Evaluation of Serum ANGPTL8/Betatrophin and Cartonectin/CTRP3 Levels in Diabetic and Non-Diabetic Retinopathy
By Vehbi Pehlivan1, Mehmet Ferit Gursu2, Onur Citak3, Erhan Onalan1, Burkay Yakar4, Emir Donder1Affiliations
doi: 10.29271/jcpsp.2023.01.70ABSTRACT
Objective: To determine serum betatrophin and cartonectin levels and their relationship with biochemical parameters in diabetic and non-diabetic retinopathy.
Study Design: Descriptive study.
Place and Duration of Study: Department of Internal Medicine and Ophthalmology, School of Medicine, Firat University, Turkey, from April to November 2020.
Methodology: Patients with diabetic retinopathy (DR), non-diabetic retinopathy (non-DR), type 2 diabetes mellitus without retinopathy, and healthy controls, were enrolled from April to November 2020. Demographic, metabolic, and biochemical parameters were evaluated. Serum betatrophin, cartonectin, IL-6, and TNFα levels were assayed by ELISA methods. One-Way Anova or Kruskal Wallis tests were applied to find out statistical significance among different variables between four groups.
Results: A total of 84 participants (DR= 21 patients; non-DR= 21 patients; 21 diabetic patients without retinopathy and 21 healthy controls) were enrolled in the study. TNF-α level was significantly higher in both DR and non-DR than in controls (p<0.001). IL-6 level was higher in DR group than T2DM and controls (p=0.013). Serum betatrophin level was significantly higher in DR group than in non-DR and T2DM groups (p=0.002). Cartonectin level was decreased in DR, non-DR, and T2DM groups compared to non-diabetic healthy controls (p=0.002).
Conclusion: Serum betatrophin levels are higher in DR, whereas cartonectin levels are lower in both DR and non-DR groups. Serum betatrophins and cartonectin estimation may be helpful in early diagnosis and differential diagnosis in cases of diabetic and non-diabetic retinopathy.
Key Words: Angiopoietin-like protein 8, Cartonectin/CTRP3, Diabetic retinopathy, Diabetes mellitus, Type 2, Diagnosis, Differential, Betatrophin.
INTRODUCTION
Diabetes mellitus is a chronic disease with pathological effects on blood vessels. Vascular pathologies are the basis of complications caused by diabetes mellitus. Diabetes mellitus is a disease that disrupts the structures of large and small blood vessels.1 Diabetic retinopathy is the result of damage to retinal vessels by high blood sugar and is specific to diabetes. It is the leading cause of acquired adult blindness today. Screening and early diagnosis are important to prevent blindness after diabetic retinopathy.2
The human body produces some biochemical substances in physiological and pathological conditions. These biochemical markers can guide physicians in diagnosis, treatment, and prognosis. It has been shown in the literature that HbA1c, which is an indicator of glycemic control, can be used as a biomarker to predict the risk of developing diabetic retinopathy. Inflammatory and angiogenetic changes play a role in the aetiology of DR. Therefore, inflammatory and angiogenic biomarkers may contribute to the follow-up and treatment of DR together with HbA1c. Although there are new studies on new biomarkers in the literature, sufficient evidence has not yet been reached for their use.3-5
Betatrophin is one of the biomarkers that have been researched for use in the diagnosis and follow-up of the disease. Recent studies have emphasised that betatrophin may be associated with lipid metabolism, autophagy, and cell proliferation. Studies showing increased levels of betarophin in the serum and vitreous humour of patients with diabetic retinopathy have brought to mind its possible relationship with diabetic retinopathy.6,7
Another biomarker likely to be associated with diabetic retinopathy may be cartonectin. It has been suggested that cartonectin has blood sugar and hepatosteatosis-reducing effects. In addition, its anti-inflammatory and endothelial cell proliferation stimulating effects have been reported. Considering the effects of cartonectin on blood sugar and lipid metabolism, it can be said that changes in cartonectin level may be related to the etiopathogenesis of DR. Although the functions of cartonectin have been investigated, the relationship between diabetic retinopathy and cartonectin has not been adequately studied in the literature.8
The current study was intended to determine the serum betatrophin and cartonectin levels and their relationship with biochemical parameters in diabetic and non-diabetic retinopathy.
METHODOLOGY
This descriptive study was performed at the department of Internal Medicine and Ophthalmology, between April and November 2020. Open access power analysis test "http://biostatapps.inonu.edu.tr/WSSPAS/" was used for power analysis. For the sample size, the power analysis test was applied by taking into account the one-way analysis of variance method and the mean standard deviation values in independent samples. Considering power 0.8, type 1 error (alpha) 0.005, and effect size 0.96, the minimum number of samples in each group was 18 (72 in total).6 Total of 84 participants were enrolled in the study and divided into four groups as the diabetic retinopathy group (DR= 21 patients), the nondiabetic retinopathy (non-DR= 21 patients), T2DM without retinopathy group (21 patients with T2DM and non diabetic retinopathy), and healthy control group (21 participants without either obesity, or T2DM, or retinopathy). The patients were excluded if they had malignancy, type 1 diabetes mellitus, cardiovascular disease, ocular disease or rheumatological diseases.
