Extrahepatic Biliary Tract Variations is an Effect for Acute Calculous Cholecystitis
By Emre Bozdag1, Suleyman Sonmez2, Erkan Somuncu1, Serhan Yilmaz1, Ceren Basaran1, Mehmet Abdussamet Bozkurt1Affiliations
doi: 10.29271/jcpsp.2022.08.991ABSTRACT
Objective: To evaluate the anatomy of the extrahepatic bile duct and to reveal its importance in the formation of acute calculous cholecystitis (ACC).
Study Design: Case-control study.
Place and Duration of Study: Department of General Surgery and Radiology, Kanuni Sultan Suleyman Training and Research Hospital of the University of Health Sciences, Turkey, between January 2016 and December 2021.
Methodology: The data of the patients treated with ACC were analysed on MRCP by an experienced radiologist. The patients were divided into two groups; asymptomatic gallstones (AsGS, control group) and ACC. The cystic duct, common hepatic duct, and common bile duct lengths and variations in cystic duct opening were measured. Receiver operating characteristics (ROC) analysis was conducted to define a cut-off value and compared categorical results of the two groups by Mann-Whitney U test.
Results: One-hundred and seventy-three patients were analysed, one-hundred and seven were females, and 66 were males. The median age was 46 years in the AsGS group and 53 years in the ACC group. It was statistically significant that ACC had a higher median age value than AsGS (p=0.014). In the analysis of extrahepatic variations, cystic duct, common hepatic duct, and common bile duct length, were statistically longer in the calculous cholecystitis group (p<0.001, p=0.022, and p=0.019 respectively). ROC analysis was performed for cystic, common hepatic, and common bile duct length, respectively. Cut-off values were 30.5 mm, 36.5 mm, and 42.5 mm.
Conclusion: Extrahepatic bile duct variations are of critical importance in ACC surgery. In the data, as the cystic duct and common bile duct lengthens, the possibility of ACC increases. There is need for studies with larger samples.
Key Words: Acute calculous cholecystitis, Extrahepatic biliary tract, Anatomical variations, Cholelithiasis.
INTRODUCTION
Acute calculous cholecystitis (ACC) is a clinical condition caused by the inflammatory/infectious processes in the gallbladder wall associated with the gallstones.1 It is the most common complication due to the gallstones in the gallbladder and constitutes one-third of all the emergency surgery applications.2 Patients exhibited one of the local signs of inflammation such as, right upper quadrant pain, fever, and leukocytosis.
Although the cause is usually gallstones, calculous cholecystitis accounts for ten percent of all the cases of acute cholecystitis and 5% to 10% of all the cases of cholecystitis.
Friedman reported the average rate of development of ACC was 6-11% in patients with symptomatic cholelithiasis during the 7-11-year follow-up period.3 Although ACC occurs due to the cystic duct obstruction, its clinical picture, unlike biliary colic, cannot be explained by the obstruction alone.4,5
Various factors are known to play a role in the etiology of ACC. However, there are not enough significant studies in the literature about the cause of cystic duct obstruction which is the main mechanism in the formation of cholecystitis. As it is known, cholecystitis does not occur in almost all the cases of calculous gallbladder.6 The relationship, between anatomical variations in the extrahepatic bile ducts and acute cholecystitis, has never been mentioned in the literature. Previous studies have generally emphasised that conditions in which the anatomy is not well- understood during surgical procedures applied to this region, may increase morbidity and mortality.7 Studies examining the effects of these anatomical structures on the formation of gallstones are also very limited.8 There is no study in the current literature examining their effects on ACC.
In this study, the aim was to examine the association of acute calculous cholecystitis with the basic anatomical structures of this region.
METHODOLOGY
In this retrospective study, the data of the patients, who underwent MRCP at the Kanuni Sultan Suleyman Training and Research Hospital of the University of Health Sciences, Turkey, between 2016 and 2021, were scanned. Patients older than 18 years were included in the study. Patients who had previous cholecystectomy and/or hepatopancreaticobiliary surgery, patients with signs of tumours in the gastrointestinal tract, pregnant women, patients without gallbladder stones, patients in the acute cholecystitis group with MRCP imaging earlier than 8 weeks, and patients whose medical history could not be reached were excluded from the study (Figure 1). Demographic data of the patients were recorded. A total of 173 patients with a history of calculous cholecystitis were divided into two groups; 106 patients in group 1 and 67 patients in group 2 (Figure 1). Ethical approval for the study was granted by the hospital in which the procedures were performed (IRB No. 2021.08.239).
