Expression of TLE-1 in Gastrointestinal Stromal Tumour and Its Relationship to Clinicopathological Parameters
By Bermal Hasbay, Fazilet KayaselCukAffiliations
doi: 10.29271/jcpsp.2023.03.286ABSTRACT
Objective: To determine the significance of the TLE-1 expression in GISTs, and evaluate the predictive value of TLE-1 expression in patient survival.
Study Design: An observational study.
Place and Duration of the Study: The archives Department of Pathology, Başkent University, Adana, Turkey, between 2010 and 2021.
Methodology: Fifty patients diagnosed with GIST were included in the study. The diagnosis of GIST was confirmed in all tumours with an immunohistochemistry panel comprising CD117, DOG1, CD34, SMA, S100, and Ki67. The expression of TLE-1 in all tumours was evaluated. Positive and negative predictive values between pathological parameters findings were evaluated and differences between methods were evaluated using 2019 Medcalc. The correlations between the immunoscore value and prognostic parameters were analysed using correlation chi-square test.
Results: Among 50 patients, 31(62%) were males, and 19 (38%) were females. The mean age was 59±13.5 (24-78) years. The tumour grade showed a significant positive correlation with the number of mitoses, tumour size, presence of metastases, and the Ki67 proliferation index. A significant positive correlation was observed between CD117 and DOG-1 immunostaining (r= 0.64, p<0.001). Ki67 index was found to be directly proportional to number of mitoses, tumour size, and DOG-1 expression. Although TLE-1 showed a significant relationship with tumour type (p=0.034), it did not demonstrate a statistically significant relationship with mitosis, tumour grade, or survival. Compared to the spindle cell type, mixed and epithelioid type tumours were immunostained with TLE-1 more frequently and intensely.
Conclusion: While GISTs showed a close relationship with classical prognostic factors, TLE-1 tumour expression was not associated with any prognostic parameter other than the tumour type. Moreover, using an immunostaining panel that includes other sarcomas in the differential diagnosis, especially in tumours with mixed or epithelioid patterns. Molecular genetic confirmation will help the patient in terms of accurate diagnosis and appropriate treatment in difficult cases.
Key Words: Gastrointestinal stromal tumour, TLE-1, Prognosis, Survival.
INTRODUCTION
Gastrointestinal stromal tumours (GIST) are mesenchymal neoplasms that originate from the interstitial cells of cagal. Mazur and Clark first used the term GIST in 1983 to describe gastrointestinal nonepithelial neoplasms; however, this group of tumours was later grouped under a more general name, GIST.1-3 GISTs can develop anywhere in the gastrointestinal tract (GIT). However, they are most often observed in the stomach (54%) and small intestine (30%), less frequently in the colon and rectum (5%), and rarely in the oesophagus and appendix (1%). Extraintestinal GISTs are commonly observed in the omentum, mesentery, and retroperitoneum.1-6
Cases of sporadic GIST can occur at any age, with the highest incidence occurring in the 6th decade. Various syndromes (such as Carney, neurofibromatosis 1, familial KIT, or platelet-derived growth factor receptor alpha (PDGFRA) mutation syndrome) may be associated with 5-10% of cases. Most patients with GIST syndrome are deficient in succinate-dehydrogenase (SDH). GISTs, which occur primarily in children and young adults, are associated with SDH deficiency.5,6
Most GISTs are well-circumscribed, with tumours ranging from a few centimetres to more than 20 centimetres. Microscopically, they may have very different morphological appearances. Three main morphological patterns were observed. Overall, about 70% of GISTs are composed of spindle cells, while 20% are composed of epithelioid cells, and 10% contain a mixture of both types.2 SDH-deficient GISTs have characteristic epithelioid morphology. Compared with spindle cell GISTs, these tumours have higher multinodularity, lymphovascular invasion, and lymph node metastasis.
Immunophenotypically, most GIST show CD117 expression in a strong, diffuse, cytoplasmic, membranous, or perinuclear dot-like pattern, with up to 5% of cases being CD117 negative. The chloride channel protein anoctamin, 1/DOG1 is a GIST marker that is both specific and sensitive. In approximately 50% of CD117-negative GISTs, DOG1 staining is positive. The CD117 and DOG1 are both expressed in the interstitial cells of cagal. The CD34 staining was also positive, particularly in the spindle cell GISTs. They may show positive staining for S100 protein and smooth muscle actin (SMA) at lower percentages and mostly focally.2-6
TLE-1 (transducer-like enhancer of split 1), is a transcriptional co-repressor protein in the Wnt signalling pathway. TLE-1 is a transducer-like enhancer gene that is involved in haematopoiesis, neuronal differentiation, and terminal epithelial differentiation. TLE-1 was initially reported as a highly sensitive and specific marker for synovial sarcomas (SS). In later studies, TLE-1 expression was also found in other soft tissue tumours, particularly schwannomas, malignant peripheral nerve sheath tumours, rhabdomyosarcomas, and SS.7,8
This study aimed to determine the significance of the TLE-1 expression in GISTs, and evaluate its predictive value in patient survival.
