Evaluation of Prognostic Factors and Trastuzumab-based Treatments in HER2/Neu-positive Metastatic Gastric Cancer
By Izzet Dogan, Senem Karabulut, Didem Tastekin, Ferhat Ferhatoglu, Nail Paksoy, Burak SakarAffiliations
doi: 10.29271/jcpsp.2022.08.1014ABSTRACT
Objective: To determine the efficacy of trastuzumab-based treatment in patients with HER2/neu-positive metastatic gastric cancer.
Study Design: Observational study.
Place and Duration of Study: Department of Medical Oncology, Istanbul University, Institute of Oncology, Istanbul, Turkey, between January 2014 and December 2020.
Methodology: The clinicopathological characteristic and treatment data of patients with HER2/neu-positive metastatic gastric cancer were recorded retrospectively. Kaplan-Meier analysis was performed to compare the chemotherapy regimens.
Results: Sixty-three patients were included in the study. The average age was 61. Female patients accounted for 27% of the total, while male patients accounted for 73%. De novo metastatic cases accounted for 44 (69.8%) of the total number of patients. The median survival time was 13.6 (8-19.3) months. Complete response was 6.3%, partial response was 39.7%, and the stable response was 9.5% with trastuzumab-based chemotherapy. The overall survival (p= 0.45) and progression-free survival (p=0.893) were similar for different chemotherapy regimens. The grade 1-2 to grade 3-4 toxicity ratio was 79.6% and 20.6%, respectively. The patients’ performance (p<0.001) and the number of metastatic sites (p=0.001) were both shown to be unfavourable predictive variables for OS in multivariate analysis.
Conclusion: The addition of taxane to trastuzumab-based combinations (with platinum and fluoropyrimidine) did not affect overall and progression-free survival in this research. Three or more metastatic sites and poor performance status were found as the unfavourable prognostic variables for overall survival.
Key Words: Gastric cancer, Trastuzumab, Chemotherapy, Prognostic factors.
INTRODUCTION
Gastric cancer is the fifth most seen cancer globally.1 The majority of individuals with gastric cancer have no symptoms. The most prevalent symptoms are weight loss and severe stomach pain.2 Many risk factors have been identified including, smoking, advanced age, obesity, family history, smoked foods, and helicobacter pylori infection. Early gastric tumours that are surgically treatable, are typically asymptomatic and only diagnosed occasionally outside of the screening programs. Many gastric cancer patients have presented with metastatic disease at diagnosis.
Fluoropyrimidine-, platinum-, and taxane-based combinations are commonly used to treat metastatic gastric cancer. Capecitabine with fluorouracil and cisplatin with oxaliplatin have a similar effect on the survival results in gastric cancer.3 As a result, they can be used interchangeably in different protocols.
The ratio of HER2 overexpression in metastatic gastroesophageal junction cancer (mGEJC) and gastric cancers (mGC) ranges from 12% to 20%.4-6 HER2 expression is less common in resectable gastric cancer than in metastatic gastric cancer.7 The HER2 receptor is a negative prognostic factor in gastric cancer and the HER2 gene amplification levels significantly predict the treatment response.8 The efficacy of trastuzumab is researched in the adjuvant and neoadjuvant therapy of the gastric cancer. Currently, trastuzumab is primarily used to treat HER2-positive metastatic cancer patients. The TOGA trial found that the addition of trastuzumab to the chemotherapy (a combination of platinum and fluoropyrimidine) was superior to only chemotherapy in metastatic HER2-positive mGEJC and mGC.9 The most appropriate chemotherapy regimens to combine with trastuzumab-based treatment are yet unknown. Whether there would be the additional benefit of adding taxane in fluoropyrimidine, platinum, and trastuzumab for PFS and OS in metastatic HER2-positive mGC is unclear. Therefore, studies comparing trastuzumab with doublet or triplet chemotherapy regimens are needed. The aim of this study was to evaluate the trastuzumab-based chemotherapy regimens in HER2-positive mGEJC and mGC patients.