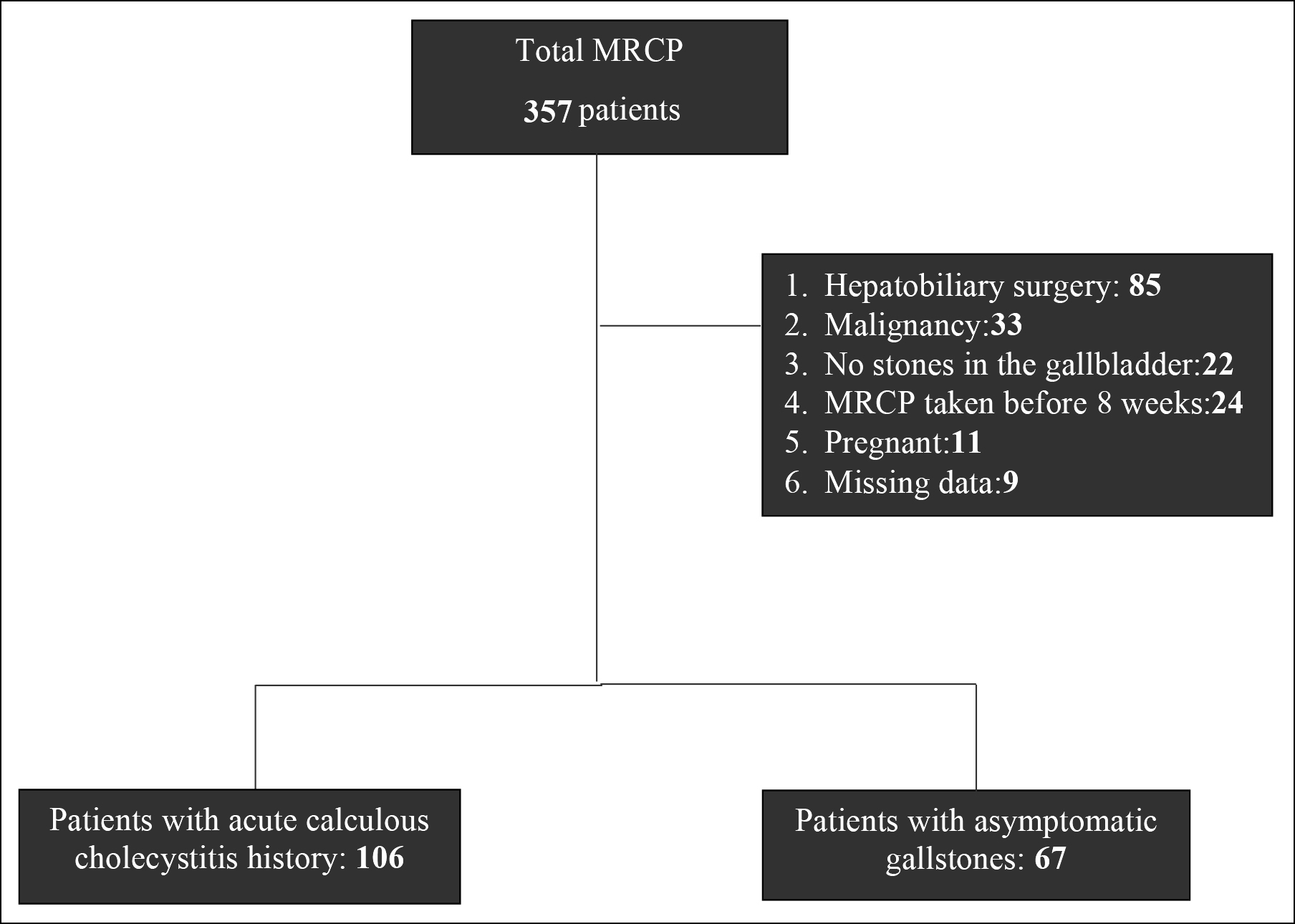 Figure 1: Selection and distribution or stratification of the patients.
Figure 1: Selection and distribution or stratification of the patients.