METHODOLOGY
The Archives Department of Pathology, Başkent University, Adana, Turkey was scanned, and 50 cases were diagnosed with GIST, between 2010 and 2021 were selected. An 11-year electronic diagnostic data search was performed in the hospital medical data management system using the keyword “Gastrointestinal stromal tumour” in the diagnostic line. Fifty cases diagnosed as GIST in the department and having paraffin blocks in the archives were included in the study. Five cases diagnosed with GIST but without paraffin blocks in the archives were excluded.
Medical records of patients with GIST were based on patients’ clinical records and retrospective clinical data collection. The patients were evaluated in terms of age, gender, localisation, tumour type, tumour size, grade, metastases, number of mitoses, survival, and the outcome of the disease. H&E-stained sections and immunostains of all patients were examined, and parameters including necrosis, mitosis, and tumour diameter were also noted. CD117, DOG1, CD34, SMA, S100, and Ki67 were applied to all the cases at the diagnostic stage, and their expression in tumours was expressed as a percentage of the prevalence and severity. The TLE-1 (clone EPR9386 (2), Abcam, US) was immunohistochemically applied to a paraffin block selected from each patient. Two pathologists blindly assessed the TLE-1 expression. Nuclear staining was considered to indicate positive TLE-1 expression. Tumour TLE-1 expression was graded as negative, when less than 5-10% of tumour cells were stained, 1 positive when 10-50% of tumour cells were stained, and 2 positive when more than 50% tumour cells were stained.
This study was approved by hospital institutional review board and supported by the research and practice hospital of university research fund. As this was a retrospective study, patients did not provide written informed consent for their clinical records to be used in this study.
All statistical analyses were conducted using SPSS software (version 25.0). In this study, positive predictive values and negative predictive values between pathological parameters findings were evaluated and differences between methods were evaluated using 2019 MedCalc. The correlations between the immunoscore value and prognostic parameters were analysed using the correlation chi-square test. Survival rates were evaluated by the Kaplan–Meier method, and the significance of differences between survival curves was determined using the log-rank test. All p-values less than 0.05 were considered statistically significant. Categorical measurements were summarised as numbers and percentages, and continuous measurements were summarised as mean and standard deviation.
RESULTS
Among the 50 patients with GIST, 31(62%) were males, and 19 (38%) were females. The mean age was 59±13.5 (24-78) years. A total of 30 (60%) patients developed GIST in the stomach, whereas only 14 (28%) patients developed GIST in the small intestine, 2 (4%) in the colon, and 2 (4%) in the oesophagus. The remaining two patients (4%) were diagnosed with liver and peritoneal metastases with unknown primary sites. The most common pattern is a spindle cell type (38/50, 76%), followed by mixed (8/50, 16%) and epithelioid (4/50, 8%) patterns (Figures 1,2). A total of 27 cases (54%) were high-grade tumours, whereas the remaining 23 cases (46%) were low-grade tumours.