METHODOLOGY
This study was a retrospective observational analysis that included the HER2-positive mGEJC and mGC patients diagnosed and treated at the outpatients clinic of Istanbul University, Oncology Institute, Istanbul, Turkey, between 2014 and 2020. The local Ethics Committee approved this study (2021/229154). Patients were identified using the hospital's database. All the patients with metastatic HER2-positive gastric cancer, who received first-line treatment and whose data could be accessed, were included in the study. Patients whose data were not suitable for the statistical analysis, were excluded from the study. The clinical, pathological, and treatment features of the patients were recorded. HER2 receptor status was tested in both immune-histochemistry (IHC) and Fluorescence In-Situ hybridisation (FISH). The patient's performance was evaluated using the Eastern Cooperative Oncology Group (ECOG) criteria.
Chemotherapy was given every three weeks. Three chemo-therapy regimens were C+T (capecitabine and trastuzumab), PF + T (platinum, fluoropyrimidine, and trastuzumab), and DCF+T (docetaxel, cisplatin, fluoropyrimidine, and trastuzumab), and were compared in the study. Trastuzumab was administered as an intravenous infusion on day 1 of the first cycle at an initial dosage of 8 mg/Kg followed by 6 mg/Kg every three weeks. Fluorouracil (800 mg/m2 per day administered by the continuous IV infusion on days 1–5 of each cycle) and capecitabine (1000 mg/m2 given orally twice a day for 14 days followed by a 1-week respite) were engaged as fluoropyrimidine. Platinum was utilised in chemotherapy regimens as oxaliplatin (130 mg/m2, IV infusion each on three weeks) or cisplatin (60 mg/m2, IV infusion each on three weeks). In the DCF + T regimen, docetaxel was administered at a dose of 60 mg/m2/day through IV infusion at every three weeks. The Response Evaluation Criteria in Solid Tumours (RECIST) were performed to detect the treatment responses. All the chemotherapy regimens were compared for overall survival (OS) and progression-free survival (PFS). All the adverse events related to chemotherapy were recorded.
The Ministry of Health's death notification system was used to check the patients' death status. The OS was defined as the time from the diagnosis to death (due to any cause). PFS was calculated as the time from the start of the treatment to progression.
SPSS 25 was used for all the analyses. Continuous data were reported as median (minimum-maximum) values, whereas categorical variables were provided as numbers and percentages. The normal distribution of continuous variables was evaluated with the Kolmogorov-Smirnov test. The log-rank test was performed to analyse OS using the Kaplan-Meier technique. The Univariate and multivariate analyses were conducted using the Cox regression model. A p-value under 0.05 was used to determine statistical significance.
RESULTS
The subjects included 63 metastatic cancer patients who were treated with first-line chemotherapy. Tumour samples of all the patients were 3+ on IHC and positive on FISH. All the patients had a tumour with adenocarcinoma histology. The average age was 60.5 ± 11.1 years with 17 (27%) female and 46 (73%) males. De novo metastatic cases accounted for 44 of the total number of patients (69.8%). The patient characteristics and treatment responses for the chemotherapy regimens are shown in Table I.
Table I: Patients’ characteristic and objective tumour responses based on RECIST.