Type 2 diabetes mellitus (T2DM) was diagnosed by an endocrinologist according to the American Diabetes Association guidelines.9 The diagnosis of diabetic retinopathy was confirmed by ophthalmologists experienced in the field and experienced in the diagnosis and treatment of diabetic retinopathy. In the ophthalmology department of Fırat University, the diagnosis of DR is made by considering the criteria of international clinical diabetic retinopathy and diabetic macular oedema disease severity scales.10 Diagnosis of non-DR was confirmed by the same ophthalmologists taking into account the absence of DR diagnostic criteria. T2DM group was defined as, patients with T2DM and without any retinopathy. Diagnosis of T2DM was confirmed by an endocrinologist, in addition, two ophthalmologists confirmed no signs of retinopathy in patients. The control group consisted of healthy volunteers who did not report diabetes mellitus, retinopathy and any chronic disease. It was confirmed by the endocrinologist and ophthalmologist that the participants in the control group did not have diabetes mellitus and retinopathy. This study was approved by the Noninvasive Ethics Committee of the Firat University (Date: 08.04.2021 no: 2021:05/41).
Demographic characteristics, which included age, gender, and comorbid diseases were questioned. Standing height of patients were measured with 0.1 cm sensitive linear height scale. The weight of the patients were measured with a sensitive digital scale. Body mass index (BMI) was calculated using the conventional Quetelet formula (BMI= Kg/m2). During overnight fasting, two venous blood samples were collected. One blood sample was sent to the clinical laboratory centre of the hospital within 1 hour for further analyses of FPG, HbA1c, insüline, serum creatinine and lipid profiles including triglyceride, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), vitamin D, AST, and ALT levels. Five-milliliter blood (5 mL) sample was obtained for analyses of IL-6, TNF-α, Betatrophin, and Cartonectin. These samples were centrifuged at 5°C at 4,000 rpm, and were stored at -80°C for further analyses. Betatrophin, Cartonectin, TNF-α, and IL-6 levels were determined by ELISA method according to the criteria recommended by the study kits.
