External Iliac Artery Anastomosis and Internal Iliac Artery Anastomosis for Artery anastomosis in Deceased-donor Kidney Transplantation and Multifactorial Analysis of Graft Survival
By Salih Kara, Ercan Korkut, Nurhak Aksungur, Necip Altundas, Gurkan Ozturk, Zuhal Yetis DemirAffiliations
doi: 10.29271/jcpsp.2022.10.1313ABSTRACT
Objective: To determine the effects of surgical techniques applied to arterial anastomosis for kidney transplantation on the graft outcome.
Study Design: Observational study.
Place and Duration of Study: Organ Transplantation Center, Ataturk University Research Hospital and School of Medicine, Erzurum, Turkey, from January 2010 to January 2020.
Methodology: In total, 143 consecutive patients who underwent deceased-donor-donor kidney transplantation during a 10-years period were retrospectively analysed. All patients were divided into two groups according to the vascular anastomosis techniques (end-to side external iliac and end-to-end internal iliac). The two groups were compared in terms of urine volume on postoperative days 1 and 7; blood creatinine levels on postoperative days 1, 2, and 7; complications; and graft survival.
Results: The mean patient age was 42.04 ± 11.1 years. No significant difference was observed between creatinine values and urine amounts for both surgical techniques (p >0.05). Only the amount of urine on the postoperative 7th day had a significant effect on graft survival (p <0.05). There was no significant difference between the two anastomosis techniques in terms of graft survival (p >0.05).
Conclusion: Both surgical techniques can be used safely in renal transplantation and arterial anastomosis. Also, decreased urine volume during follow-up can be considered as an early indicator of graft loss in the long-term.
Key Words: Kidney transplantation, Surgical anastomosis, Delayed graft function, Graft survival.
INTRODUCTION
Kidney transplant is the treatment of choice for most patients with end-stage renal disease. A successful kidney transplant improves the quality of life and reduces the risk of mortality for most patients compared to dialysis.1,2 Kidney transplant is the most common vascularised solid organ transplant. Therefore, negative effects such as graft loss, morbidity, and mortality occur in cases of problems with surgical techniques. However, with the development of surgical instruments and immunosuppressive treatments in recent years, surgical complications have decreased and graft survival has increased.
Technical problems in kidney transplantation are significantly less common compared with those in other solid organ transplants. Arterial anastomosis, one of the three types of anastomoses of kidney transplantation (renal artery, renal vein, and ureter), is a critical step as it directly impacts graft survival. Two arterial anastomosis techniques are typically used in transplantation centres across the world. These include end-to-side anastomosis of the donor renal artery to the recipient external iliac artery and end-to-end anastomosis of the proximal end of the donor renal artery to the distal end of the recipient internal iliac artery. Although end-to-side anastomosis to the external iliac artery is more commonly used, the number of studies in the literature comparing the two techniques is insufficient. However, a randomised controlled trial comparing these techniques suggested that there was no difference between them.3,4 In this study, the aim was to determine the demographic and clinical characteristics and postoperative results of patients who underwent arterial anastomosis due to deceased-donor kidney transplantation.
METHODOLOGY
The approval for the study was obtained from the Ethics Committee of Erzurum Ataturk University, Faculty of Medicine (27/05/2021 No. 4-24), in accordance with Declaration of Helsinki. All patients who underwent deceased-donor kidney transplantation between January 2010 and January 2020 in Ataturk University Transplantation Center were included in the study. These patients were divided into two groups: patients who underwent end-to-end anastomosis to the internal iliac artery and patients who underwent end-to-side anastomosis to the external iliac artery. Both surgical procedures were performed by the same surgical team. The two groups were compared in terms of urine volume on postoperative days 1 and 7, blood creatinine levels on postoperative days 1, 2, and 7; complications; and graft survival. In addition, factors affecting graft survival were analysed. Pediatric kidney transplantation were excluded.