|
Characteristics |
Number of patients |
Percent (%) |
|
Gender Male Female |
46 17 |
73 27 |
|
ECOG performance status 0 1 2 |
34 25 4 |
54 39.7 6.3 |
|
Primary tumour location EGJ Cardia Corpus Antrum Unknown |
10 15 17 17 4 |
15.9 23.8 27 27 6.3 |
|
De novo metastatic Yes No |
44 19 |
69.8 30.2 |
|
Prior therapy Surgery Adjuvant radiotherapy |
19 9 |
30.2 14.3 |
|
Metastatic locations Liver Lymph nodes Lung Other (Ovary, bone, pancreas) |
39 27 16 12 |
61.9 42.9 25.4 19 |
|
Number of metastatic sites 1-2 >2 Unknown |
46 13 4 |
73.1 20.6 6.3 |
|
First-line CTx regimens DCF-T PF-T C-T |
13 40 10 |
20.6 63.5 15.9 |
|
Tumour responses Complete response Partial response Stable response Progression |
4 25 6 28 |
6.3 39.7 9.5 44.4 |
|
Second-line treatment Yes No Unknown |
15 46 2 |
23.8 73 3.2 |
Thirteen (20.6%) patients received the DCF-T regimen, 40 (63.5%) patients, the PF-T regimen, and 10 (15.9%) patients, the C-T regimen. The number of patients, who developed any toxicity, was 52 (82.5%). Grade 1-2 toxicity was observed in 50 (79.6%) patients, and grade 3-4 toxicity in 13 (20.6%) patients. Haematological grades 1-2 and 3-4 toxicities were observed in 35 (55.6%) patients and 13 (20.6%) patients, respectively. The most observed adverse events were fatigue, nausea, vomiting, anaemia, and thrombocytopenia. There was no marked differences between chemotherapy regimens for the adverse event. There was no Cardiac events. Only two (3.2%) patients had a drop > 5% left ventricular ejection fraction (LVEF).