SPSS 22 (Statistical Package for Social Sciences; SPSS Inc., Chicago, IL) program was used for statistical analysis. Frequency (percentage) and mean (standard deviation) or median (Q25-Q75) were used for the descriptive properties of the variables. The statistical relationship between categorical variables was analysed using the Pearson Chi-Square test. The distribution property of continuous variables was analysed using the Shapiro-Wilk test. One Way ANOVA or Kruskal Wallis tests were used for comparisons between groups, taking into account the distribution characteristics of the variables. Correlation between continuous variables was analysed with Pearson correlation or Spearman correlation tests as appropriate. Statistical significance was accepted as p<0.05.
RESULTS
A total of 84 participants (21 diabetic retinopathy, 21 non-diabetic retinopathy, 21 T2DM without retinopathy and 21 control without diabetes mellitus and retinopathy) were included in the study. There was no significant difference sex (p=0.305), ALT (p=0.462), total cholesterol (p=0.265), triglyceride (p=0.204) and VLDL (p=0.411) levels between different goups. The AST level is significantly lower in DR group than in others (p=0.021). TNF-α level was significantly higher in both DR and non-DR than control (p<0.001). IL-6 level was higher in DR group than T2DM and control (p=0.013, Table I).
There are significant differences in betatrophin and cartonectin levels between groups (p=0.002). Betatrophin level is higher in the DR group than in non-DR and T2DM groups (Figure 1).
Serum cartonectin level is decreased in DR, non-DR, and T2DM groups compared to controls (Figure 2).
Table I: Demographics and biochemical characteristic of study participants.|
Variables |
Control (n=21) |
DR (n=21) |
Non-DR (n=21) |
T2DM (n=21) |
p-value |
|
Gender |
|
|
|
|
|
|
Female |
6 (28.6) |
9 (42.9) |
10 (47.6) |
12 (57.1) |
0.305* |
|
Male |
15 (71.4) |
12 (57.1) |
11 (52.4) |
9 (42.9) |
|
|
Age (year) |
42.0 (37.0-48.5)a |
62.0 (60.0-65.0)b |
64.0 (54.0-68.5)b |
58.0 (46.0-65.5)b |
<0.001** |
|
FPG mg/dl |
87.0 (80.5-95.50)a |
175.0 (132.0-242.50)b.c |
90.0 (84.5-101.0)a |
177.0 (123.0-210.5)b.c |
<0.001** |
|
BMI (kg/m2) |
27.0 (23.0-29.0)a |
28.0 (25.0-30.0) |
27.0 (26.0-28.5)b |
30.0 (28.0-31.5)ab |
0.005** |
|
HbA1c ( %) |
5.2 (4.85-5.70)a |
9.9 (7.85-11.30)b.c |
5.70 (5.15-5.80)a |
9.70 (6.0-12.60)b.c |
<0.001** |
|
HOMA-IR |
1.80 (0.94-2.21)a |
6.50 (3.40-19.18)b.c |
1.50 (1.15-2.50)a |
5.80 (2.54-10.24)b.c |
<0.001** |
|
Triglyceride (mmol/L) |
123.0 (88.5-171.5) |