MRCP data were analysed retrospectively. All the MRI examinations were performed with the whole body Siemens Magnetom Aera device using a 1.5 Tesla unit abdominal coil. Imaging parameters for T2W-TSE slices were set to 1100 ms / 620 ms / 1 [(repetition time (TR) / echo time (TE) / mean number of the signals (NSA)]. In addition, MRCP 3D images were acquired. Cystic duct, common hepatic duct, and common bile duct lengths and variations in cystic duct opening were evaluated retrospectively by an experienced radiologist (Figure 2). Cystic duct: the part of the gallbladder from the end of the infundibulum to the beginning of the common bile duct. Common hepatic duct: the part from bifurcation to the part where the cystic duct opens into the common bile duct. Common bile duct: this structure was considered as the part from the junction of the cystic duct with the common hepatic duct to the point where it opens into the duodenum. These anatomical structures were marked on MRI and measured as a straight line (2D).
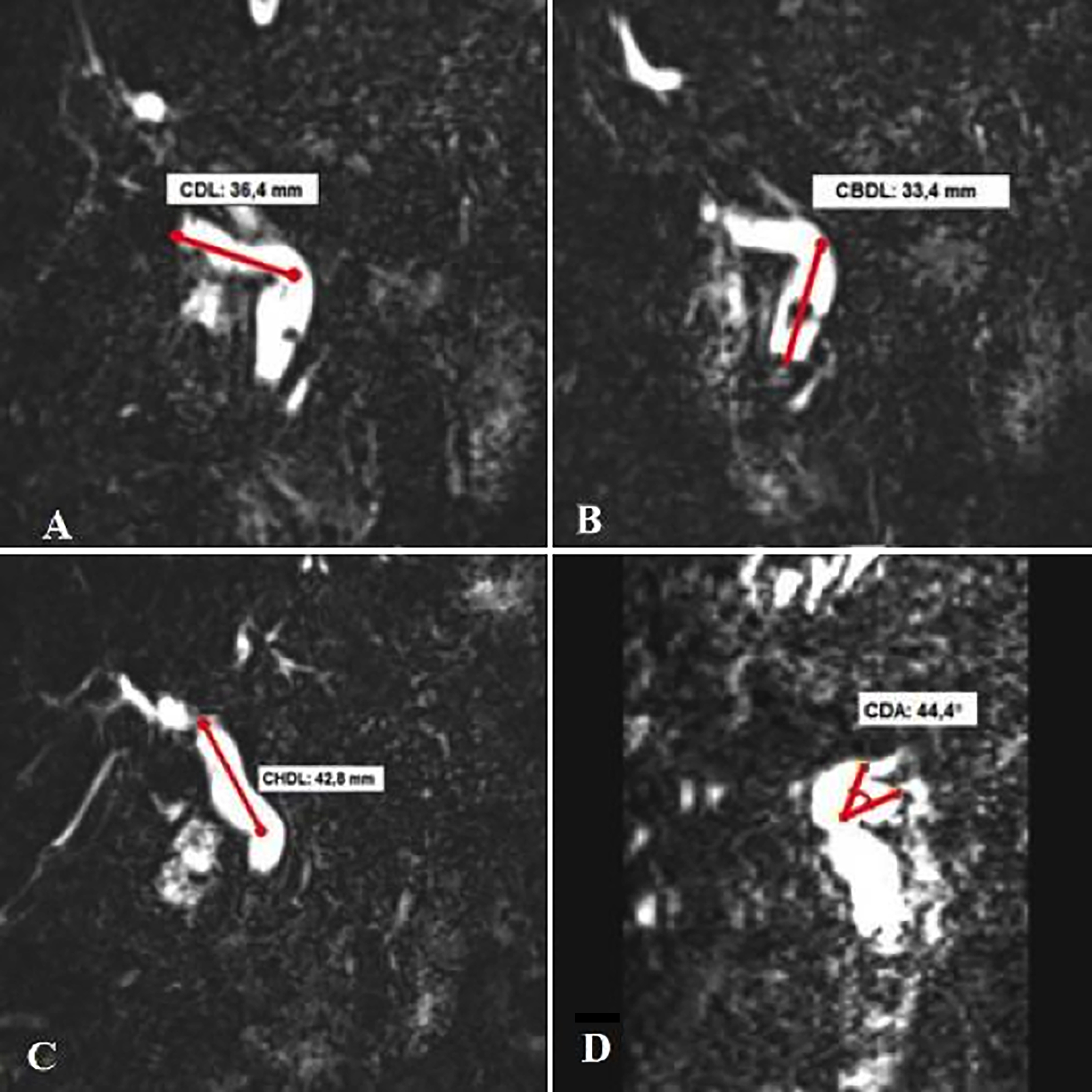 Figure 2: Anatomical structures measured on MRCP image: (a) Cystic duct length (CDL), (b) Common hepatic duct length (CHDL), (c) Common bile duct length (CBDL), and (d) Cystic duct angle (CDA).