Partial response was detected in 25 (39.7%) patients, stable response in 6 (9.5%) patients, and complete response in 4 (6.3%) patients with trastuzumab-based chemotherapy during a median follow-up of 12.9 (1.2-80.2) months. Fifty-five (87.2%) patients died during the study period (Figure 1). The median OS was 13.6 (8-19.3) months, whereas the median PFS was 8.6 (4.9-12.2) months. Chemotherapy regimens were not different for OS (p=0.452) and PFS (p=0.893) according to the Kaplan-Meier curve (Figure 2). Performance status (p<0.001) and three or more sites of metastases (p=0.001) were shown to be unfavourable prognostic variables for OS in multivariate analysis. The site (liver, lung, and lymph nodes) of metastasis was not a prognostic factor. DCF+T regimen was not superior to the PF+T regimen (p=0.202). The analysis of prognostic factors is presented in Table II.
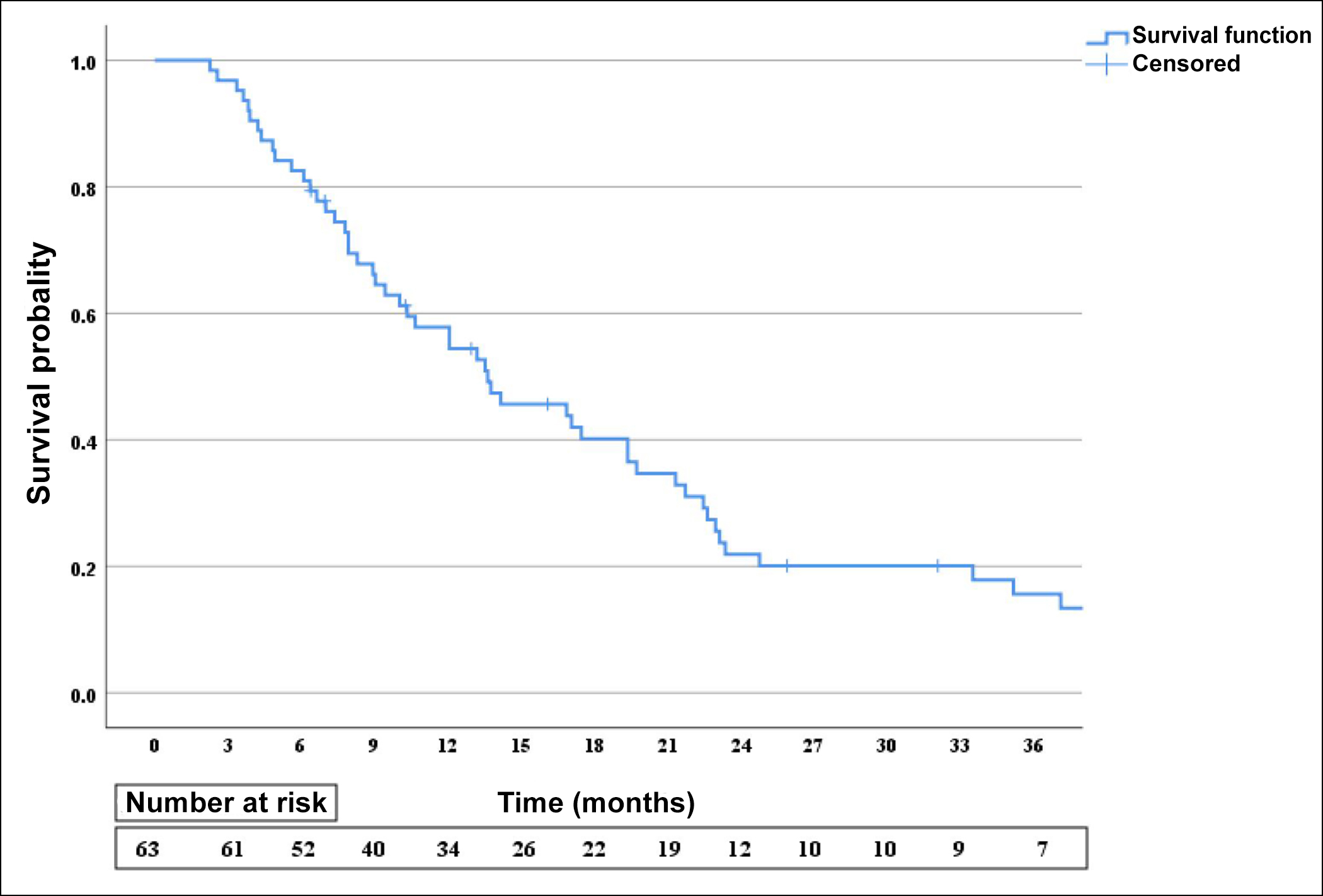 Figure 1: Overall survival of the patients by Kaplan-Meier curve.
Figure 1: Overall survival of the patients by Kaplan-Meier curve.
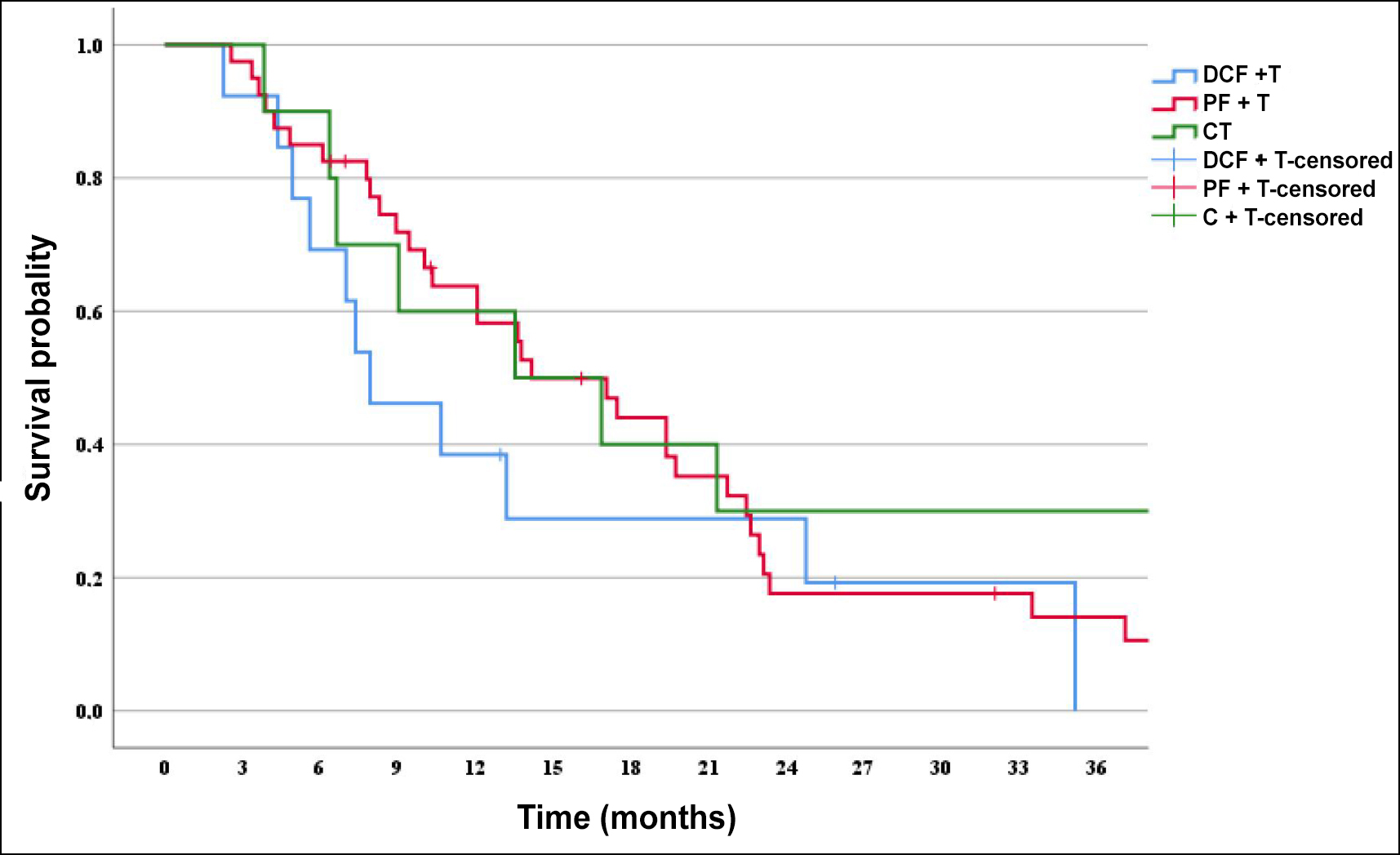 Figure 2: Comparison of the overall survival for chemotherapy regimens.
Figure 2: Comparison of the overall survival for chemotherapy regimens.