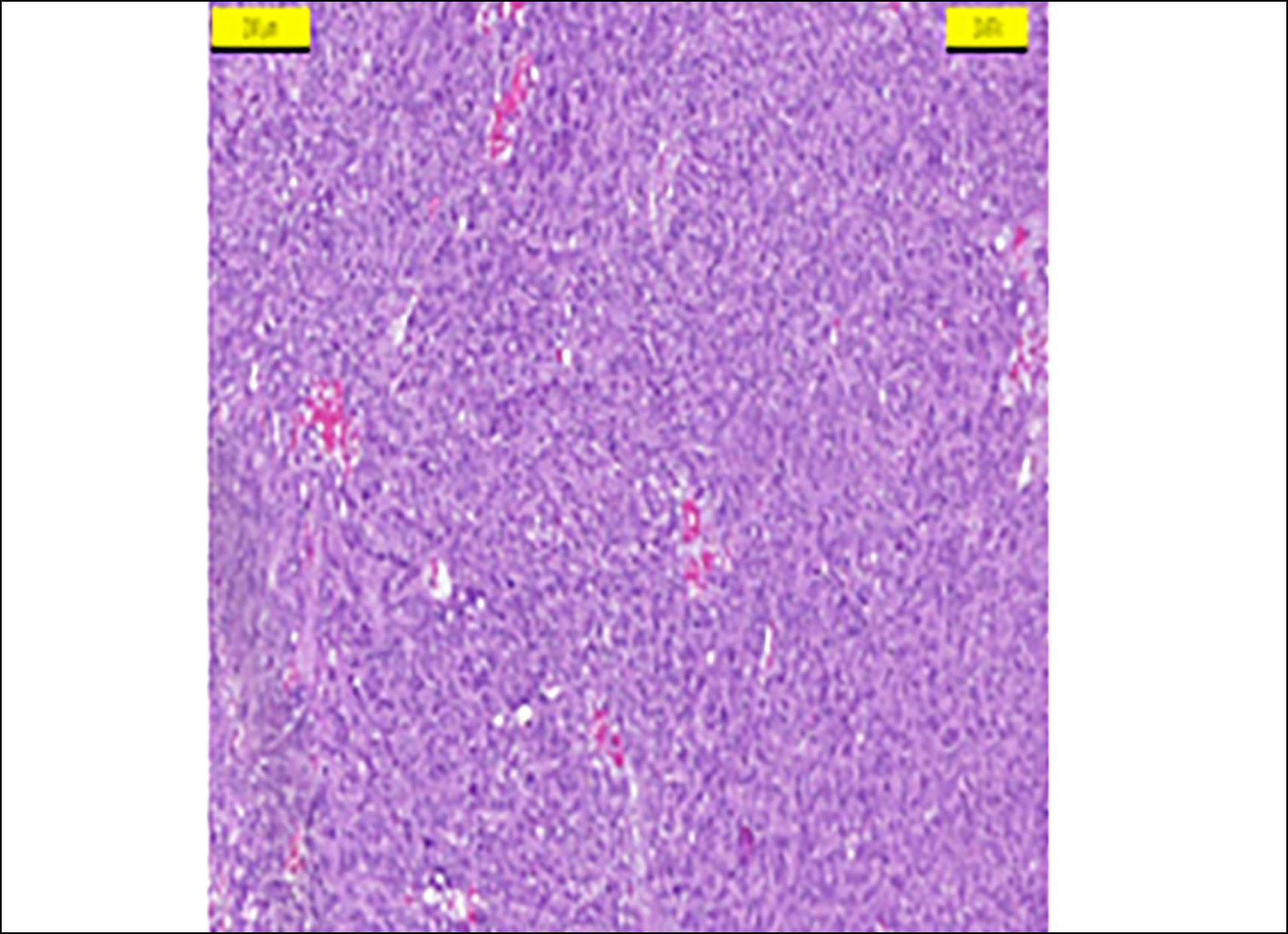 Figure 1: Spindle-shaped gastrointestinal stromal tumour (HEX200).
Figure 1: Spindle-shaped gastrointestinal stromal tumour (HEX200).
Half of the gastric GISTs (15/30) were diagnosed as high-grade and half as low-grade. Similarly, six (42.9%) of 14 cases located in the small intestine were diagnosed as high grade and eight (57.1%) as low-grade. However, all tumours in the colon and oesophagus were diagnosed as high grade. In addition, high-grade GIST was observed in both cases located in the liver and peritoneum, which were diagnosed as metastases of unknown primary sites.
