adjusted HR (95% CI) |
p-value |
Multivarivariate Analyses Adjusted HR (95% CI) |
p-value
|
|
Age, years |
|
|
|
|
|
|
<65 years >65 years |
8.969 9.856 |
6.450-11.488 6.640-13.072 |
0.251 |
|
|
|
Sex, n (%) |
|
|
|
|
|
|
Male Female |
8.674 |
6.450-10.897 |
0.563 |
|
|
|
Surgery on primary tumor, n (%) |
|
|
|
|
|
|
Yes No |
9.889 8.641 |
4.265-15.514 5.892-11.390 |
0.367 |
|
|
|
RAS status, n (%) |
|
|
|
|
|
|
Wild Mutant |
13.667 |
8.207-19.127 |
0.298 |
|
|
|
Number of metastatic site(s), n (%) |
|
|
|
|
|
|
<3 >3 |
13.667 6.571 |
11.324-16.011 3.558-9.584 |
0.004 |
1.853 (1.190-2.884) |
0.006 |
|
Prior systemic therapies, n (%) |
|
|
|
|
|
|
<3 |
8.674 |
6.908-10.439 |
0.049 |
0.407 (0.191-0.865) |
0.019 |
|
Tumor sideness, n (%) |
|
|
|
|
|
|
Right Colon Left Colon |
6.571 |
4.279-8.863 |
0.238 |
|
|
|
1. Line targeted therapy, n (%) |
|
|
|
|
|
|
Anti-VEGF None |
8.969 12.452 |
6.746-11.193 5.511-19.392 |
0.678 |
|
|
|
2. Line targeted therapy, n (%) |
|
|
|
|
|
|
Anti-VEGF Anti-EGFR None |
8.214 |
4.343-12.084 |
0.148 |
1.411 (0.879-2.267) |
0.154 |
|
The multivariate analysis model was made with number of metastatic site, prior systemic therapies and second line targeted therapy. Omnibus tests of model coefficients: -2 log likelihood, 702.822, chi-square, 16.189, p: 0.003. |
|||||
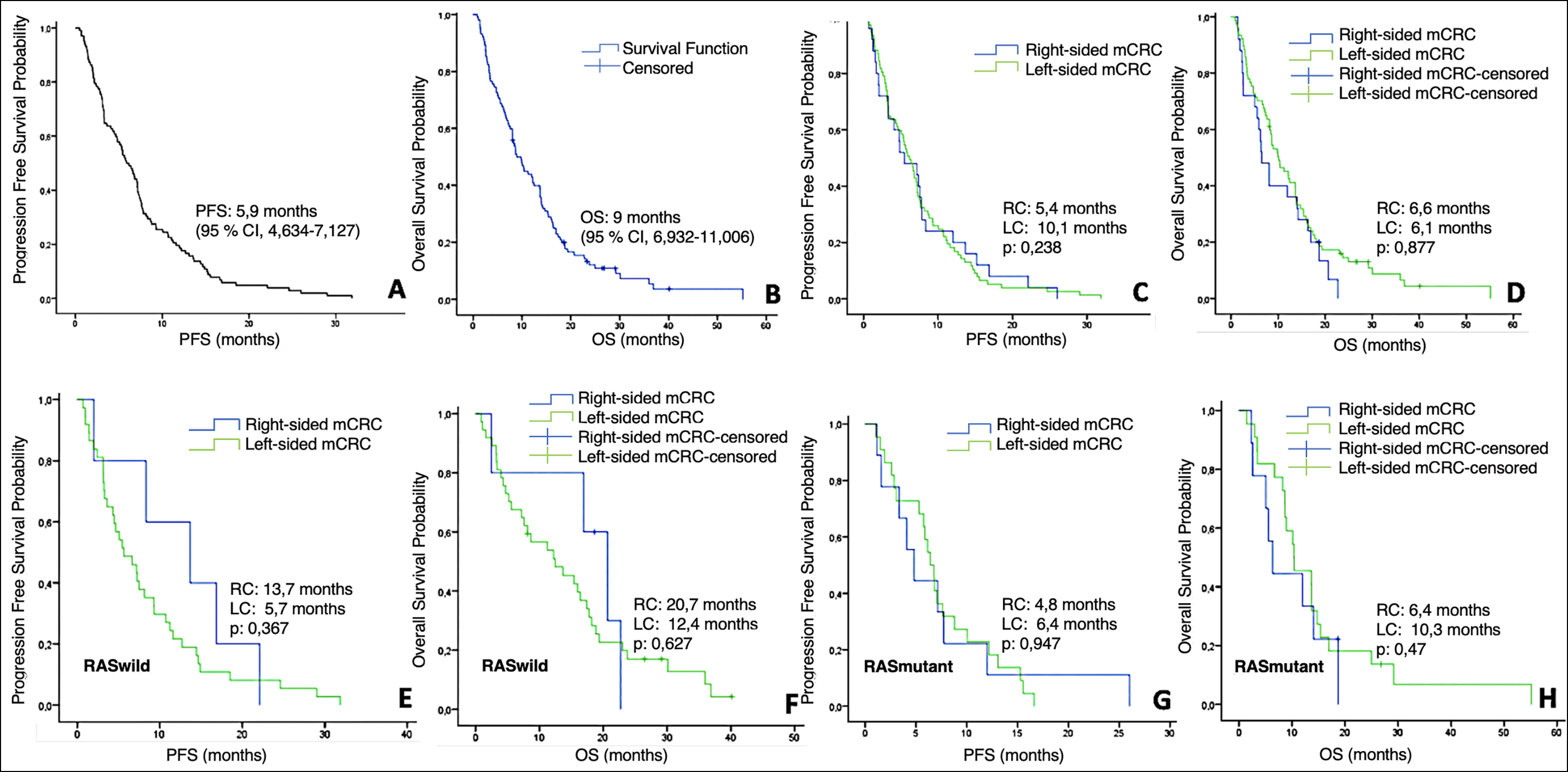 Figure 1: Kaplan-Meier Analysis of PFS and OS with regorafenib in mCRC patients and subgroups. (A) PFS in all patients. (B) OS in all patients. (C) PFS according to tumor sideness. (D) OS according to tumor sideness. (E) PFS according to tumor sideness in RAS-wild type subgroup. (F) OS according to tumor sideness in RAS-wild type subgroup. (G) PFS according to tumor sideness in RAS-mutant type subgroup. (H) OS according to tumor sideness in RAS wild-mutant subgroup.
Figure 1: Kaplan-Meier Analysis of PFS and OS with regorafenib in mCRC patients and subgroups. (A) PFS in all patients. (B) OS in all patients. (C) PFS according to tumor sideness. (D) OS according to tumor sideness. (E) PFS according to tumor sideness in RAS-wild type subgroup. (F) OS according to tumor sideness in RAS-wild type subgroup. (G) PFS according to tumor sideness in RAS-mutant type subgroup. (H) OS according to tumor sideness in RAS wild-mutant subgroup.