All patients underwent deceased-donor kidney transplantation. Surgical procedures were performed by the same surgical team for all patients. First, the right iliac fossa was prepared, and the external iliac vein, internal iliac artery, and external iliac artery were isolated. The deceased-donor kidney was placed in the right iliac fossa. Subsequently, a side clamp was placed on the external iliac vein, and end-to-side anastomosis of the graft renal vein to the recipient external iliac vein was performed. This was followed by arterial anastomosis. In some patients, a side clamp was placed on the external iliac artery, and end-to-side anastomosis of the graft renal artery to the external iliac artery was performed. In the group using the internal iliac artery the recipient internal iliac artery was isolated, closed with a bull-dog clamp to cut off the flow, and its distal end was located and ligated. Further, the proximal end of the artery was prepared for anastomosis. End-to-end anastomosis of the graft renal artery to the internal iliac artery was performed. Following arterial anastomosis, the graft was perfused in all patients, and bleeding was controlled from both the graft and the anastomotic sites. Following completion of vascular anastomoses, the anastomoses were found to be successful, and urine output from the graft ureter was observed. The graft ureter was anastomosed to the bladder with a double-J stent using the Modified Lich-Gregoir technique.5 All patients were followed up in the transplant intensive care unit (ICU) during the postoperative period and then transferred to the organ transplant ward.
Urine output was monitored hourly from the first hour after surgery. The fluid regimen was based on the calculation of the patient’s urine output, weight, and other losses. In all patients, urinary catheters were removed on postoperative day 3 or 4. The double-J catheter inserted during surgery was removed on day 21. Oral regimen intake was initiated 8 hours after the surgery. All patients were mobilised in the early period (12 hours), and respiratory and lung exercises were routinely performed.
The immunosuppression regimen followed for all recipient patients during the induction phase included a dose of 15 mg/Kg methylprednisolone during anaesthesia and 1–2 mg/Kg anti-thymocyte globulin (ATG) initiated 6 hours after declamping. For the maintenance regimen, when the creatinine values fell below 3 mg/dl, 0.15 mg/Kg tacrolimus (divided into two doses; target value 8–12) and 5 mg/Kg cyclosporine (divided into two doses; target value C0: 150–300) were administered. On the last day of ATG treatment, mycophenolate mofetil was started at night (1 g in the morning and evening).
Independent samples t-test was performed to analyse the difference between urine volume and creatinine values of the two groups. The differences between complications requiring surgery were analysed using the chi-square test. Survival analysis was performed using the Kaplan–Meier test. The normality test performed before the survival analysis revealed that the follow-up time data did not have a normal distribution Hence, median values were used for the survival analysis. Cox-regression hazard analysis was performed to evaluate the effects of the parameters on graft survival. Data were analysed at a 95% confidence interval, and p significance value was accepted as <0.05. SPSS 22.00 statistics software was used.
RESULTS
In total, 143 patients underwent deceased-donor kidney transplantation between January 2010 and January 2020. The mean patient age was 42.04 ± 11.1 (18–68) years. Further, 47 (32.9%) of these patients were females and 96 (67.1%) were males. In total, 119 (83.2%) of these patients underwent hemodialysis (HD) before transplantation and 24 (16.8%) underwent peritoneal dialysis.
End-to-side anastomosis of the graft renal artery to the external iliac artery was performed in 106 (74.1%) patients, and end-to-end anastomosis to the internal iliac artery was performed in 37 (25.9%) patients. The mean cold ischemia time was 953.30 ± 19.42 minutes for the patients who underwent anastomosis to the external iliac artery, whereas it was 846.49 ± 26.99 minutes for the patients who underwent anastomosis to the internal iliac artery. Urine output at discharge was 3146.75±133.81 in patients who underwent end-to-side anastomosis, while it was 3063.38 ± 249.78 in patients who underwent end-to-end anastomosis. The mean discharge creatinine value was 1.87±0.12 in patients who underwent anastomosis to the external iliac artery, whereas it was 1.69±0.25 in patients who underwent anastomosis to the internal iliac artery (Table I).
Table I: Patients details.
|
|
External iliac artery anastomosis (n=106) |
Internal iliac artery anastomosis (n=37) |
|
Mean age (years) |
42 ± 11 |
41 ± 11 |
|
Gender (male: female) |
70 (48.9%):36 (25.2%) |
26 (18.2%):11 (7.7%) |
|
Dialyse program |
|
|
|
HD |
88 (61.5 %) |
31 (21.7 %) |
|
PD |
18 (12.6 %) |
6 (4.2 %) |
|
Cold ischemia (min) |
953.30 ± 19,42 |
846.49 ± 26.99 |
|
Warm ischemia (min) |
41.36 ± 0.43 |
40.27 ± 0.19 |
|
Outcome creatine |
1.87 ± 0.12 |
1.69 ± 0.25 |
|
Outcome urine (ml) |
3146.75 ± 133.81 |
3063.38 ± 249.78 |
|
Hospital stay (days) |
20.17 ± 1.00 |
17.22 ± 1.07 |
There were no surgical complications in 123 (86%) patients. Arterial thrombosis was not reported in anastomoses performed with either anastomosis technique. However, various surgical complications were reported. Five patients (3.5%) had hematomas requiring surgical intervention, seven (4.9%) required ureteral revision during the follow-up period, three (2.1%) underwent nephrectomy for the transplanted kidney, one (0.7%) had iliac artery aneurysm, and two (1.4%) underwent fenestration due to lymphocele development around the transplanted kidney.