138.0 (105.5-262.0) |
121.0 (91.5-164.0) |
148.0 (113.5-190.5) |
0.204** |
|
TC (mmol/L) |
188.2±30.0 |
189.7±53.9 |
169.0±44.8 |
192.6±36.8 |
0.265*** |
|
LDL-C (mmol/L) |
108.6±21.5 |
110.1±39.9 |
96.5±30.7 |
127.4±31.2 |
0.021*** |
|
VLDL (mg/dl) |
26.0 (17.60-42.50) |
27.0 (21.0-60.0) |
25.0 (17.0-37.0) |
29.6 (23.0-39.5) |
0.411** |
|
D vit (nmol/L) |
23.40 (16.35-29.25) |
12.40 (7.75-17.30) |
16.20 (8.05-19.60) |
17.40 (10.05-22.95) |
0.008** |
|
AST (U/L) |
19.0 (17.0-23.5)a.b |
16.0 (15.0-20.5)a |
25.0 (17.5-31.0)b |
19.0 (16.5-24.5)a.b |
0.021** |
|
ALT (U/L) |
19.0 (14.0-29.5) |
18.0 (14.0-26.5) |
21.0 (15.0-32.0) |
25.0 (16.0-32.0) |
0.462** |
|
Hb |
14.9±1.1a |
13.1±1.8b |
13.0±2.2b |
13.7±1.8a.b |
0.003*** |
|
BUN (mg/dl) |
30.0 (25.0-33.5)a |
47.0 (32.5-56.0)b.c |
42.0 (32.0-60.5)a |
32.0 (25.5-40.0)b.c |
<0.001** |
|
Creatine (mg/dl) |
0.86 (0.75-0.92)a.b |
1.01 (0.76-1.35)a.b |
1.0 (0.81-1.53)a |
0.77 (0.66-0.94)b |
0.009** |
|
IL-6 (pg/ml) |
295.0 (264.5-410.0)a |
432.0 (295.5-557.5)b |
366.0 (355.5-473.5)a.b |
368.0 (274.0-415.5)a.b |
0.013** |
|
TNF-α (pg/ml) |
1055.77 (801.99-1187.85)a |
1436.60 (1242.70-2244.00) |
1807.57 (1512.30-3149.61)ab |
1268.70 (909.08-1530.23)b |
<0.001** |
|
Betatrophin (ng/L) |
377.27 (337.55-439.47)b |
455.37 (364.29-541.14)a |
354.12 (342.09-418.13)b |
345.94 (295.10-414.5)b |
0.002** |
|
Cartonectin (ng/L) |
1.64 (1.04-6.92)a |
0.80 (0.50-1.37)b |
0.57 (0.30-2.25)b |
0.68 (0.56-1.38)b |
0.002** |
|
* Chi-square test; ** Kruskal Wallis test; *** One-Way Anova test; T2DM: Diabetes without diabetic retinopathy; DR: Diabetic retinopathy; non-DR: Retinopathy without T2DM; BMI: Body mass index; FPG: Fasting plasma glucose; HbA1c: Glycated hemoglobin; TC: Total cholesterol; LDL-C: Low density lipoprotein cholesterol; VLDL: Very low density lipoprotein cholesterol; IL-6: Interleukin 6; TNF-α: Tumour necrosis factor-alpha. |
|||||
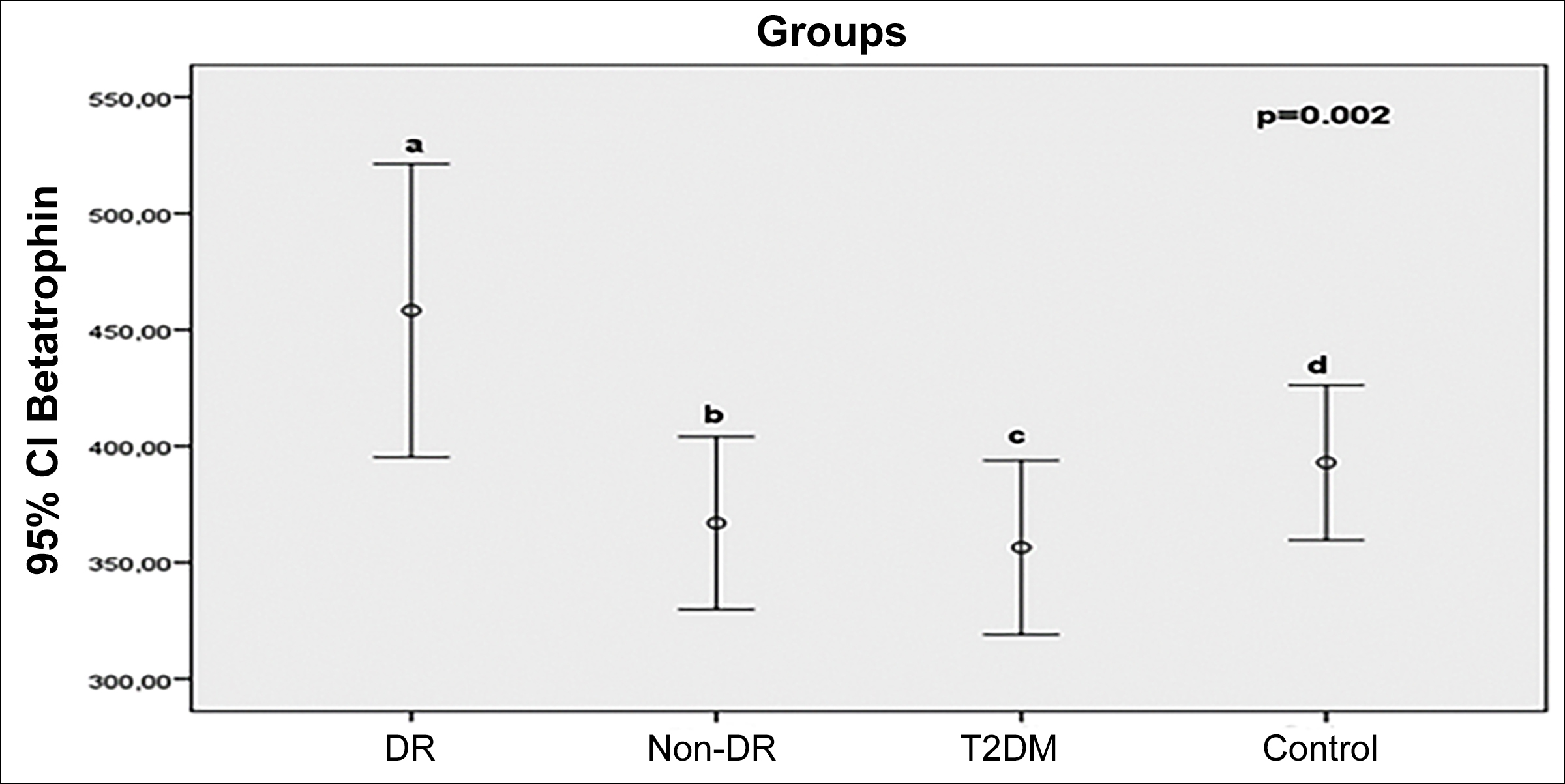 Figure 1: Comparasion of serum betatrophin (ng/L) levels between groups (p=0.002; a-b: 0.037; a-c:0.006; a-d:0.199; b-c:0.507; b-d:0.425; c-d:0.144).
Figure 1: Comparasion of serum betatrophin (ng/L) levels between groups (p=0.002; a-b: 0.037; a-c:0.006; a-d:0.199; b-c:0.507; b-d:0.425; c-d:0.144).
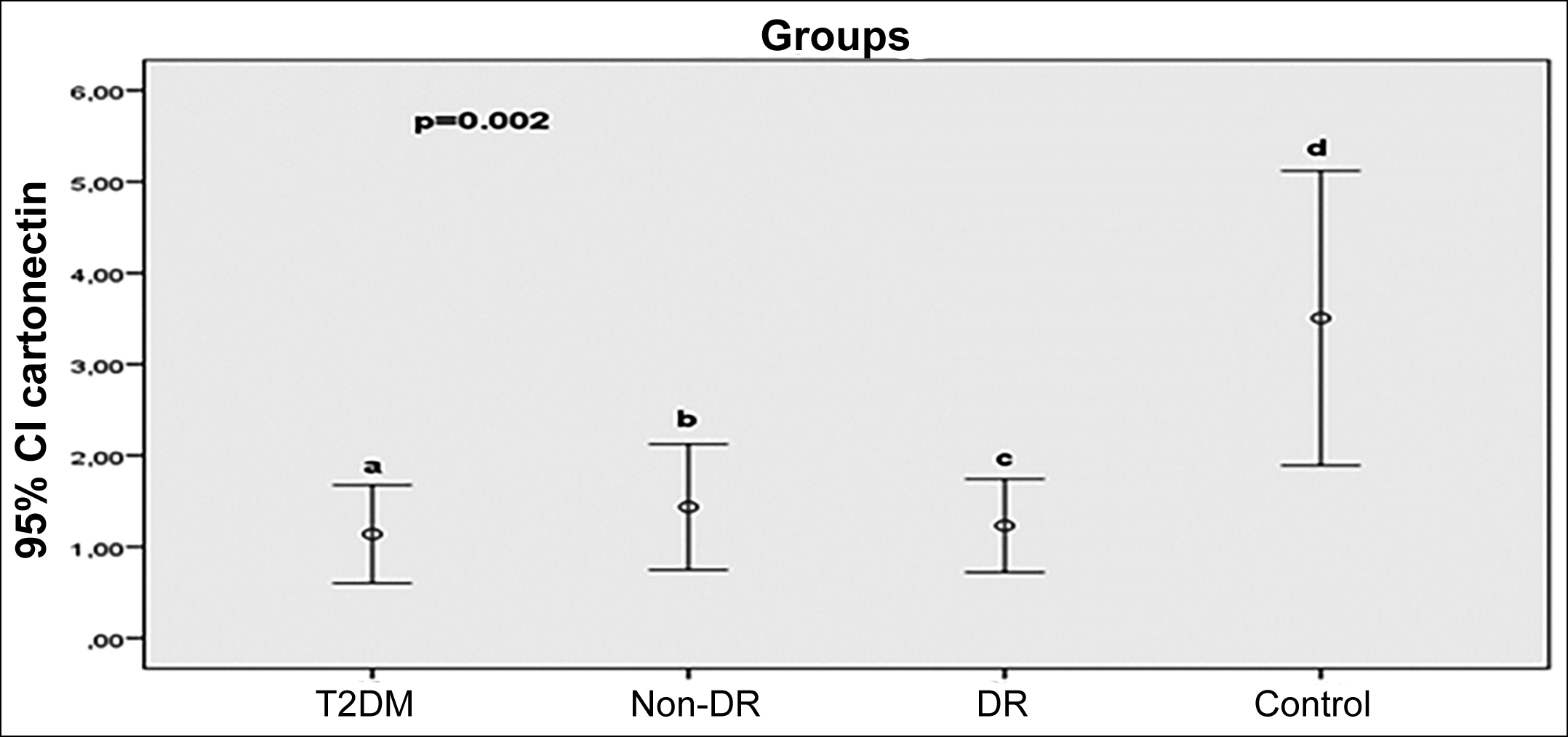 Figure 2: Comparasion of serum cartonectin (ng/L) levels between groups (p=0.002; a-b:0.810; a-c:0.872; a-d:0.002; b-c:0.937; b-d:0.001; c-d:0.001).