Figure 2: Anatomical structures measured on MRCP image: (a) Cystic duct length (CDL), (b) Common hepatic duct length (CHDL), (c) Common bile duct length (CBDL), and (d) Cystic duct angle (CDA).
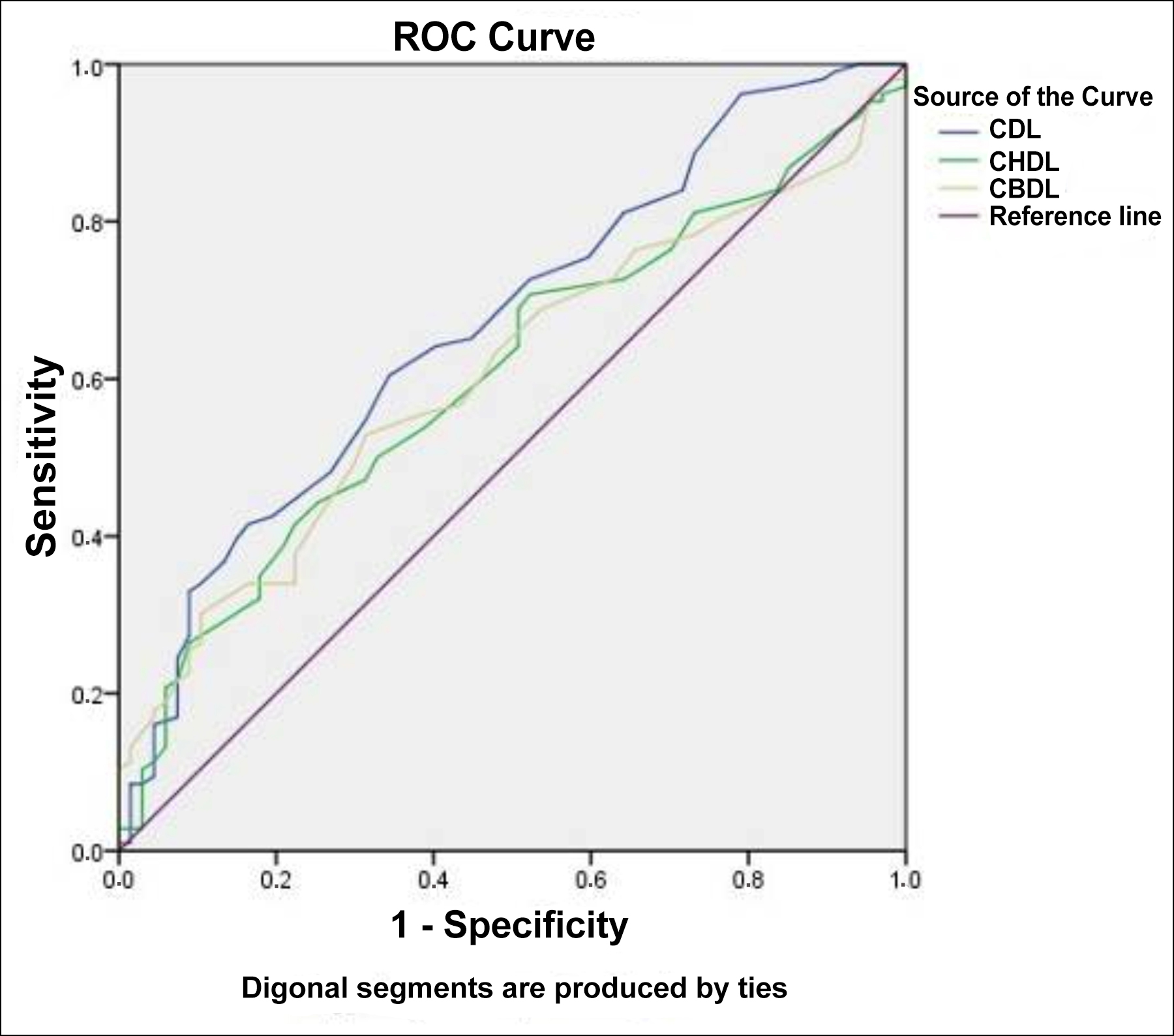 Figure 3: ROC analysis.