Among the patients whose disease progressed under trastuzumab-based chemotherapy; 15 (24.6%) patients received second-line chemotherapy, six (40%) patients continued to receive a trastuzumab-based chemotherapy regimen, and nine (60%) patients received a different chemotherapy regimen. OS results were similar between the two groups (p=0.877).
Table II: Univariate and multivariate analysis for overall survival.
|
|
Univariate analysis |
Multivariate analysis |
|
|
p-value |
p-value |
HR (95% CI) |
|
|
Age (<65 vs. >65) |
0.542 |
|
|
|
Gender (Male vs. Female) |
0.653 |
|
|
|
ECOG PS (0-1 vs. 2) |
<0.001 |
<0.001 |
15.06 (3.41-66.46) |
|
Primary tumor location (EGJ vs. Gastric) |
0.489 |
|
|
|
De novo metastatic (Yes vs. No) |
0.573 |
|
|
|
Prior radiotherapy (Yes vs. No) |
0.688 |
|
|
|
Number of metastatic sites (1-2 vs. >2) |
0.001 |
0.016 |
2.65 (1.19-5.85) |
|
Treatment responses (Yes vs. no) |
<0.001 |
<0.001 |
4.35 (2.05-9.24) |
|
First-line CTx regimens DCF-T PF-T C-T |
0.221 |
0.027 0.202 0.008 |
1 1.88 (0.71-4.96) 3.44 (1.39-8.53) |
|
Second-line treatment (Yes vs. No) |
0.007 |
0.001 |
4.30 (1.79-10.32) |
DISCUSSION
Chemotherapy is used to relieve symptoms (including malignant dysphagia), enhance the quality of life, and extend survival in patients with mGEJC and mGC. In this study, adding taxane to fluoropyrimidine, platinum, and trastuzumab did not improve PFS or OS in patients with metastatic HER2-positive mGEJC and mGC. The good performance status of the patients before the treatment and the low tumour burden has affected the course of the disease.