Figure 2: Comparasion of serum cartonectin (ng/L) levels between groups (p=0.002; a-b:0.810; a-c:0.872; a-d:0.002; b-c:0.937; b-d:0.001; c-d:0.001).
Betatrophin levels were significantly positively correlated with ALT (r:0.545, p=0.011) and haemoglobin (r:0.508, p=0.019) in diabetic patients. In the control group, betatrophin levels were significantly negatively correlated with IL-6 (r:-0.524, p=0.015) and the age (r:-0543, P=0.011) of the participants. There was no significant correlation between cartonectin level and continuous variables of the participants in the study population (Table II).
A significant positive correlation was found between betatrophin and TNF-α (r:0.608, p=0.010), IL-6 (r:0.554, p=0.021), BMI (r:0.590, p=0.013), and AST (r:0.511, p=0.036) levels in the DR group. There is a significant negative correlation between betatrophin and haemoglobin (r:-0.601, p=0.011). Cartonectin level only showed a negative correlation with ALT (r:-0.477, p=0.029) level. In the non-DR group, there was a significant negative correlation between betatrophin and glucose levels (r:-0.446, 0.043), a positive significant correlation between cartonectin and VLDL (r:0.480, p=0.028), and a significant negative correlation between cartonectin and IL-6 (r:-0.481, p=0.027), respectively (Table III).
DISCUSSION
The current study investigated the serum betatrophin and cartonectin levels in diabetic patients with and without retinopathy. The results of this study showed that serum betatrophin level is higher in diabetic retinopathy than in both non-DR and patients with T2DM without retinopathy. Previous studies reported that increased betatrophin levels are associated with diabetic retinopathy.6,11,12 Betatrophin increases insulin secretion. Due to this mechanism of action, increased betatrophin levels are seen in insulin resistance and T2DM states. The insulin secretory effect of betatrophin has an inhibitory effect on lipoprotein lipase activity, thus causing an increase in triglycerides. Previous literature data showed that the duration of diabetes and poor blood glucose regulation are major risk factors for the development of DR.13 The mechanism of DR development may cause more betatrophin production from the liver and may explain the increased betatrophin levels in patients with DR.
Table II: Correlation of serum betatrophin and cartonectin between biochemical variables in control and T2DM groups.|
Variables |
Control group |
|
T2DM group |
|||
|
Betatrophin |
Kartonektin |
|
Betatrophin |
Kartonektin |
||
|
Cartonectin (ng/L) |
r |
0.102 |
1.000 |
r |
-0.240 |
1.000 |
|
p |
0.662 |
. |
p |
0.294 |
. |
|
|
TNF-α (pg/ml) |
r |
0.114 |
0.138 |
r |
-0.219 |
0.009 |
|
p |
0.623 |
0.550 |
p |
0.340 |
0.968 |
|
|
IL-6 (pg/ml) |
r |
-0.524* |
-0.136 |
r |
0.173 |
0.083 |
|
p |
0.015 |
0.556 |
p |
0.454 |
0.720 |
|
|
Vitamin D (nmol/L) |
r |
-0.023 |
-0.045 |
r |
0.068 |
0.012 |
|
p |
0.922 |
0.846 |
p |
0.769 |
0.958 |
|
|
Age (year) |
r |
-0.543* |
-0.309 |
r |
-0.176 |
0.022 |
|
p |
0.011 |
0.173 |
p |
0.445 |
0.925 |
|
|
BMI (kg/m2) |
r |
0.066 |
-0.232 |
r |
-0.263 |
-0.017 |
|
p |
0.776 |
0.311 |
p |
0.250 |
0.942 |
|
|
HbA1c (%) |
r |
-0.024 |
0.097 |
r |
0.128 |
-0.174 |
|
p |
0.917 |
0.675 |
p |
0.580 |
0.452 |
|
|
Glucose (mg/dl) |
r |
0.106 |
0.112 |
r |
0.142 |
0.369 |
|
p |
0.649 |
0.630 |
p |
0.538 |
0.100 |
|
|
Insulin |
r |
0.104 |
-0.293 |
r |
-0.188 |
0.103 |
|
p |
0.653 |
0.198 |
p |
0.414 |
0.656 |
|
|
HOMA-IR |
r |
0.100 |
-0.289 |
r |
-0.051 |
0.108 |
|
p |
0.666 |
0.204 |
p |
0.825 |
0.642 |
|
|
T. cholesterol (mg/dl) |
r |
-0.285 |
0.052 |
r |
-0.067 |
0.291 |
|
p |
0.211 |
0.823 |
p |
0.773 |
0.201 |
|
|
LDL (mg/dl) |
r |
-0.166 |
-0.097 |
r |
0.058 |
0.202 |
|
p |
0.472 |
0.676 |
p |
0.801 |
0.381 |
|
|
Triglyceride (mg/dl) |
r |
0.265 |
0.150 |
r |
0.255 |
-0.092 |
|
p |
0.245 |
0.517 |
p |
0.265 |
0.691 |
|
|
VLDL (mg/dl) |
r |
0.242 |
0.223 |
r |
0.034 |
0.037 |
|
p |
0.290 |
0.332 |
p |
0.884 |
0.874 |
|
|
AST (U/L) |
r |
-0.044 |
0.060 |
r |
0.398 |
-0.102 |
|
p |
0.851 |
0.797 |
p |
0.074 |
0.658 |
|
|
ALT (U/L) |
r |
-0.064 |
0.033 |
r |
0.545* |
-0.101 |
|
p |
0.783 |
0.886 |
p |
0.011 |
0.664 |
|
|
Hgb (g/dl) |
r |
0.239 |
0.307 |
r |