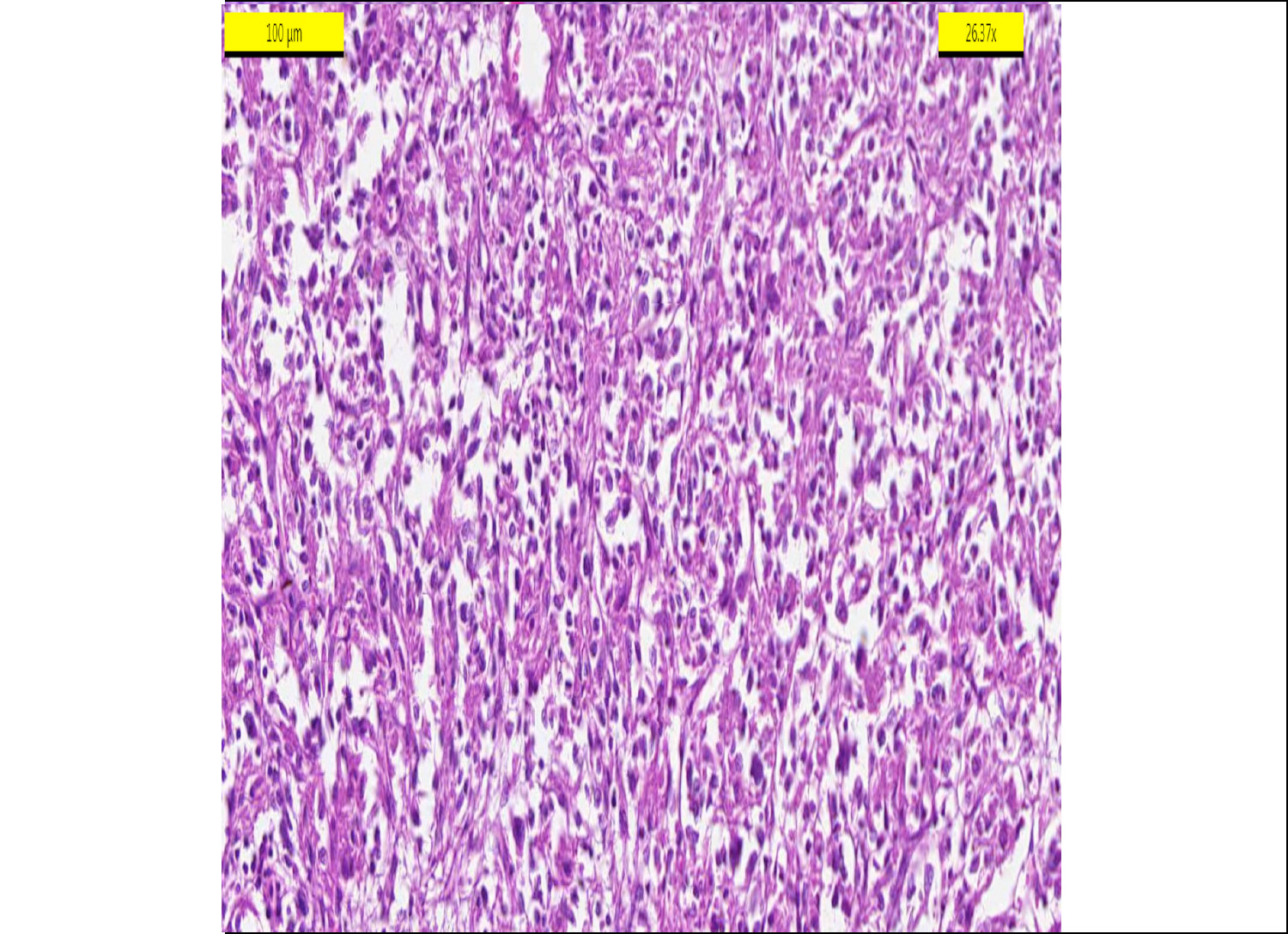 Figure 2: Mix pattern gastrointestinal stromal tumour (HEX200).
Figure 2: Mix pattern gastrointestinal stromal tumour (HEX200).
While, 23 (76.7%) of all gastric GISTs (n=30) showed a spindle cell pattern, only three cases (10%) showed epithelioid, and four patients (13.3%) showed a mixed type GIST. Similarly, a spindle cell pattern was observed in most small intestinal localised tumours (71.4%). The epithelioid pattern was found in only one case (7.2%), and a mixed pattern was found in three patients (21.4%) with small intestinal involvement. A spindle cell pattern was observed in one of the two tumours located in the colon, and a mixed-type pattern was observed in the other. In addition, a spindle cell pattern was observed in tumours located in the oesophagus and in both tumours located metastatically in the liver and peritoneum.
All patients were followed up for 71.4±39 months, and only seven patients died secondary to GIST. Almost all of these patients (6/7) had high-grade GIST, and only one patient had low-grade GIST in the stomach. The other six tumours with high-grade GIST were located in the stomach in two patients, small intestine in three patients, and colon in one patient.
Among all patients, GIST was found incidentally in one patient as a result of the exploration performed during the surgical operation for rectal adenocarcinoma. In addition, two patients diagnosed with rectum cancer were excised during the operation because a mass (leiomyoma or stromal tumour) was described in the stomach (3 cm and 5 cm in diameter) in the abdominal USG. The tumour was localised in the stomach in these three patients; one was diagnosed with low-grade GIST and two with high-grade GIST. In addition, multifocal pancreatic necrosis, common bile duct inflammation, and low-grade GIST located in the stomach were detected during Whipple’s operation performed on a patient with clinical suspicion of pancreatic carcinoma. Moreover, simultaneous gastric adenocarcinoma and low-grade GIST in the stomach were detected in one patient. In another patient, hepatocellular carcinoma (HCC) was detected during follow-up, and the patient died secondary to HCC complications.