In combination with chemotherapy, trastuzumab is a standard option for treating the HER2-positive mGEJC and mGC patients.9 The combination of trastuzumab and many different chemotherapy regimens have been investigated. Two different multi-centre phases II study showed that trastuzumab with S‑1 plus oxaliplatin or cisplatin had a hopeful activity with low toxicities for the patients with HER2-positive mGC.10,11 Another phase II study showed that trastuzumab combined with capecitabine and oxaliplatin in the first-line treatment of HER2-positive mGC was effective and tolerable with low cardiac toxicity.12 Kurokawa et al. found that trastuzumab with cisplatin was an active regimen and had a favourable toxicity profile in advanced HER2-positive mGEJC and mGC patients.13 Mondaca et al. showed that trastuzumab with triplet chemotherapy combination (5-fluorouracil, cisplatin, and docetaxel) in HER2-positive mGC was effective and safe.14 The KEYNOTE-811 trial found that combining pembrolizumab with trastuzumab and chemotherapy reduced tumour volume and significantly improved complete responses and the objective response rate.15
It is known that a multi-drug chemotherapy regimen is superior to a single-drug chemotherapy regimen in patients with mGC. The data comparing the different trastuzumab-based chemotherapy regimens for HER2-positive mGC is limited. The present study found that the DCF+T regimen was superior to the C+T regimen but not the PF + T regimen for OS. All the chemotherapy regimens were well-tolerated, and the authors did not observe any cardiac adverse events. Unlike this study, a Turkish Oncology Group (TOG) study published by Gurbuz et al. showed that adding taxane to trastuzumab-based therapy increased OS, although it did not affect PFS in the HER2- positive mGEJC and mG patients.16 In this study, no significant difference was found between the groups in terms of toxicity. This can be explained by the limited number of patients included in the study.
The ECOG performance status at the diagnosis, receiving second-line treatment, and having three or more metastasis sites were prognostic factors for the OS. There are yet no predictive biomarkers for trastuzumab response. The level of HER2 gene amplification was correlated with the efficacy of trastuzumab-based chemotherapy in the advanced gastric cancer patients.8 The median OS of the patients in this study was similar to the TOGA trial. Tumour samples of all the patients were 3+ on IHC and positive on FISH in this research. In the TOGA trial, the IHC3+/ FISH positive patient group benefited more from the trastuzumab-based chemotherapy. From this perspective, survival results seem somewhat low. This situation might explain that the heterogeneous patient groups.
In case of disease progression after trastuzumab treatment, there was a marked difference in the disease prognosis in patients who continued with trastuzumab-based therapy and patients using a different chemotherapy regimen. In a study conducted on the patients with HER2 positive mGC and resistant to the treatment, loss of HER2 receptors was observed in 60.6% of the patients.17 In preclinical investigations, activation of the PI3K-AKT-mTOR signalling pathway by the deletion of the PTEN suppressor and mutation activation of PI3K has been shown to induce trastuzumab resistance in the breast cancer patients.18 Trastuzumab resistance may result from the intratumoural heterogeneity in gastric cancer.19,20 In case of response to trastuzumab at first-line therapy, a retrospective study found that trastuzumab continuation after six treatment cycles were effective and safe in HER2-positive mGC patients.21
Many studies have evaluated the efficacy and adverse events of different HER2 targetted agents in patients with HER2 positive mGEJC and mGC. A Phase III study demonstrated that adding lapatinib to capecitabine and oxaliplatin did not improve overall survival except for the subgroup included Asian patients.22 The JACOB trial showed that adding pertuzumab to trastuzumab and chemotherapy did not improve OS in patients with HER2-positive mGC and mGEJC compared to placebo.23 In the patients with HER2-positive mGC, who were previously treated, the GATSBY trial found that trastuzumab emtansine had no better results than taxane for the survival.24 In previously treated HER2-positive mGC patients, trastuzumab deruxtecan therapy has improved the results of response and OS compared to the standard treatments.25
There were several limitations to this research. The research was retrospective and the patient groups studied were heterogeneous. The number of patients was low, and some information about clinicopathological features was lacking.