0.508* |
0.034 |
|
p |
0.297 |
0.176 |
p |
0.019 |
0.882 |
|
|
Plt |
r |
0.178 |
0.239 |
r |
-0.233 |
-0.107 |
|
p |
0.440 |
0.297 |
p |
0.309 |
0.644 |
|
|
Urea (mg/dl) |
r |
0.088 |
0.149 |
r |
0.044 |
-0.285 |
|
p |
0.705 |
0.520 |
p |
0.849 |
0.210 |
|
|
Creatinine (mg/dl) |
r |
-0.157 |
-0.183 |
r |
0.313 |
-0.001 |
|
p |
0.496 |
0.428 |
p |
0.167 |
0.998 |
|
Although betatrophin increases insulin secretion and sensitivity, its benefit in T2DM and metabolic diseases is controversial. Hyperinsulinemia, such as that develops in T2DM, stimulates betatrophin expression and secretion. In studies conducted with diabetic patients, it has been reported that high betatrophin levels both increase the risk of diabetic retinopathy and are associated with an increase in all-cause mortality.14,15 The current study showed that betatrophin levels are higher in DR patients compared to both T2DM patients without retinopathy and non-DR patients. This finding is consistent with literature data that associates high betatrophin levels with the risk of retinopathy. Recent studies have reported that dyslipidemia may contribute to DR susceptibility.16 The current study showed that LDL-c levels were significantly increased in diabetic retinopathy but no significant difference in triglyceride levels between DR and controls. Betatrophin is an adipokine that causes both dyslipidemia and dysglycemia. Therefore, increased betatrophin levels in the DR group may cause retinopathy through dyslipidemia.
The current study determined that the cartonectin level was lower in the experimental group (DR, non-DR, and T2DM) compared to the controls. There were no significant differences in serum cartonectin levels between DR and non-DR groups. Cartonectin is a recently discovered cytokine with anti-diabetic, anti-inflammatory and neo-vascular effects. It has been stated that CTRP3 is associated with DR and may be a new biomarker for DR severity.17 Previous studies have reported that cartonectin levels are decreased in T2DM and diabetic retinopathy.8,18,19 Current study data are consistent with previous literature reporting decreased cartonectin levels in T2DM and diabetic retinopathy. In addition, the authors showed that the levels of cartonectin are decreased in non-diabetic retinopathy. In their study examining the possible role of cartonectin in the pathophysiology of diabetic retinopathy, Zhang et al. reported that CTRP3 expression was significantly reduced in vitreous samples taken from DR patients. They suggested that this situation may cause diabetic retinopathy by causing increased oxidative stress and apoptosis.
Table III: Correlation of serum betatrophin and cartonectin with biochemical variables in DR and non-DR groups.|
Variables |
|
DR group |
|
Non-DR group |
||
|
|
|
Betatrophin |
Kartonektin |
|
Betatrophin |
Kartonektin |
|
Cartonectin (ng/L) |
r |
0.335 |
1.000 |
r |
-0.124 |
1.000 |
|
p |
0.189 |
. |
p |
0.594 |
. |
|
|
TNF-α (pg/ml) |
r |
0.608** |
0.205 |
r |
0.000 |
-0.085 |
|
p |
0.010 |
0.374 |
p |
1.000 |
0.714 |
|
|
IL-6 (pg/ml) |
r |
0.554* |
0.186 |
r |
0.212 |
-0.481* |
|
p |
0.021 |
0.418 |
p |
0.357 |
0.027 |
|
|
Vitamin D (nmol/L) |
r |
-0.157 |
-0.168 |
r |
0.085 |
-0.196 |
|
p |
0.547 |
0.466 |
p |
0.714 |
0.395 |
|
|
Age (year) |
r |
-0.507* |
-0.121 |
r |
-0.049 |
0.297 |
|
p |
0.038 |
0.603 |
p |
0.831 |
0.192 |
|
|
BMI (kg/m2) |
r |
0.590* |
0.062 |
r |
-0.047 |
0.033 |
|
p |
0.013 |
0.789 |
p |
0.840 |
0.887 |
|
|
HbA1c (%) |
r |
-0.464 |
-0.121 |
r |
-0.077 |
0.239 |
|
p |
0.061 |
0.600 |
p |
0.740 |
0.297 |
|
|
Glucose (mg/dl) |
r |
-0.316 |
-0.155 |
r |
-0.446* |
0.371 |
|
p |
0.216 |
0.503 |
p |
0.043 |
0.098 |
|
|
Insulin |
r |
0.444 |
0.084 |
r |
-0.354 |
0.064 |
|
p |
0.074 |
0.716 |
p |
0.115 |
0.781 |
|
|
HOMA-IR |
r |
0.346 |
-0.097 |
r |
-0.368 |
0.226 |
|
p |
0.174 |
0.676 |
p |
0.101 |
0.324 |
|
|
T. cholesterol (mg/dl) |
r |
-0.145 |
0.048 |
r |
0.214 |
-0.147 |
|
p |
0.580 |
0.836 |
p |
0.352 |
0.525 |
|
|
LDL (mg/dl) |
r |
0.211 |
0.345 |
r |
-0.141 |
-0.237 |
|
p |
0.416 |
0.125 |
p |
0.542 |
0.300 |
|
|
Triglyceride (mg/dl) |
r |
-0.174 |
0.019 |
r |
-0.061 |
0.414 |
|
p |
0.504 |
0.936 |
p |
0.793 |
0.062 |
|
|
VLDL (mg/dl) |
r |
-0.184 |