Figure 3: ROC analysis.
The cystic duct was assessed as low, middle, and high according to the location where it opened to the extrahepatic duct. These variations have been classified into 6 subgroups; mid-lateral (ML), mid-medial (MM), mid-anterior (MA), mid-posterior (MP), low-medial (LM), and high- lateral (HL).9
All the statistical analyses were performed with the Social Science Statistical Package 22.0 for Windows (SPSS 22) Frequencies and percentage were given for the categorical variables, median (minimum-maximum), Q1-Q3, and IQR (Interquartile Range) values were given for non-normally distributed continuous variables. The normality of continuous variables was checked with the Shapiro-Wilk test. Mann-Whitney U test was used to compare continuous variables that did not show normal distribution, and the Chi-square test was used to compare categorical variables. In order to determine the mechanism of acute cholecystitis, the ROC curve was used to determine the optimum cut-off values. Variables showing a significant relationship in univariate analysis were evaluated with multivariate binary logistic regression analysis to determine the risk factors for acute cholecystitis, and the odds ratio was calculated. Spearman correlation test was used for correlation analysis. Results with a p-value of <0.05 were considered significant.
RESULTS
A total of 173 patients were included in the study. The median age of the patients was 50 years (IQR: 39-63.5). The female/male ratio in the patients was 61.8% (n=107) / 38.2% (n=66).
Group 1 had 61.3% (106) patients and Group 2 had 38.7% (67) patients. The median age of the ACC group (Group 1) was 53 years (IQR: 26.5), and the median age of AsGS group (control group 2) was 46 years (IQR: 20). Age was significantly advanced in the ACC group p=0.014.
The female/male ratio in the ACC group was 58.5% (n= 62) / 41.5% (n=44), and the female/male ratio in the control group was 67.2% (n=45) / 32.8% (n=22, p=0.253). The cystic duct variance was 49.1% (85) ML, 23.1% (40) MP, 16.2% (28) HL, 8.1% (14) MM, 2.3% (4) MA, and 1.2% (2) LM. The cystic duct variance in the ACC group was 47.2% (50) ML, 23.6% (25) MP, 17.0% (18) HL, 8.5% (9) MM, 1.9% (2) MA, and 1.9% (2) LM.
The cystic duct variance in the control group was 52.2% (35) ML, 22.4% (15) MP, 14.9% (10) HL, 7.5% (5) MM, and 3.0% (2) MA. There was no significant difference between the groups in terms of cystic duct variance (p=0.766, Table I).
The median cystic duct angle of the participants was 42 degrees (IQR: 13.5). In the ACC group, the cystic duct angle was 42 degrees (IQR: 13.25). In the control group, the cystic duct angle was 41 degrees (IQR: 13.25). There was no significant difference between the groups in terms of cystic duct angle (p=0.317, Table I). The median cystic duct length was 31 mm. In the ACC group was 32 mm (IQR: 13.5), and in the control group was 28 mm (IQR: 10). The cystic duct was significantly longer in the ACC group (p<0.001, Table I).
Median common hepatic duct length was 33.5 mm in the ACC group (IQR: 15) and in the control group was 31 mm (IQR: 10). The common hepatic duct was significantly longer in the ACC group (p=0.022).
The median common bile duct length was 43 mm (IQR: 18) in the ACC group and 38 mm (IQR: 12) in the control group. The common bile duct was significantly longer in the ACC group (p=0.019, Table I).
ROC analysis was performed for the cystic duct, common hepatic duct, and common bile duct lengths. In the formation of acute cholecystitis, the cut-off value of 30.5 mm cystic duct length was found with a sensitivity of 60.4% and a specificity of 65.7%, an accuracy of 0.668 (95% CI: 0.587-0.750, p<0:001). In an acute cholecystitis, 41.5% sensitivity and 77.6% specificity were found to be cut-off value of 36.5 mm common hepatic duct length with an accuracy rate of 0.604 (95% CI: 0.519-0.688, p=0.022). In an acute cholecystitis, the cut-off value of 42.5 mm common bile duct length was found with 52.8% sensitivity and 68.7% specificity, an accuracy rate of 0.606 (95% CI: 0.522-0.689) (p=0.019, Figure 3).