Both groups were compared in terms of postoperative day 1, 2, and 7 creatinine values and postoperative day 1 and 7 urine volumes. Analysis was performed using the independent samples t-test. Equal distribution was reported for all variances based on Levene's test. Based on these evaluations, there was no significant difference in the creatinine values and urine volumes between the two surgical techniques (outcome creatinine p: 0.483; outcome urine p: 0.758).
The two anastomosis techniques were compared in terms of surgical complications using the chi-square test. Based on our data, the accuracy of this test was arguable as there were values with an expected label of <5 in the chi-square test. However, no significant difference was found between the two techniques in terms of complication rates (Pearson’s chi-square test, p = 0.839).
Kaplan–Meier analysis was performed to calculate graft survival; graft function was reported in 122 (85.3%) of 143 patients, whereas graft loss was reported in 21 (14.7%) patients. Based on the examination of the survival results of the Kaplan–Meier analysis, the median was 50.00 months, standard deviation was 4.40, and confidence interval was 41.37–55.62.
Table II: Cox regression analysis of factors affecting graft survival.
|
|
HR |
95% CI |
p |
|
Anastomosis |
0.244 |
0.047-1.271 |
0.094 |
|
Cold ischemia |
1.001 |
0.998-1.003 |
0.498 |
|
Warm ischemia |
0.926 |
0.818-1.047 |
0.219 |
|
Postop 7 day creatine |
0.889 |
0.711-1.110 |
0.299 |
|
Urine postop 1 day |
1.000 |
0.999-1.000 |
0.375 |
|
Urine postop 7 day |
0.999 |
0.999-1.000 |
0.001 |
|
Postop 1 day creatine |
1.081 |
0.833-1.403 |
0.559 |
|
Postop 2 day creatine |
0.781 |
0.629-0.970 |
0.025 |
Cox-regression analysis was performed to analyse the factors affecting graft survival. Although graft survival for end-to-end anastomosis to the internal iliac artery appeared better in the survival graph obtained from the analysis, no significant difference was observed between the two anastomosis techniques based on the test analysis (Figure 1; p = 0.094). In addition, cold ischemia time, warm ischemia time, and postoperative 1 and 7 day creatinine values did not significantly affect graft survival. However, urine volume on postoperative day 7 and postoperative day 2 creatinine value had a significant effect on graft survival (Table II; HR: 0.99 p <0.05; HR: 0.78 p <0.005). Accordingly, graft loss decreased depending on the increase in urine volume on postoperative day 7.