0.007 |
r |
0.015 |
0.480* |
|
p |
0.479 |
0.974 |
p |
0.949 |
0.028 |
|
|
AST (U/L) |
r |
0.511* |
-0.048 |
r |
-0.181 |
0.147 |
|
p |
0.036 |
0.836 |
p |
0.433 |
0.524 |
|
|
ALT (U/L) |
r |
-0.072 |
-0.477* |
r |
-0.109 |
0.078 |
|
p |
0.785 |
0.029 |
p |
0.637 |
0.736 |
|
|
Hgb (g/dl) |
r |
-0.601* |
-0.359 |
r |
-0.246 |
-0.072 |
|
p |
0.011 |
0.110 |
p |
0.283 |
0.756 |
|
|
Plt |
r |
0.108 |
0.160 |
r |
-0.178 |
0.357 |
|
p |
0.680 |
0.487 |
p |
0.440 |
0.112 |
|
|
Urea (mg/dl) |
r |
-0.331 |
0.105 |
r |
0.107 |
0.111 |
|
p |
0.194 |
0.652 |
p |
0.646 |
0.631 |
|
|
Creatinine (mg/dl) |
r |
-0.121 |
0.130 |
r |
0.071 |
0.178 |
|
p |
0.642 |
0.573 |
p |
0.758 |
0.441 |
|
Overexpression of CTRP3 has been shown to improve cell viability, reduced oxidative stress, and improve cell apoptosis in HG-induced retinal cells.19 Previous literature data and current data suggest that cartonectin may be a new biomarker for diabetic retinopathy and a new weapon for the treatment of diabetic retinopathy.
Diabetic retinopathy is an inflammatory process. Previous studies have reported that both betatrophin and cartonectin have anti-inflammatory effects. For this reason, these adipokines’ levels may be associated with DR through the inflammatory process. In the current study, a positive significant correlation was found between betatrophin levels and TNF-α and IL-6 levels in DR patients. There was no significant correlation between Cartonectin level and inflammation markers. Previous studies have shown that the increase in TNF-α and IL-6 correlates with the increase in betatrophin levels.7 Although betatrophin, which has an anti-inflammatory effect, is expected to increase inflammation, there are studies showing that there is no relationship between anti-inflammatory parameters and betatrophin level.20 The literature data examining the relationship between Cartonectin and inflammatory markers is insufficient. Current literature data is insufficient to explain the relationship between anti-inflammatory parameters and betatrophin and cartonectin levels. The authors believe that new studies are needed.
The present study has some limitations. The study was designed as a descriptive study and conducted in a single centre. The diabetic retinopathy risk factors of the participants could not be standardised. Another limitation of the study is the ignoring of confounding factors such as age and duration of diabetes, which may have an impact on the study results. The effect of long-term glycemic control, which is the most important factor in the development of diabetic retinopathy, on the results could not be evaluated. Considering the limitations of the current study, prospective cohort studies with DR risk factors and long-term blood pressure and blood glucose monitoring are required to elucidate the role of betatrophin and cardboardectin in the aetiopathogenesis of diabetic retinopathy.
CONCLUSION
The present study concludes that both serum betatrophin and cartonectin may be new markers for diabetic retinopathy but serum cartonectin may be insufficient to distinguish between diabetic retinopathy and non-diabetic retinopathy. Further studies are needed to use betatrophin and cartonectin as a new marker for DR and in early diagnosis.
DISCLOSURE:
This study is part of the thesis project of Pehlivan V. Internal Medicine and funded by the Firat University.
ETHICAL APPROVAL:
This cross-sectinal study was approved by the Noninvasive Ethics Committee of the Firat University (Date: 08.04.2021 no: 2021:05/41).
PATIENTS’ CONSENT:
Informed and written consents were taken from each patient.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
VP: Study design, data collection, interpretation, and drafting of research work.
MFG, OC: Contribution in data collection, acquisition, and drafting of work.
EO, BY: Analysis, drafting of work, Critical revision, and final approval of the draft.
ED: Critical revision and final approval of the draft.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Motala AA, Mbanya JC, Ramaiya K, Pirie FJ, Ekoru K. Type 2 diabetes mellitus in sub-Saharan Africa: challenges and opportunities. Nat Rev Endocrinol 2022; 18(4):219-29. doi.org/10.1038/s41574-021-00613-y.