CONCLUSION
In conclusion, OS and PFS were not affected by adding taxane into trastuzumab with a doublet chemotherapy combination (including platinum and fluoropyrimidine). Trastuzumab and combination fluoropyrimidine or platin in treating HER2-positive mGC was effective and well-tolerated. Different agents targeting HER2 receptors have been used to treat HER2-positive metastatic stomach cancer; the combination of trastuzumab and doublet chemotherapy is still the standard approach in first-line therapy. Comparative studies are limited in the literature to determine the most appropriate trastuzumab-based therapy in mGEJC and mGC. This study is one of the rare studies on this issue. But, randomised prospective studies with the large numbers of patients are required to determine the appropriate treatment combination with trastuzumab in treating HER2-positive mGEJC and mGC.
ETHICAL APPROVAL:
The ethics committee of the Faculty of Medicine, Istanbul University approved this study (2021/229154).
PATIENTS’ CONSENT:
For this type of research, informed consent is not required.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
ID, SK, DT, BS: Conception and design of the study.
ID, NP, FF: Statistical analyses and drafting of the manuscript.
NP, FF, SK, DT, BS: Acquisition and interpretation of the data.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71(3): 209-49. doi: 10.3322/caac.21660.
- Wanebo HJ, Kennedy BJ, Chmiel J, Steele G, Winchester D, Osteen R. Cancer of the stomach. A patient care study by the American College of surgeons. Ann Surg 1993; 218(5):583-92. doi: 10.1097/00000658-199321850-00002.
- Sumpter K, Harper-Wynne C, Cunningham D, Rao S, Tebbutt N, Norman AR, et al. Report of two protocol planned interim analyses in a randomised multicentre phase III study comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric cancer receiving ECF. Br J Cancer 2005; 92(11):1976-83. doi: 10.1038/sj.bjc.6602572.
- Kunz PL, Mojtahed A, Fisher GA, Ford JM, Chang DT, Balise RR, et al. HER2 expression in gastric and gastroeso-phageal junction adenocarcinoma in a US population: Clinicopathologic analysis with proposed approach to HER2 assessment. Appl Immunohistochem Mol Morphol 2012; 20(1):13-24. doi: 10.1097/PAI.0b013e31821c821c.
- Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes: A systematic review. Int J Cancer 2012; 130(12):2845-56. doi: 10.1002/ijc.26292.
- Janjigian YY, Werner D, Pauligk C, Steinmetz K, Kelsen DP, Jager E, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: A European and USA International collaborative analysis. Ann Oncol 2012; 23(10):2656-62. doi: 10.1093/annonc/ mds104.
- Kataoka Y, Okabe H, Yoshizawa A, Minamiguchi S, Yoshimura K, Haga H, et al. HER2 expression and its clinicopathological features in resectable gastric cancer. Gastric Cancer 2013; 16(1):84-93. doi: 10.1007/s10120- 012-0150-9.
- Gomez-Martin C, Plaza JC, Pazo-Cid R, Salud A, Pons F, Fonseca P, et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J Clin Oncol 2013; 31(35):4445-52. doi: 10.1200/JCO.2013. 48.9070.
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010; 376(9742): 687-97. doi: 1016/S0140-6736(10)61121-X.
- Takahari D, Chin K, Ishizuka N, Takashima A, Minashi K, Kadowaki S, et al. Multicenter phase II study of trastuzumab with S-1 plus oxaliplatin for chemotherapy-naive, HER2-positive advanced gastric cancer. Gastric Cancer 2019; 22(6):1238-46. doi: 10.1007/s10120-019- 00973-5.