DCR with regorafenib were similar in both RS and LS colon tumours (60% vs. 61%, respectively, p>0.99). No patients achieved complete response was in both arms, partial response achieved in 2 patients with the RS tumour and 9 patients with the LS tumour. Stable response was observed in 52% of patients with RS colon tumour and 49% of patients with LS colon tumour. Progressive disease rate was similar in both arms (p=0.874).
At a median follow-up of 9.4 months, the median PFS was 5,9 months (95% CI, 6.3-7.1) and the median OS was 9.0 months with regorafenib (95% CI, 6.4-11.5; shown in Figure 1). When evaluating the survival results by tumour sidedness, the median PFS was 5.4 months in patients with RS colon tumours and 6.1 months in patients with LS colon tumours. The median OS was 6.6 months in patients with RS colon tumours and 10.1 months in patients with LS colon tumours, without statisticially significant level (p=0.238).
Univariate analysis of different subgroups of age, gender, surgery on the primary tumour, RAS status, and first and second-line targeted therapies showed that there was no difference for PFS. Also, no difference was observed in PFS, according to the tumour sidedness. When comparing RS and LS mCRC patients by RAS status, there was an increase in favour of the RS mCRC in both PFS and OS, but this was not statistically significant which was attributed to the unbalanced distribution of patients between groups (5 patients in RS vs. 37 patients in LS) (shown in Figure 1). Patients with metastatic sites <3 had significantly higher both PFS and OS than those with ≥3 (for PFS 7.1 vs. 4.7, respectively, p=0.038, and for OS 13.7 vs. 6.6 respectively, p=0.004). For the number of prior systemic therapies, there was no difference for PFS (p=0.15), but statistically significant difference was found for OS in univariate analysis in favour of >3 line therapies (p=0.049, Tables II and III).