No correlation was found between the formation of acute cholecystitis and lengths of the cystic duct and common bile duct by multivariate binary logistic regression analysis evaluation (r = -0.038, p=0.623 / r = -0.112, p=0.143). There was no correlation between weight and cystic duct length and common bile duct length (r = -0.097, p=0.205 / r = 0.018, p=0.817). No correlation was found between BMI, cystic duct length, and common bile duct length (r = -0.082, p=0.283 / r = 0.076, p=0.321). Regression analysis was not performed due to the variables did not show correlation.
Multivariate binary logistic regression analysis was performed for the variables that showed a significant relationship in univariate analysis. The cystic duct and common bile duct lengths were found to be risk factors for the development of acute cholecystitis (p=0.012, OR=1.057, 95% CI: 1.012 – 1.103), (p=0.004, OR=1.051, 95% CI: 1.016 – 1.088, Table II).
DISCUSSION
ACC is a common health problem known for its various complications. However, symptoms related to the cholelithiasis are a leading gastrointestinal problem for hospitalisation and healthcare use.10 There are many etiological factors in the formation of gallstones that cause this scenario. The main ones are age, female gender, high-fat diet, insufficient fiber intake, obesity, genetic, and environmental factors.11 Taştemuru published in 2020 that anatomical variations are etiologically effective in the formation of gallstones.8 The present study was designed to reveal the effect of anatomical structures on the etiology of ACC. In the study, it was shown that especially cystic duct length and common bile duct length could play a role in the etiology. However, the authors observed that there is no study examining the relationship of a similar condition with ACC.
Primarily, physical examination and laboratory methods are used in the diagnosis of acute cholecystitis.12 In spite of the fact that ultrasonography (USG) and computed tomography (CT) imaging are the primary choices, bile duct anatomy cannot be evaluated clearly with these techniques, while MRCP, Percutaneous transhepatic cholangiography (PTC), and endoscopic retrograde cholangiopancreatography (ERCP) can clearly demonstrate the anatomy.13 Invasive imaging techniques such as ERCP and PTC are often preferred in the cases where biliary tract drainage is required due to the various complications (perforation, pancreatitis, and significant radiation exposure).
Table I: Comparison of categorical results between group 1 and group 2.
|
Cystic duct variance |
Group 1 (n=106) |
Group 2 (n=67) |
p-value
|
||||||
|
% (n) |
% (n) |
||||||||
|
Normal |
49.1% (85) |
52.2% (35) |
0.766* |
||||||
|
Posterior |
23.1% (40) |
22.4 % (15) |
|||||||
|
Anterior |
2.3% (4) |
3.0% (2) |
|||||||
|
Medial |
8.1% (14) |
7.5% (5) |
|||||||
|
High |
16.2% (28) |
14.9% (10) |
|||||||
|
Low |
1.2% (2) |
- |
|||||||
|
|
Median (min - max) |
Q1 |
Q3 |
IQR |
Median (min - max) |
Q1 |
Q3 |
IQR |
|
|
Cystic duct angle (°) |
42 (23-78) |
36.75 |
50 |
13.25 |
41 (23-67) |
35 |
49 |
13.25 |
0.317** |
|
Cystic duct length (mm) |
32 (17-64) |
25 |
37.5 |
13.5 |
28 (4-63) |
23 |
33 |
10 |
<0.001** |
|
Common hepatic duct length (mm) |
33.5 (10-65) |
27 |
42 |
15 |
31 (17-56) |
25 |
36 |
10 |
0.022** |
|
Common bile duct length (mm) |
43 (20-77) |
35 |
53 |
18 |
38 (24-60) |
33 |
45 |
12 |
0.019** |
|
Group 1: Acute calculous cholecystitis group. Group 2: Asymptomatic gallstones. Note.* Chi-square tests, ** Mann-whitney U test. |
|||||||||
Table II: Determining the severity of the risk factors for acute calculous cholecystitis.