The tumour grade showed a significant positive correlation with the number of mitosis, tumour size, presence of metastases, and Ki67 proliferation index. A significant positive correlation was observed between CD117 and DOG-1 immunostaining (R: 0.64, p <0.001). The Ki67 index was found to be directly proportional to the number of mitoses, tumour size, and DOG-1 expression (Table I).
The mean survival time was 91.4±6.4 and 48.2±8.9 months for patients with tumours showing low-grade (<5%) and high-grade (>5%) Ki-67 index, respectively. In addition, the mean survival time was 93.4±10.2 months in patients with tumour size less than 5 cm and 63.9±7.3 months in patients with tumour size more than 5 cm. Moreover, the mean survival time was 82.8±8.7 and 64.3±8.5 months for patients with low (<5 mitoses) and high mitoses (>5 mitoses) rates. The 10-year patient survival was 92.1%, 75%, and 62.5% for tumours with a spindle cell, an epithelioid, and mixed type of GIST, respectively.
Table I shows the clinicopathological statistical values of the parameters. Table II shows the distribution of TLE-1 immunostaining according to the prognostic parameters. Although TLE-1 showed a significant relationship with the tumour type (p = 0.034), it did not demonstrate a statistical relationship with mitosis, tumour grade, or survival. Compared to a spindle cell type GIST, mixed and epitheloid types showed more frequent and intensive staining with TLE-1 (Figure 3). No TLE-1 staining was found in 55.3%, 50%, and 12.5% of tumours with a spindle cell, epithelioid, and a mixed type GIST, respectively.
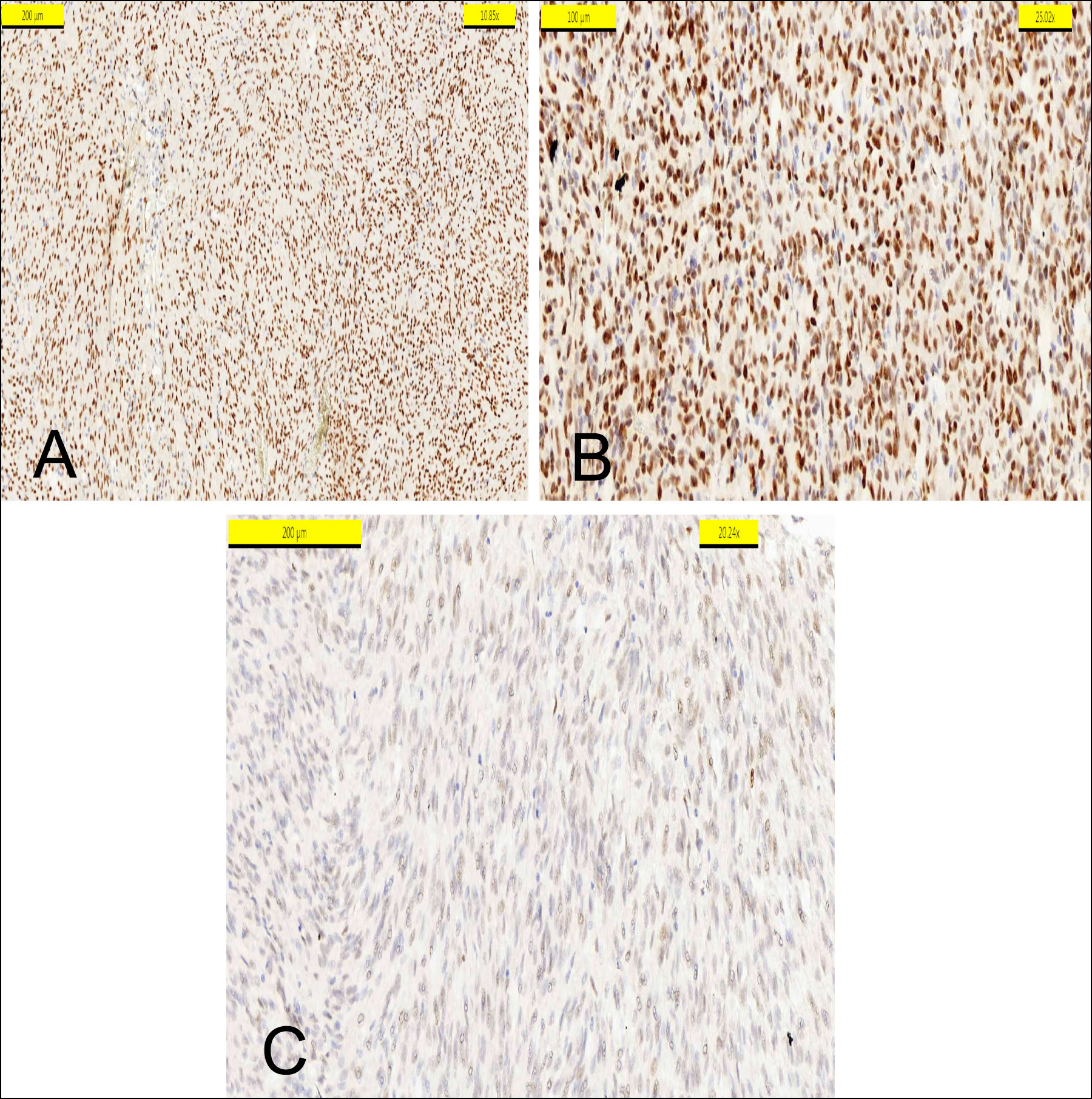 Figure 3: (A) TLE1 positivity in epitheloid type. (B) TLE1 positivity in mix type. (C) Pale positivity of TLE1 in a spindle pattern.