Multivariate analysis was performed with Cox regression model. Patients with metastatic sites <3 had significantly higher PFS and OS rates than those with ≥3 (p=0.017 and p=0.006, respectively). For the number of prior systemic treatments, there was no difference for PFS (p=0.071), but a statistically significant difference was found in multivariate analysis favouring patients who received >3 lines of therapy for OS. (p=0.019, Tables II and III).
A comparison of PLT, neutrophil, lymphocyte, PLR, NLR, CEA and bilirubin levels before and after the use of regorafenib was performed. No significant change was observed in all parameters before and after treatment.
DISCUSSION
This study aimed to investigate the outcomes of regorafenib treatment in refractory mCRC patients, by primary tumour sidedness, the effects of previously targeted therapies, RAS status and inflammatory markers. The median PFS and OS as 5.9 months and 9.0 months, respectively with regorafenib. In multivariate analysis, there was no any significance of tumour sidedness and prior systemic therapies in terms of survival, while the patients with metastatic sites <3 and the number of prior systemic therapies ≤3 line had significantly higher survival.
Colorectal cancers are heterogenous tumours, and the importance of tumour location has increased in recent years. The frequency, pathogenesis, prognosis and treatment responses differ between the proximal and distal of the colon. Right and left colon tumours show distinct molecular features. DNA mismatch repair gene mutations are more common in right-sided tumours, while mutations related to chromosomal instability are more common in LS tumours. Regarding treatment responses, LS mCRC patients benefit more from chemotherapies and EGFR-targeted therapies, while RS mCRC patients do not respond well to conventional chemotherapies and derive numerically greater benefit from anti-VEGF therapies such as bevacizumab than with anti-EGFR agents.14 In addition, right colon tumours show more promising results with immunotherapies due to their high antigenic load and higher probability of high microsattellite instability (MSI-H).
In this study, the median PFS and median OS were found as 5.9 and 9.0 months, respectively at a median follow-up of 9.4 months. In the CORRECT trial, median PFS and OS were 1.9 and 6.4 months respectively; in the CONCUR trial median PFS and OS were 3.2 and 8.8 months respectively.9,10 Higher median PFS and OS values were found in this study than the results reported in the literature. Since the RAS analyses of the patients were performed later due to the access problem, some of them received regorafenib before anti-EGFR treatment, which may have caused higher PFS and OS values compared to the pivotal studies.
The sequence of anti-angiogenic agents and anti-EGFR agents is another issue in the treatment of mCRC patients. A meta-analysis by Khattak et al. showed that right-sided colon cancer patients benefited from an anti-VEGF therapy such as bevacizumab, with a trend for longer survival compared to anti-EGFR agents in first-line therapy (HR:1.3, p=0.081).14 Similarly, in a phase II REVERSE study that evaluated the sequence of regorafenib and cetuximab, it was shown that regorafenib followed by cetuximab suggested a longer OS in RAS and B-RAF wild-type mCRC patients compared to the reverse.12 Therefore, it was planned to evaluate the efficacy of regorafenib, an anti-VEGF agent, in right colon cancer. Because there are opinions that ongoing VEGF inhibition in the 1st and 2nd steps may be more effective due to the low anti-EGFR treatment response in right colon cancer. Saab et al. agreed that tumour sidedness in third-line therapy of mCRC does not affect the response to trifluridine/tipiracil or regorafenib in an expert panel.15 A subanalysis of the prospective observational CORRELATE study with regorafenib in mCRC did not show an association of primary tumour location with the response to regorafenib; OS is 6.7 and 6.3 months for left and right colon carcinomas, respectively (p=0.278).16 In another study evaluating trifluridine/tipiracil and regorafenib, OS and time to treatment failure were not affected by tumour sidedness for both agents.17 Similar PFS results were found in left and right colon tumours (p=0.877) and there was also no difference in response rates. There was no complete response in both RS and LS colon tumours, similar to the results of the CONCUR and CORRECT trials. However, OS was numerically lower in the RS mCRC than in the LS mCRC as expected, but no statistical difference was observed (p=0.33). In the literature, the 5-year cancer-specific survival rate for right and left-sided mCRC was 79.8% and 82.9%, respectively.18 In the present study, the number of patients with RS colon tumour was considerably less than the number of patients with LS colon tumour, as reported in the literature. If the number of patients was close in both groups, it is thought that there would be a statistical difference. Yoon et al. showed that LS mCRC patients had significantly better PFS with regorafenib treatment than RS mCRC patients (2.6 months and 1.9 months, respectively, p=0.04).19