DISCUSSION
To achieve successful transplantation and graft survival in kidney transplants, vascular anastomoses must be performed safely. Arterial anastomosis is a type of vascular anastomoses, and the application of the appropriate technique for the appropriate artery is one of the first steps for achieving adequate graft function. Therefore, artery anastomosis sites should be selected according to the number and length of donor arteries. Prior to anastomosis, it is critical to ensure that the recipient arteries have no atheromas and intact intima. External iliac artery and internal iliac artery can be used in the recipient for arterial anastomoses. As there is more atherosclerosis in the internal iliac arteries, external iliac arteries are more commonly used by surgeons. In addition, external iliac arteries are preferred due to their superficial location, their larger size, and the possibility of anastomosis in the presence of multiple arteries in the donor.6 Furthermore, when internal iliac arteries are used, the distal end of the internal iliac artery is ligated and the blood flow to the distal end is blocked, resulting in complications such as erectile dysfunction; therefore, the use of the external iliac artery is more common.7 Issues regarding which area and technique are superior are still debated. While there was no difference in graft and graft survival in kidney transplantation between the two techniques in a study conducted by Daowd and Al Ahmad, the study by Dogan reported that graft survival was better in the group that underwent anastomosis to the internal iliac artery.8,9 Based on the examination of similar studies,10 it was observed that the two techniques did not differ in terms of short-term graft function and complications. In the present study, although end-to-end anastomosis to the internal iliac artery appeared to have better graft survival in the early period based on the survival graph, no significant difference was noted between the two anastomosis techniques based on the test analysis (Figure 1). Therefore, the arterial anastomosis area may not be important for graft survival. However, graft survival may decrease and vascular complications may increase in multiple artery reconstructions for kidney transplantation.11-14
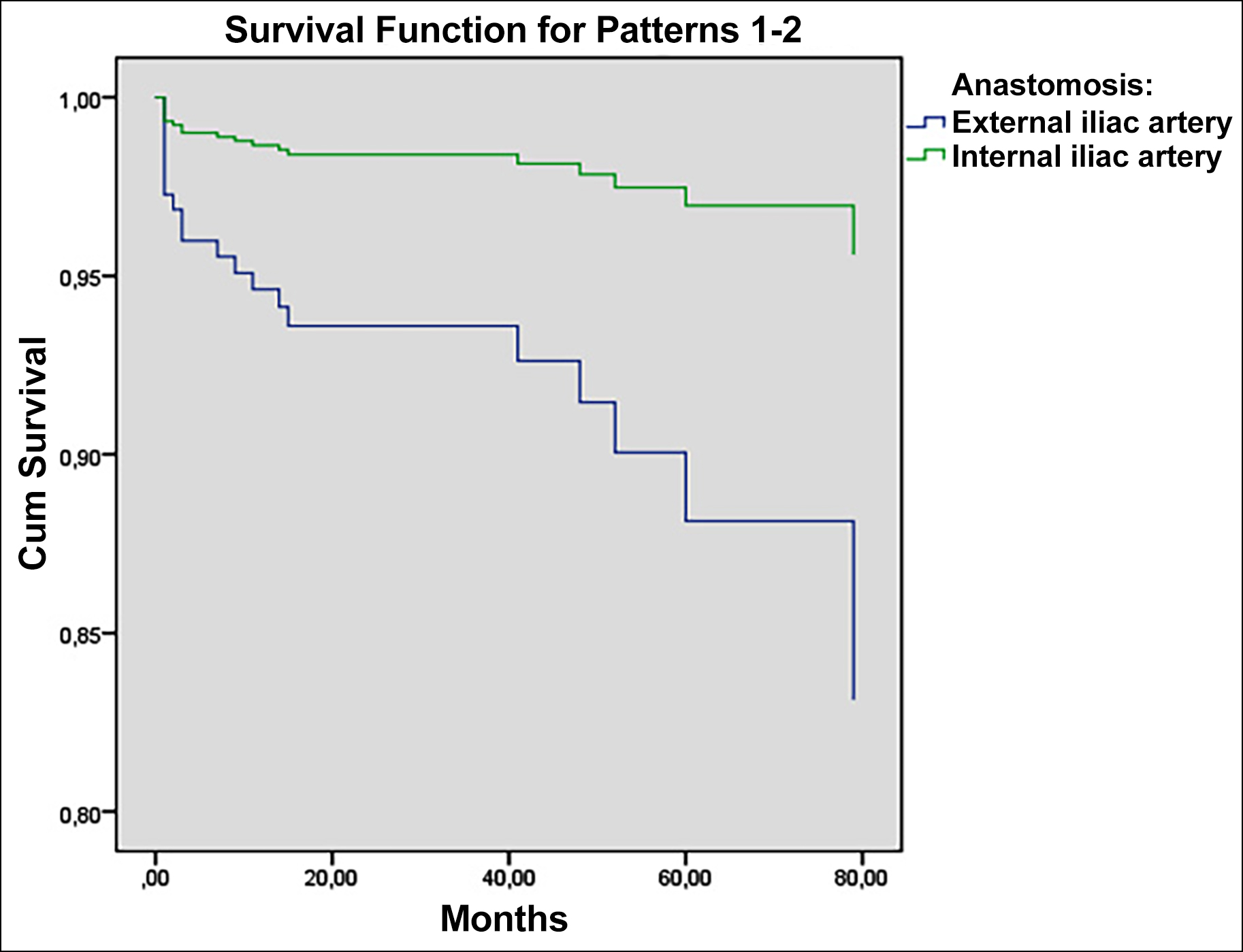 Figure 1: Graft survival graphic.
Figure 1: Graft survival graphic.