Figure 3: (A) TLE1 positivity in epitheloid type. (B) TLE1 positivity in mix type. (C) Pale positivity of TLE1 in a spindle pattern.
DISCUSSION
The GISTs are the most common mesenchymal gastrointestinal tract tumours. It is believed that the majority of tumours in the GIT referred to as leiomyoma and leiomyosarcoma, in earlier years, were GIST.1-6,9-12 The annual incidence of GISTs has been reported to be 6-15 per million individuals.13 They can be found anywhere in the GIT from the oesophagus to the anus.1-5 The liver and abdominal membranes (peritoneum, mesentery, and omentum) are the most common sites of GIST metastases.
Table I: Clinicopathological statistical values of the parameters (chi-square test and long rank test).
|
Tumour grade Number of mitoses <5 >5
|
N
24 (48%) 26(52%) p<0.001 |
R:0.96 p<0.001
|
Ki67
<%5 >%5
|
N
31 (62%) 19 (38%) |
10 year survival
91.4+- 6.4 month 48.2+- 8.9 month p<0.001 |
|
Tumour size <5 cm >5 cm
|
18 (36%) 32 (64%)
|
R:0.38 p=0.007 |
Tumour size <5 cm > 5cm
|
18 (36%) 32 (64%) |
93.4±10.2 month 63.9 ± 7.3 month p=0.041 |
|
Metastases No Yes Unknown |
29 (58%) 16 (32%) 5 (10%) |
R:0.3 p=0.036 |
Tumour type Spindle type Epiteloid type Mix type |
|
74.75 +34.6 67 +49.4 59.85 +58.1 |
|
Ki67 <%5, >%5
|
31 (62%) 19 (38%)
|
R:0.64 p<0.001 |
Number of mitosis <5 >5
|
24 (48%) 26 (52%) |
82.8±8.7 month 64.3±8.5 month p=0.16 |
|
Tumour type Spindle type Epiteloid type Mix type |
38 (76%) 4 (8%) 8 (16%) |
R:0.64 p=0.65 |
Grade Low grade High grade
|
23 (46%) 27 (54%) |
82.8±8.7 month 64.4±8.5 month p=0.16 |
Table II: The distribution of TLE-1 immunostaining according to the prognostic parameters (Chi-Square test and Fisher’s exact test).
|
|
Total No. of cases |
TLE1 |
|
|
+ |
- |
||
|
Tumour type |
|
|
|
|
Spindle Type Epiteloid Type Mixed type |
38 (76%) 4 (8%) 8 (16%) |
17 (44.7%) 2 (50%) 7 (87.5%) |
21 (57.5%) 2 (50%) 1 (12.5%) |
|
p |
0.034 |
||
|
Grade |
|
|
|
|
Low grade High grade |
23 (46%) 27 (54%) |
14 (28%) 12 (24%) |
9 (18%) 15 (30%) |
|
P |
0.17 |
||
|
Ki67 |
|
|
|
|
<5% >5% |
31 (62%) 19 (38%) |
18 (36%) 8 (16%) |
13 (26%) 11 (22%) |
|
p |
0.2 |
||
|
Tumour size |
|
|
|
|
<5 cm >5 cm |
18 (36%) 32 (64%) |
10 (20%) 16 (32%) |
8 (16%) 16 (32%) |
|
p |
0.63 |
||
|
Mitoses |
|
|
|
|
<5 >5 |
24 (48%) 26 (52%) |
15 (30%) 11 (22%) |
9 (18%) 15 (30%) |
|
p |
0.1 |
||
The mean age of the patients at diagnosis was 60 years.1-5,9-12 The stomach was the most prevalent location in this series, followed by the small intestine, with a mean age of 59 years.