When the RAS status was examined, a significant rate of RAS wild type was detected in LS colon cancers compared to the RS colon cancers (48% vs. 20%, respectively, p=0.034), but there were no statistical differences in terms of PFS and OS. In a similar study conducted by Yoon et al., a statistically significant increase in PFS was observed in patients with LS tumours only in the KRAS wild-type subgroup compared to patients with RS colon tumours.19
It was found that the patients with metastatic sites <3 and the number of prior systemic therapies >3 had significantly higher survival. In patients with low tumour burden, better results are also obtained in the third and next-line treatments. In some studies, only lung metastases have been shown to be associated with a better prognosis.20-22 Prior systemic therapies were evaluated in many studies, in the CONCUR study, patients who received >3 lines of therapies had better overall survival than patients who received ≤3 lines of therapies. In the observational single-arm CONSIGN trial, 46% of patients received >3 lines of systemic treatment and the PFS with regorafenib was 2.7 months, similar to the pivotal studies.22 In this study, the number of prior systemic therapies >3 had significantly higher survival. Nevertheless, the number of patients who received more than 3 lines of treatment in our study constituted 15% of the entire population. According to these results, the efficacy of regorafenib continues in heavily treated patients. The prognostic and predictive value of inflammatory biomarkers has been evaluated in some studies.23-25 In this study, inflammatory biomarkers were measured before treatment and they were compared during the response evaluation, and no significant difference was found in any parameter between responding and non-responding patients.
This study has some limitations, primarily being a retrospective study and the fact that the number of patients was very different between the RC and LC arms, which is thought to affect the statistical analysis. In addition, the number and order of treatment received before regorafenib is heterogeneous due to some problems in accessing targeted medicine in Turkiye.
However, this situation also helped to obtain different data, for example, the continued effectiveness of regorafenib after the 3rd line treatment. As a single-center study and since the median follow-up period is longer from similar studies, it is thought that these results will shed light on future studies.
CONCLUSION
Right and left-sided colon tumours are very different from each other in molecular, clinical and prognostic terms and there are different recommendations for treatment sequencing according to tumour sidedness in analyses performed with targeted therapies. Although there is no distinction yet for regorafenib, first-line anti-VEGF therapies (bevacizumab) in right colon tumours have superior results, regorafenib should be evaluated for treatment sequencing in more comprehensive studies in this respect. Both the PFS and OS were increased in the right colon in the RAS wild-type group, however, this advantage did not reach statistical significance. The tumour burden also affected the response in subsequent treatments and regorafenib was also effective in after third line treatment.
ETHICAL APPROVAL:
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional) and with the Helsinki Declaration of 1975, as revised in 2000. This study protocol was reviewed and approved by Karadeniz Technical University Scientific Research Ethics Committee, approval number 2020/331.
PATIENTS’ CONSENT:
The study is retrospective study. For this reason, patient consent is not available.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
SNK: Conception, design, interpretation, wrote the paper and final approval.
ACO: Data acquisition
NE: Analysis, critical revision
AY: Data acquisition.
EF: Critical revision.
FO: Critical revision.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- https://gco.iarc.fr/today/online-analysis-multi-bas (The last date accessed: 11 september 2022).
- van der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, de Wilt JH. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis 2015; 32(5):457-65. doi: 10.1007/s10585-015-9719-0.
- Venook AP, Niedzwiecki D, Lenz H-J, Innocenti F, Mahoney MR, O’Neil BH, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). American Society of Clinical Oncology (ASCO). Chicago, Illinois, USA: J Clin Oncol 2014; 32:LBA3- LBA3.
- Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15(10):1065-75. doi: 10.1016/S1470- 2045(14) 70330-4.
- Peeters M, Price T, Taieb J, Geissler M, Rivera F, Canon JL, et al. Relationships between tumour response and primary tumour location, and predictors of long-term survival, in patients with RAS wild-type metastatic colorectal cancer receiving first-line panitumumab therapy: Retrospective analyses of the PRIME and PEAK clinical trials. Br J Cancer 2018; 119(3):303-12. doi: 10.1038/s41416-018-0165-z.
- Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur J Cancer 2017; 70:87-98. doi: 10.1016/ j.ejca.2016.10.007.
- https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (The last date accessed: 11 september 2022).
- https://clinicaltrials.gov/ct2/show/NCT02934529 (The last date accessed: 11 september 2022).
- Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. CONCUR investigators. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015; 16(6):619-29. doi: 10.1016/S1470- 2045(15)70156-7.
- Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Correct study group. regorafenib mono-therapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013; 381 (9863):303-12. doi: 10.1016/S0140-6736(12)61900-X.
- Sato Y, Matsusaka S, Suenaga M, Shinozaki E, Mizunuma N. Cetuximab could be more effective without prior bevacizumab treatment in metastatic colorectal cancer patients. Onco Targets Ther 2015; 8:3329-36. doi: 10.2147/OTT. S89241.
- Shitara K, Yamanaka T, Denda T, Tsuji Y, Shinozaki K, Komatsu Y, et al. REVERCE: A randomised phase II study of regorafenib followed by cetuximab versus the reverse sequence for previously treated metastatic colorectal cancer patients. Ann Oncol 2019; 30(2):259-65. doi: 10. 1093/annonc/mdy526.
- Chen D, Gu K, Wang H. Optimizing sequential treatment with anti-EGFR and VEGF mAb in metastatic colorectal cancer: Current results and controversies. Cancer Manag Res 2019; 11:1705-16. doi: 10.2147/CMAR.S196170.
- Khattak MA, Martin H, Davidson A, Phillips M. Role of first-line anti-epidermal growth factor receptor therapy compared with anti-vascular endothelial growth factor therapy in advanced colorectal cancer: A meta-analysis of randomized clinical trials. Clin Colorectal Cancer 2015; 14(2):81-90. doi: 10.1016/j.clcc.2014.12.011
- Bekaii-Saab T, Kim R, Kim TW, O'Connor JM, Strickler JH, Malka D, et al. Third- or later-line therapy for metastatic colorectal Cancer: Reviewing best practice. Clin Colorectal Cancer 2019; 18(1):e117-e129. doi: 10.1016/j.clcc.2018. 11.002.
- Ducreux M, Öhler L, Scheithauer W, Metges J, Dourthe L, Groot J, et al. Impact of tumor location on outcomes in patients with metastatic colorectal cancer (mCRC) treated with regorafenib (REG): An interim analysis from the prospective, observational Correlate Study. J Clin Oncol 2017; 35(15):3567.
- Nagaoka T, Wakatsuki T, Shinozaki E, Nakayama I, Suenaga M, Fukuda N, et al. Prognostic impact of primary tumor location in patients with metastatic colorectal cancer (mCRC) at the salvage lines. J Clin Oncol 2017; 35(4):741.
- Warschkow R, Sulz MC, Marti L, Tarantino I, Schmied BM, Cerny T, et al. Better survival in right-sided versus left-sided stage I - III colon cancer patients. BMC Cancer 2016; 16: 554. doi: 10.1186/s12885-016-2412-0.
- Yoon SE, Lee SJ, Lee J, Park SH, Park JO, Lim HY, et al. The ımpact of primary tumor sidedness on the effect of regorafenib in refractory metastatic colorectal cancer. J Cancer 2019; 10(7):1611-5. doi: 10.7150/jca.29106.
- Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep 2016; 6:29765. doi: 10.1038/srep29765.
- Ge Y, Xiang R, Ren J, Song W, Lu W, Fu T. A Nomogram for predicting multiple metastases in metastatic colorectal cancer patients: A large population-based study. Front Oncol 2021; 11:633995. doi: 10.3389/fonc.2021.633995.
- Van Cutsem E, Martinelli E, Cascinu S, Sobrero A, Banzi M, Seitz JF, et al. A regorafenib for patients with metastatic colorectal cancer who progressed after standard therapy: Results of the large, single-arm, open-label phase IIIb consıgn study. Oncologist 2019; 24(2):185-92. doi: 10. 1634/theoncologist.2018-0072.
- Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): Neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res 2019; 8(6):886-94. doi: 10.21037/tlcr.2019. 11.16.
- Eren T, Karacin C, Ucar G, Ergun Y, Yazici O, Imamoglu GI, et al. Correlation between peripheral blood inflammatory indicators and pathologic complete response to neoadjuvant chemotherapy in locally advanced breast cancer patients. Medicine (Baltimore) 2020; 99(22):e20346. doi: 10.1097/MD.0000000000020346.
- Rossi S, Toschi L, Finocchiaro G, Santoro A. Neutrophil and lymphocyte blood count as potential predictive indicators of nivolumab efficacy in metastatic non-small-cell lung cancer. Immunotherapy 2020; 12(10):715-24. doi: 10.2217/imt- 2019-0154.