- Kurokawa Y, Sugimoto N, Miwa H, Tsuda M, Nishina S, Okuda H, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer 2014; 110(5): 1163-8. doi: 11038/bjc.2014.18.
- Rivera F, Romero C, Jimenez-Fonseca P, Izquierdo-Manuel M, Salud A, Martinez E, et al. Phase II study to evaluate the efficacy of trastuzumab in combination with capecitabine and oxaliplatin in first-line treatment of HER2-positive advanced gastric cancer: HERXO trial. Cancer Chemother Pharmacol 2019; 83(6):1175-81. doi: 10.1007/s00280-019-03820-7.
- Gravalos C, Gomez-Martin C, Rivera F, Ales I, Queralt B, Marquez A, et al. Phase II study of trastuzumab and cisplatin as first-line therapy in patients with HER2-positive advanced gastric or gastroesophageal junction cancer. Clin Transl Oncol 2011; 13(3):179-84. doi: 10.1007/s12094-011-0637-6.
- Mondaca S, Margolis M, Sanchez-Vega F, Jonsson P, Riches JC, Ku GY, et al. Phase II study of trastuzumab with modified docetaxel, cisplatin, and 5 fluorouracil in metastatic HER2-positive gastric cancer. Gastric Cancer 2019; 22(2):355-62. doi: 10.1007/s10120-018-0861-7.
- Janjigian YY, Kawazoe A, Yanez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021; 600(7890):727-30. doi: 10.1038/s41586-021-04161-3.
- Gurbuz M, Akkus E, Sakin A, Urvay S, Demiray AG, Sahin S, et al. Combination of trastuzumab and taxane-containing intensified chemotherapy in first-line treatment of HER2-positive advanced gastric cancer. Tumori 2021; 107(5):416-23. doi: 10.1177/0300891 620969823.
- Saeki H, Oki E, Kashiwada T, Arigami T, Makiyama A, Iwatsuki M, et al. Re-evaluation of HER2 status in patients with HER2-positive advanced or recurrent gastric cancer refractory to trastuzumab (KSCC1604). Eur J Cancer 2018; 105:41-9. doi: 10.1016/j.ejca.2018.09.024.
- Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 2007; 12(4):395-402. doi: 10.1016/j.ccr.2007.08.030.
- Wakatsuki T, Yamamoto N, Sano T, Chin K, Kawachi H, Takahari D, et al. Clinical impact of intratumoral HER2 heterogeneity on trastuzumab efficacy in patients with HER2-positive gastric cancer. J Gastroenterol 2018; 53(11):1186-95. doi: 10.1007/s00535-018-1464-0.
- Kaito A, Kuwata T, Tokunaga M, Shitara K, Sato R, Akimoto T, et al. HER2 heterogeneity is a poor prognosticator for HER2-positive gastric cancer. World J Clin Cases 2019; 7(15):1964-77. doi: 10.12998/wjcc.v7. i15.1964.
- Gurbuz M, Akkus E, Sakin A, Urvay S, Demiray AG, Sahin S, et al. Trastuzumab +/- capecitabine maintenance after the first-line treatment of HER2-positive advanced gastric cancer: Retrospective observational real-life data of Turkish oncology group. J Gastrointest Cancer 2022; 53(2):282-88. doi: 10.1007/s12029-021-00594-1.
- Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC - A randomised phase III trial. J Clin Oncol 2016; 34(5): 43-51. doi: 10.1200/JCO.2015.62.6598.
- Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol 2018; 19(10):1372-84. doi: 10.1016/ S1470-2045(18)30481-9.
- Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017; 18(5):640-53. doi: 10.1016/S1470- 2045(17)30111-0.
- Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated her2-positive breast cancer. New Eng J Med 2020; 382(7): 610-21. doi: 10.1056/NEJMoa1914510.