Although various classifications have been used to define the malignant potential of GISTs, these tumours have generally been divided into very low-, low-, intermediate-, and high-risk groups.4-6,12,13 Tumour diameter (maximum tumour diameter in cm), mitotic rate (number of mitoses/in 50 high magnification fields), and tumour location were the most important features for determining malignant potential. These tumours are classified as low- or high-grade, according to the 2019 world health organization classification of tumours (WHO) classification based on the number of mitoses and their progression curves. In the French intergroup clinical practice guidelines, the risk factors for GIST are the number of mitoses, tumour size, location, and tumour rupture. In addition, the ESMO/EURACAN guidelines list histologic type, tumour grade, depth of invasion, and presence of metastases as prognostic factors.6 Although the Ki67 index, tumour diameter, and presence of metastases were associated with survival in this series, no correlation was found with the number of mitoses. Among the histological types, survival was found to be lower in the mixed pattern than in the others. Six of the ex-seven patients had high-grade tumuors.
TLE-1 belongs to a family of four genes, is located on chromosome 9q21, and consists of 19 exons. TLE-1 is a 770-amino-acid transcriptional protein that does not bind to DNA and serves as a co-repressor of other transcription factors and signalling pathways. The TLE-1 protein inhibits transcriptional activity, especially in the Wnt signalling pathway, where it interacts with β-catenin and T-cell factors.14 Although the TLE-1 protein does not bind directly to DNA, it acts on transcriptional factors in the cell by downregulating transcriptional activators, enhancing transcriptional repressors, and converting transcriptional activators into repressors. Su et al., found that, SS18-SSX forms a functional endogenous complex with TLE-1 in SS which represses activating transcription factor 2 (ATF2) and ATF2 target genes, resulting in abnormal transcriptional activity that ultimately leads to malignant transformation.15 Although the TLE-1 immunostaining was originally considered specific for SS, positive TLE-1 immunostaining has also been demonstrated in several other soft tissue tumours (neurofibroma, schwannoma, malignant peripheral nerve sheath tumour, solitary fibrous tumour, rhabdomyosarcoma, leiomyosarcoma, Ewing sarcoma family, and high-grade chondrosarcoma).7,8,14 In other words, a spindle cell soft tissue tumour that is TLE-1 positive is not automatically an SS.14 In recent studies, significant associations were found between high expression of TLE1 and poor prognosis in lung adenocarcinoma, while significant associations were found between low expression and poor prognosis in T-ALL.16, 17In this series, 44.7% of tumours with a spindle cell pattern, 50% of tumours with epithelioid pattern, and 87.5% of tumours with a mixed pattern were diagnosed with GIST with CD117 and DOG-1 positive had TLE-1 staining. No correlation was found between TLE-1 expression and any prognostic parameter. However, tumours with epithelioid and mixed patterns were of a higher grade and showed more extensive TLE-1 immunostaining. Compared to high-grade sarcomas such as SS, GISTs, especially if small and low-grade, usually consist of cells with benign cytological characteristics and include rare mitotic activity. However, many soft tissue tumours, such as SS, primitive neuro-ectodermal tumours, and epithelioid sarcomas, are part of the differential diagnosis of high-grade GIST, especially in mixed or epithelioid patterns.3,4
In these tumours, which may also show TLE-1 staining, an immunostaining panel without DOG1 and CD117 may lead to diagnostic errors. The possibility of GIST should also be considered, especially in high-grade sarcomas that cannot be precisely localised or clinically unknown.
GISTs have been demonstrated to be chemosensitive to varying degrees, particularly those with KIT exon 11, 9,13, 17 mutations and PDGFRA 18,14 mutations. Long, recurrence-free survival rates have been achieved in patients treated with tyrosine kinase inhibitors, such as imatinib, sunitinib, and regorafenib.18,19 The main limitations of this study are small sample size and retrospective data collection.
CONCLUSION
Although TLE-1 expression was only related to histological tumour types in this study, no statistically significant correlation was found with prognostic factors. This may be due to the relatively small number of patients. Using an immunostaining panel that includes other sarcomas in the differential diagnosis, especially in tumours with a mixed or epithelioid pattern, and molecular genetic confirmation in difficult cases will help with patient prognoses.
ETHICAL APPROVAL:
The Non-Interventional Clinical Research Ethics Committee of Baskent University granted the ethics committee approval for the current research (Decision No. KA21/537 dated: 20.01.2022).
PATIENTS’ CONSENT:
Since this is a retrospective study, written informed consent was not given by patients for their clinical records to be used in this study.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
BH, FK: Conceived and designed the study, involved in data collection, analyzed the data, prepared the figures and tables, and drafted the manuscript.