In addition to the survival effects, there was no significant difference in postoperative creatinine values and urine volume between the two techniques. Considering that urine volume is an important parameter in terms of graft function, studies comparing the two techniques in terms of urine volume are not sufficiently numerous; therefore, this study is particularly valuable. As per present findings, no difference in urine volume was noted between the two techniques on postoperative day 1, postoperative day 7, and at discharge.
Although 20 patients (13.9%) required surgical intervention due to various complications, no significant difference was found between the two techniques in terms of surgical complications. Among the surgical interventions, the ureteral revision was the most common surgical intervention (4.9%). While the surgical procedures were typically performed using the open technique, laparoscopic fenestration was performed in patients who developed lymphocele due to the low rate of relapse and comorbidity.15
In this study, wherein two anastomotic techniques were compared, other factors affecting graft survival were also investigated. In addition, the effects of cold ischemia time, warm ischemia time, early postoperative creatinine value, and postoperative urine volume on graft survival were investigated. In fact, in the evaluation of the factors affecting graft loss, it would be more appropriate to reach a conclusion by not only focusing on a few factors but by investigating the connection of various factors individually and with each other. Ahmadpoor et al. reported that the risk of graft loss was higher in patients with lower body mass index after 5 years of follow-up. Increased cold ischemia time also increases mean creatinine values. Likewise, increased warm ischemia time and anastomosis time have been reported to have a negative impact on the graft.16-19 However, their effect on long-term graft function remains uncertain.20 Based on the results, cold ischemia time and warm ischemia time in the early period and postoperative 1st and 7th day creatinine values have no effect on graft loss. Further, increased urine volume on postoperative day 7 increased graft survival. Accordingly, increased urine volume during postoperative follow-up can provide an indication on graft survival. Therefore, decreased urine volume during follow-up can be considered as an early indicator of graft loss in the long term. Also postoperative day 2 creatinine value affected graft failure based on our results. Accordingly, the increase in creatinine value on the postoperative day 2 reduces graft loss. Although this result may seem interesting in the first evaluation, the increase in creatinine value on the 2nd day may be significant in terms of long-term graft survival. Surely, for this evaluation, it should be documented with different studies.
This study had certain limitations and strengths. The main limitation was the retrospective design of the study. The main strength of this research was that it was a comprehensive study compared to the limited literature. Similar results were found in the literature. However, the information obtained on the effect of the amount of urine on the 7th postoperative day on graft survival is a unique result.
CONCLUSION
Both surgical techniques can be used safely in renal transplantation and arterial anastomosis. The decreased donor-kidney’s urine volume during follow-up can be considered as an early indicator of graft loss in the long-term.
ETHICAL APPROVAL:
The approval for the study was obtained from the Ethics Committee of Erzurum Ataturk University, Faculty of Medicine (27/05/2021 No. 4-24), in accordance with Declaration of Helsinki.
PATIENTS’ CONSENT:
Written informed consents were obtained from the patients.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
SK: Substantial contribution to the conception, design of the work, analysis, and interpretation.
EK: Design of the work.
NA, NA: Data analysis.
GO: Drafting the work and revising it critically for important intellectual content.
ZYD: Acquisition of data.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Vincenzi PE, Gonzalez JA, Guerra GC, Gaynor BF JJ, Alvarez ADE A, Ciancio Corresponding Author G, et al. Complex surgical reconstruction of upper pole artery in living-donor kidney transplantation. 2020; Available from: www.annals oftransplantation.com/abstract/index/idArt/926850
- Scheuermann U, Rademacher S, Wagner T, Lederer A, Hau HM, Seehofer D, et al. Clinical medicine influence of multiple donor renal arteries on the outcome and graft survival in deceased-donor donor kidney transplantation. J Clin Med 2021; 10(19):4395. doi: 10.3390/jcm10194395.
- Breda A, Budde K, Figueiredo A, Lledó García E, Olsburgh J, Regele H, et al. Renal Transplantation EAU Guidelines on. 2022.
- View of kidney transplant anastomosis: Internal or external iliac artery? 2022 May 19. Available from: https:journals. sbmu.ac.ir/urolj/index.php/uj/article/view/501/402.
- Astolfi RH, Aguiar WF, Viana L, Cristelli M, Junior HTS, Pestana JM. A stentless modified lich-gregoir technique for safe early bladder catheter removal in living and deceased-donor kidney transplants. Urology 2022; 0(0). Available from: www.goldjournal.net/article/S0090429522000309/ fulltext.