- Lin KY, Hsih WH, Lin YB, Wen CY, Chang TJ. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig 2021; 12(8): 1322-25. doi.org/10.1111/jdi.13480.
- Kaštelan S, Orešković I, Bišćan F, Kaštelan H, Gverović Antunica A. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochem Med (Zagreb) 2020; 30(3): 030502. doi.org/10.11613/bm.2020.030502.
- Kwan CC, Fawzi AA. Imaging and biomarkers in diabetic macular edema and diabetic retinopathy. Curr Diab Rep 2019; 19(10):95. doi.org/10.1007/s11892-019-1226-2.
- Jenkins AJ, Joglekar MV, Hardikar AA, Keech AC, O'Neal DN, Januszewski AS. Biomarkers in diabetic retinopathy. Rev Diabet Stud 2015; 12(1-2):159-95. doi.org/10.1900/rds. 2015.12.159.
- Lu Q, Lu L, Chen W, Lu P. Expression of angiopoietin-like protein 8 correlates with VEGF in patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2017; 255(8):1515-23. doi.org/10.1007/s00417-017-3676-z.
- Onalan E, Bozkurt A, Gursu MF, Yakar B, Donder E. Role of betatrophin and ınflammation markers in type 2 diabetes mellitus, prediabetes and metabolic syndrome. J Coll Physicians Surg Pak 2022; 32(3):303-307. doi.org/10. 29271/jcpsp.2022.03.303.
- Ban B, Bai B, Zhang M, Hu J, Ramanjaneya M, Tan BK, et al. Low serum cartonectin/CTRP3 concentrations in newly diagnosed type 2 diabetes mellitus: In vivo regulation of cartonectin by glucose. PLoS ONE 2014; 9(11): e112931. doi.org/10.1371/journal.pone.0112931.
- American diabetes association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2020. Diabetes Care 2020; 43(Supp1):14-31. doi.org/10.2337/ dc20-s002.
- Wilkinson CP, Ferris FL, Klein RE, Lee PP, Agardh CD, Davis M, et al. Global diabetic retinopathy project group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003; 110(9):1677-82. doi.org/10.1016/s0161-6420(03) 00475-5.
- Wang YY, Zhang D, Jiang ZY, Lu XQ, Zheng X, Yu YJ, et al. Positive association between betatrophin and diabetic retinopathy risk in type 2 diabetes patients. Horm Metab Res 2016; 48(3):169-73. doi.org/10.1055/s-0035-1550009.
- Dong CX, Song CP, Zhang CP, Dong M, Gong XR, Gao HY, et al. Clinical and experimental study on angiopoietin-like protein 8 associated with proliferative diabetic retinopathy. Int J Ophthalmol 2017; 10(12):1819-23. doi.org/10.18240/ ijo.2017.12.05.
- Hainsworth DP, Bebu I, Aiello LP, Sivitz W, Gubitosi-Klug R, Malone J, et al. Diabetes control and complications trial (DCCT)/epidemiology of diabetes ınterventions and complications (EDIC) research group. Risk factors for retinopathy in type 1 diabetes: The DCCT/EDIC study. Diabetes Care 2019; 42(5):875-82. doi.org/10.2337/ dc18-2308.
- Zou H, Duan W, Zhang Z, Chen X, Lu P, Yu X. The circulating ANGPTL8 levels show differences among novel subgroups of adult patients with diabetes and are associated with mortality in the subsequent 5 years. Sci Rep 2020; 10(1):12859. doi.org/10.1038/s41598-020- 69091-y.
- Guo C, Wang C, Deng X, He J, Yang L, Yuan G. ANGPTL8 in metabolic homeostasis: More friend than foe? Open Biol 2021; 11(9):210106. doi.org/10.1098/rsob.210106.
- Rao H, Jalali JA, Johnston TP, Koulen P. Emerging roles of dyslipidemia and hyperglycemia in diabetic retinopathy: Molecular mechanisms and clinical perspectives. Front Endocrinol (Lausanne) 2021; 12:620045. doi.org/10.3389/ fendo.2021.620045.
- Yan Z, Wang C, Meng Z, Gan L, Guo R, Liu J, et al. C1q/TNF-Related protein 3 prevents diabetic retinopathy via AMPK-Dependent stabilisation of blood-retinal barrier tight junctions. Cells 2022; 11(5):779. doi.org/10.3390/cells110 50779.
- Yan Z, Zhao J, Gan L, Zhang Y, Guo R, Cao X, et al. CTRP3 is a novel biomarker for diabetic retinopathy and inhibits HGHL-induced VCAM-1 expression in an AMPK-dependent manner. PLoS One 2017; 12(6):e0178253.doi.org/10. 1371/journal.pone.0178253.
- Zhang J, He J. CTRP3 inhibits high glucose-induced oxidative stress and apoptosis in retinal pigment epithelial cells. Artif Cells Nanomed Biotechnol 2019; 47(1):3758-64. doi.org/10.1080/21691401.2019.1666864.
- Keikha F, Shahrokh Tehraninejad E, Rakhshkhorshid M, Afiat M, Hagholahi F, Ghasemi F. The association of the betatrophin level with metabolic and inflammatory parameters in infertile women with polycystic ovary syndrome: A case-control study. Int J Reprod Biomed 2022; 20(1):29-36. doi.org/10.18502/ijrm.v20i1.10406.