Both the authors have read and approved the final version of the manuscript to be published.
REFERENCES
- Suster S. Gastrointestinal stromal tumours. Semin Diag Pathol 1996; 13(4):297-313.
- Hornick JL, Lazar A. Gastrointestinal stromal tumours, understanding your GIST Pathology Report. Edited by Julia Doswell Royster 2010; 1-25.
- Dei Tos AP, Hornick JL, Miettinen. Gastrointestinal stromal tumour. In: The international agency for research on cancer: WHO classification of tumours of the digestive system. Lyon: ed 5th 2018, pp.439-43.
- Downs-Kelly E, Rubin BP, Goldblum JR. Mesenchymal tumours of the gastrointestinal tract. Odze RD and Goldblum JR: Surgical Pathology of the GI tract,liver, biliary tract, and pancreas. Philadelphia: ed 3rd 2015, pp.822-45.
- Schaefer IM, Mariño-Enríquez A, Fletcher JA. What is new in gastrointestinal stromal tumour? Adv Anat Pathol 2017; 24(5):259-67. doi: 10.1097/PAP.0000000000000158.
- Zhang H, Liu Q. Prognostic indicators for gastrointestinal stromal tumours: A Review. Transl Oncol 2020; 13(10):100812. doi: 10.1016/j.tranon.2020.100812.
- Kosemehmetoglu K, Vrana JA, Folpe AL. TLE1 expression is not specific for synovial sarcoma: A whole section study of 163 soft tissue and bone neoplasms. Mod Pathol 2009; 22(7):872-8. doi: 10.1038/modpathol.2009.47.
- Ali Z, Khan A, Rehman U, Faisal M, Ahmad IN, Mamoon N, et al. Is TLE1 expression limited to synovial sarcoma? Our experience at Shifa International Hospital, Pakistan. Cureus 2019; 11(11):6259. doi: 10.7759/cureus.6259.
- Akkurt G, Uluş H, Aydın A. Gastrointestinal stromal tumours: Review. Turkish Med J 2012; 6:101-7.
- Sturgeon C, Chejfec G, Espat N. Gastrointestinal stromal tumours: A spectrum of diseases. Surg Oncol 2003; 12(1):21-6. doi: 10.1016/s0960-7404(02)00074-9.
- Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumours (GISTs): A review. Eur J Cancer 2002; 38(Suppl 5):39-51. doi: 10.1016/ s0959-8049(02)80602-5.
- Joensuu H, Fletcher C, Dimitrijevic S, Silberman S. Management of malignant gastrointestinal stromal tumours. Lancet Oncol 2002; 3(11):655-64. doi: 10.1016/s1470- 2045(02)00 899-9.
- Miettinen M, El-Rifai W, Sobin HL, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumours: A review. Hum Pathol 2002; 33(5):478-83. doi: 10.1053/hupa.2002.124123.
- Pinto K, Chetty R. Gene of the month: TLE 1. J Clin Pathol 2021; 74(3):137-40. doi: 10.1136/jclinpath-2020-207174.
- Su L, Sampaio AV, Jones KB, Pacheco M, Goytain A, Lin S, et al. Deconstruction of the SS18-SSX fusion oncoprotein complex: Insights into disease etiology and therapeutics. Cancer Cell 2012; 21(3):333-47. doi: 10.1016/j.ccr.2012. 01.010.
- Qianli M, Fei X, Yang Hao, Song Z, Zhang J, Si C, et al. The prognostic role of the transducin –like enhancer of split protein family in lung adenocarcinoma. Transl Lung Cancer Res 2021; 10(7):3251-63. doi: 10.21037/tlcr-21-582.
- Salah A, Mohamed G, Doaa A, Elbaiomy M, Abdelsalam SA, Tawfik A, et al. Prognostic value of TLE1 gene expression in patients with T-cell acute lymphoblastic leukemia. Asian Pacific J Cancer Prev 2021; 22(5):1653-58. doi: 10. 31557/APJCP.2021.22.5.1653.
- Silva MV, Reid R. Gastrointestinal stromal tumours (GIST): C-kit mutations, CD117 expression, differential diagnosis and targeted cancer therapy with imatinib. Pathol Oncol Res 2003; 9(1):13-9. doi: 10.1007/BF03033708.
- Roberts PJ, Eisenberg B. Clinical presentation of gastrointestinal stromal tumours and treatment of operable disease. Eur J Cancer 2002; 38(Suppl 5):37-8. doi: 10.1016/ s0959-8049(02)80601-3.