|
|
S.E |
Exp (β) (95% CI) |
p-value |
|
Cystic duct length (mm) |
0.022 |
1.057 (1.012-1.103) |
0.012 |
|
Common hepatic duct length (mm) |
0.022 |
1.033 (1.033-0.989) |
0.146 |
|
Common bile duct length (mm) |
0.018 |
1.051 (1.016-1.088) |
0.004 |
|
SE: Standard error Exp (β): Odds ratio CI: Confidence interval. |
|||
MRCP is used as the gold standard since it is a non-invasive method and has high-sensitivity in revealing the biliary tract anatomy.14 Despite the fact that its clinical significance has not yet fully explained, there are various anatomical variations in the intra and extrahepatic bile ducts.15 Although various factors in the etiology as well as anatomical variations were mentioned in the formation of gallstones, sufficient evidence could not be presented. ACC is a surgical emergency with critical management.16,17 It is mentioned in the literature that symptoms occur in only 10% of the patients with cholelithiasis during their follow-up.3,18
Cholecystitis does not occur in every case with gallstones. In this study, it was evaluated for the first time whether anatomical variables have a relationship with the formation of cholecystitis with gallstones in each case. The authors’ evaluated the anatomical features including the distal portion of the extra biliary system. The main reason for examining this position is that ACC is associated with the distal biliary tract and biliary system. For this purpose, the anatomical sections that examined were; opening site of the cystic duct into the common bile duct, cystic duct length, the angle between the cystic duct and the common bile duct, and the length of the main hepatic duct, and the common bile duct. Although the hepatic duct is located proximal in terms of anatomical factors that may constitute acute cholecystitis, it was evaluated because it is a basic anatomical structure. The length of the hepatic duct was determined longer in the ACC group compared to the control group. While no statistical significance was detected as an independent risk factor for the hepatic duct length. Increase in the length of the common bile duct and cystic ducts was observed as a statistically independent risk factor.
The measurements can be topographically anatomic guiding and radiologically effective in the acute cholecystitis clinics in terms of the diagnosis and treatment. In this study, when the patients were evaluated according to the gender, it was observed that both ACC and AsGS were more common in women. This situation was found to be compatible with the literature.19,20 Another finding was that the patients in the acute cholecystitis group were older than the control group.
Considering the anatomical structures in general, an elongated cystic duct may delay the discharge of the gallbladder compared to a short one, and bends in a long cystic duct can be observed more frequently than a short one. When considered from a clinical point of view, this situation may cause a delay in the emptying of the gallbladder and difficulty in the removal of the formed stone from the cystic duct which may lead to the formation of the acute cholecystitis. Another hypothesis is that prolonged cystic duct may cause obstruction by causing angulation disorder with the common bile duct. Since a similar relationship may be valid for other anatomical structures, it is planned to apply this hypothesis in another study. All these hypotheses need to be supported by the different studies with large series. The authos believe that this study will be a guide for other studies on this subject.
The limitations of this study are that it is a single-centred retrospective study, the diameters of the bile ducts could not be examined within the evaluated anatomical structures, and other factors such as the number of stones in the gallbladder were not evaluated in the study. Another limitation of this study is the flat 2D measurement of the defined anatomical structures (Cystic duct, common hepatic duct, and common bile duct) in measurements made with MRCP. The actual length could not be calculated exactly because the 2D measurement was insufficient to calculate the bends in the anatomical structures. The authors think that the lack of study on this subject in the literature will be the basis for the other studies on this subject and will guide them.
CONCLUSION
As cystic duct and common bile duct lengthened, the possibility of ACC increased. It is recommended that patients with long cystic duct and common duct on imaging should be followed closely in terms of ACC. However, the authors believe that the present study results should be confirmed by the large multi-centre studies.
ACKNOWLEDGEMENTS:
The authors sincerely thank the medical students for participating in the study.
ETHICAL APPROVAL:
Ethical approval for the study was granted by the Hospital in which the procedures were performed (IRB No. 2021.08.239).
PATIENTS’ CONSENT:
Informed consent was obtained from all the patients or relatives before the study began.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
EB: Project development, data collection, manuscript writing, manuscript editing, and data analysis.
SS: Project development, data collection, and manuscript editing.
ES: Manuscript writing and editing.
SY: Data collection and analysis.
CB, MAB: Manuscript editing.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Liu Z, Kemp TJ, Gao YT, Corbel A, McGee EE, Wang B, et al. Association of circulating inflammation proteins and gallstone disease. J Gastroenterol Hepatol 2018; 33(11): 1920-4. doi: 10.1111/jgh.14265.
- Gomes CA, Junior CS, Di Saverio S, Sartelli M, Kelly MD, Gomes CC, et al. Acute calculous cholecystitis: Review of current best practices. World J Gastrointest Surg 2017; 9(5):118-126. doi: 10.4240/wjgs.v9.i5.118.
- Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg 1993; 165(4):399-404. doi: 10.1016/s0002-9610(05)80930-4.
- Ibrahim M, Sarvepalli S, Morris-Stiff G, Rizk M, Bhatt A, Walsh RM, et al. Gallstones: Watch and wait, or intervene. Cleve Clin J Med 2018; 85(4):323-31. doi: 10.3949/ccjm. 85a.17035.
- Adachi T, Eguchi S, Muto Y. Pathophysiology and pathology of acute cholecystitis: A secondary publication of the Japanese version from 1992. J Hepatobiliary Pancreat Sci 2022; 29(2):212-16. doi: 10.1002/jhbp.912.
- Shabanzadeh DM. Incidence of gallstone disease and complications. Curr Opin Gastroenterol 2018; 34(2):81-9. doi: 10.1097/MOG.0000000000000418.
- LeCompte MT, Robbins KJ, Williams GA, Sanford DE, Hammill CW, Fields RC, et al. Less is more in the difficult gallbladder: Recent evolution of subtotal cholecystectomy in a single HPB unit. Surg Endosc 2021; 35(7):3249-57. doi: 10.1007/s00464-020-07759-2.
- Taştemur Y. Anatomical variations of the cystic duct in Turkish population and their association with biliary track stone. J Coll Physicians Surg Pak 2020; 30(10):1005-8. doi: 10.29271/jcpsp.2020.10.1005.
- Gunduz N, Doğan MB, Alacagoz M, Yagbasan M, Orhan Soylemez UP, Atalay B. Anatomical variations of cystic duct insertion and their relationship with choledocholithiasis: An MRCP study. Egypt J Radiol Nucl Med 2021; 52(1):1-7. doi:10.1186/s43055-021-00579-x.
- Peery AF, Crockett SD, Barritt AS, Dellon ES, Eluri S, Gangarosa LM, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterol 2015; 149(7):1731-41. doi: 10.1053/j.gastro.2015.08.045.
- Swarne E, Srikanth MS, Shreyas A, Desai S, Mehdi S, Gangadharappa HV, et al. Recent advances, novel targets and treatments for cholelithiasis: A narrative review. Eur J Pharmacol 2021; 908:174376. doi: 10.1016/j.ejphar.2021. 174376.
- Gutt C, Schlafer S, Lammert F. The treatment of gallstone disease. Dtsch Arztebl Int 2020; 117(9):148-58. doi: 10.3238/arztebl.2020.0148.
- Pieracci FM, Jaouen BM, Stovall RT. Management of choledocholithiasis. Acute Cholecystitis Springer Switzerland 2015:p.169-86.
- Hanif H, Khan SA, Muneer S, Adil SO. Diagnostic accuracy of ultrasound in evaluation of obstructive jaundice with MRCP as gold standard. Pak J Med Sci 2020; 36(4):652-6. doi: 10.12669/pjms.36.4.1665.
- Lamah M, Karanjia ND, Dickson GH. Anatomical variations of the extrahepatic biliary tree: Review of the world literature. Clin Anat 2001; 14(3):167-72. doi: 10.1002/ca. 1028.
- Gutt CN, Encke J, Koninger J, Harnoss JC, Weigand K, Kipfmüller K, et al. Acute cholecystitis: Early versus delayed cholecystectomy, a multicenter randomised trial (ACDC study, NCT00447304). Ann Surg 2013; 258(3):385-93. doi: 10.1097/SLA.0b013e3182a1599b.
- Williams AM, Biesterveld BE, Alam HB. Acute cholecystitis. In: Brown C, Inaba K, Martin M. Salim A. (eds) Emerg General Surg 2019; Springer, Cham. doi:10.1007/978- 3-319-96286-3_10.
- Schwab ME, Braun HJ, Feldstein VA, Nijagal A. The natural history of fetal gallstones: A case series and updated literature review. J Matern Fetal Neonatal Med 2020; 1-8. doi: 10.1080/14767058.2020.1863366.
- Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterol 1999; 117(3):632-9. doi: 10.1016/ s0016-5085(99)70456-7.
- Figueiredo JC, Haiman C, Porcel J, Buxbaum J, Stram D, Tambe N, et al. Sex and ethnic/racial-specific risk factors for gallbladder disease. BMC Gastroenterol 2017; 17(1): 153. doi:10.1186/s12876-017-0678-6.