- Kumar Pal D, Kumar Sanki P, Roy S. Analysis of outcome of end-to-end and end-to-side internal iliac artery anastomosis in renal transplantation: Our initial experience with a case series. 2017; www.urologyannals.com.
- El-Bahnasawy MS, El-Assmy A, Dawood A, Abobieh E, Ali-El Dein B, Shehab El-Din AB, et al. Effect of the use of internal iliac artery for renal transplantation on penile vascularity and erectile function: A prospective study. J Urology 2004; 172(6I):2335-9. www.auajournals.org/doi/full/10.1097/01. ju.0000144403.03734.11.
- Daowd R, Al Ahmad A. Saudi journal of kidney diseases and transplantation. Saudi J Kidney Dis Transplan 2015; 26(5): 1009. www.sjkdt.org/article.asp?issn=1319-2442;year= 2015;volume=26;issue=5;spage=1009;epage=1012;aulast=Daowd.
- Dogan SM, Dogan G, Simsek C, Okut G, Berktas B, Simsek A, et al. Transplantation using renal grafts with multiple renal arteries: A putative study on the impact of arterial reconstruction technique and site of implantation on outcomes. Transplant Proc 2021; 53(3):920-6. doi: 10. 1016/j.transproceed.2020.08.024.
- Rana A, Agarwal N, Hanumanthappa V, Dokania M. A prospective comparison of end-to-side and end-to-end renal transplant arterial anastomosis in living donor transplants from an Indian centre. Indian J Transplant 2020; 14(2):125.
- Yamanaga S, Rosario A, Fernandez D, Kobayashi T, Tavakol M, Stock PG, et al. Inferior long-term graft survival after end-to-side reconstruction for two renal arteries in living donor renal transplantation. PLOS One 2018; 13(7): 0199629. doi: 10.1371/journal.pone.0199629.
- Afriansyah A, Rasyid N, Rodjani A, Wahyudi I, Mochtar CA, Susalit E, et al. Laparoscopic procurement of single versus multiple artery kidney allografts: Meta-analysis of comparative studies. Asian J Surg 2019; 42(1):61-70. doi: 10.1016/j.asjsur.2018.06.001.
- Iwami D, Hotta K, Sasaki H, Hirose T, Higuchi H, Takada Y, et al. A 2-mm cutoff value is reasonable and feasible for vascular reconstruction in a kidney allograft with multiple arteries. Transplant Proc 2019; 51(5):1317-20. doi: 10.1016/j.transproceed.2019.01.137.
- Sevmis M, Demir ME, Merhametsiz O, Aktas S, Sevmis S, Uyar M. Grafts with multiple renal arteries in kidney transplantation. Transplant Proc 2021; 53(3):933-40. doi: 10.1016/j.transproceed.2020.07.019.
- Lucewicz A, Wong G, Lam VWT, Hawthorne WJ, Allen R, Craig JC, et al. Management of primary symptomatic lymphocele after kidney transplantation: A systematic review. Transplant 2011; 92(6):663-73. doi: 10.1097/TP. 0b013e31822a40ef.
- Ahmadpoor P, Seifi B, Zoghy Z, Bakhshi E, Dalili N, Poorrezagholi F, et al. Time-varying covariates and risk factors for graft loss in kidney transplantation. Transplant Proc 2020; 52(10):3069-73. doi: 10.1016/j.transproceed. 2020.06.015.
- Zavacky P. The impact of the angioplasty of the renal artery and cold ischemia time in kidney transplantation on graft function. Science citation index expanded and in journal citation reports/science edition. Bratisl Lek Listy 2018; 119(7). Available from: www.elis.sk.
- Heylen L, Pirenne J, Samuel U, Tieken I, Naesens M, Sprangers B, et al. The impact of anastomosis time during kidney transplantation on graft loss: A eurotransplant cohort study. Am J Transplant 2017; 17(3):724-32. doi: 10.1111/ajt.14031.
- Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: A critical reappraisal. Am J Transplant 2011; 11(3):450-62. doi: 10.1111/j. 1600-6143.2010.03283.x.
- Weissenbacher A, Oberhuber R, Cardini B, Weiss S, Ulmer H, BC Osme Uller C, et al. The faster the better: anasto-mosis time influences patient survival after deceased-donor donor kidney transplantation. Transpl Int 2015; 28(5): 535-43. doi: 10.1111/tri.12516.