Establishment and Validation of a Novel Nomogram for Predicting Distant Metastasis in Patients with Invasive Lung Adenocarcinoma
By Zhanyue Pang1, Haibo Liu2, Zhitao Chen2, Liangming Zhu1Affiliations
doi: 10.29271/jcpsp.2022.12.1563Objective: To establish and verify a nomogram for predicting distant metastasis in invasive lung adenocarcinoma (IAC).
Study Design: Observational study.
Place and Duration of Study: Department of Thoracic Surgery, Jinan Central Hospital, Jinan, China, from December 2021 to May 2022.
Methodology: To create a nomogram, univariate and multivariate logistic regression analyses were used to identify the independent predictors of distant metastasis. The calibration, discrimination, and clinical performance of the nomogram were tested by calibration plots, area under receiver operating characteristic curve (AUC), and decision curve analysis (DCA).
Results: Age at diagnosis (<70 years), histological type (invasive mucinous adenocarcinoma), T stage, N stage, surgical approach, and lymph node dissection were independent predictors for the development of nomogram. Compared with the American Joint Committee on Cancer-8th edition staging system, AUC showed that this prediction model has a higher predictive performance (training set: 0.922 vs. 0.790; verification set: 0.919 vs. 0.779). In addition, the overall survival time (OS) of IAC patients was meaningfully different among the three groups of different risks stratified based on model score (p <0.001).
Conclusion: The prediction model constructed according to factors such as histological type and surgical approach in this study can accurately predict distant metastasis in IAC patients and define high-risk patients according to nomogram score.
Key Words: Invasive adenocarcinoma IAC, Distant metastasis, Nomogram, Surveillance, Epidemiology and end results SEER.
INTRODUCTION
With an approximated 2206771 new cases and 1796144 fatalities worldwide in 2020, the most common kind of cancer that causes mortality is lung cancer.1 Non-small cell lung cancer (NSCLC), which accounts for around 85% of occurrences, is the most prevalent kind.2 Adenocarcinoma (LUAD), the most typical histological type of lung cancer, was further divided into atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and invasive adenocarcinoma (IAC).3 IAC is the most common type of LUAD, mainly composed of invasive non-mucinous adenocarcinoma and invasive mucinous adenocarcinoma (IMA).4 IAC is one of the highly invasive and lethal types of tumours, usually showing diffuse multi-organ metastasis in the advanced stage.5
The probability of distant metastasis largely determines the treatment strategy and the possibility of long-term survival. Treatments for LUAD have advanced recently, including surgical resection, immunotherapy, and targeted therapy. Currently, the best treatment for IAC is surgical resection. The different effects of lobectomy and sublobectomy on distant metastasis and long-term survival have received a lot of attention. With the development of gene detection technology and the discovery of more molecular targets, targeted therapy has made remarkable progress. The research on tumour microenvironment has promoted the progress of immunotherapy, which was also an effective intervention for patients with IAC.6 Nevertheless, the prognosis of IAC was still not satisfactory, whose 5-year survival rate was less than 20%,7 and metastatic lesions were the main cause of death.
After surgery, a precise estimate of the probability of distant metastasis in IAC patients can help patients and doctors decide adjuvant treatment options and follow-up frequency, as well as provide patients and their families with information about long-term survival outcomes. Some studies have shown that clinical features of NSCLC patients were associated with their propensity to distant metastasis.8 Unfortunately, there is no reliable prediction model to accurately predict IAC patients' distant metastasis. The method generally used to evaluate the risk of metastasis and long-term survival is the American Joint Committee on Cancer (AJCC) staging system. Although the AJCC staging system may be helpful for general survival prediction, it may not be applicable for a personalised assessment of patients. Nomogram is a new statistical prediction model, which can predict accurately individuals.9 Some studies have indicated that nomogram has good application value in various cancers.10 In order to improve the prognosis of IAC, this study aimed at evaluating various clinical features of different IAC patients and created a nomogram that integrates essential clinical and pathological features to improve the prediction effect of traditional methods and guide clinical decision-making.
METHODOLOGY
The SEER database, which has information on about 30% of the US population, provided the data for this study.11 SEER*STAT 8.3.9.2 software was used to retrieve the characteristics of patients who received an IAC diagnosis between 2010 and 2015 from the database (1975-2018). The IAC was identified by the site codes [8230, 8250, 8252, 8253, 8260, 8550, 8551] of the International Classification of Diseases for Oncology third edition (ICD-O-3). The inclusion criteria were precise pathological diagnosis; IAC was the single primary malignancy; age above 18 years when diagnosed; and the desired clinical information of the patients was known. Figure 1 depicts the flowchart for patient selection.
Demographic and clinicopathological data included histological type, tumour site, T stage, N stage, distant metastasis, surgical approach, whether lymph node dissection was performed, gender, race, age at diagnosis, as well as overall survival (OS). A median age of 69 years was used to convert the variable age from a continuous to a categorical one. The histological type was classified as invasive non-mucinous adenocarcinoma and invasive mucinous adenocarcinoma (IMA). The classification of the tumour site included main bronchus, upper, middle, and lower lobes, overlapping and others. The surgical approach included non-operation, lobectomy and sublobectomy. The study's outcome variable was chosen to be distant metastasis.
SPSS 19.0 software was used to complete the statistical analysis. Continuous variables were reported as median (quartile spacing) and Mann-Whitney U test was applied to compare them across the groups. Categorical variables were written as integers (proportions) and Chi-sq. or Fisher’s exact test were applied to compare them across the groups. The survival was examined using the Kaplan-Meier method and the Log-rank test. All patients were allocated at random into training and verification sets with a 7:3 ratio. Variables connected to distant metastasis in IAC patients were identified using univariate logistic regression analysis. To screen out independent predictors, multivariate analysis was used to examine factors having p-values < 0.05 in the univariate study. A nomogram was developed on the basis of these characteristics, to forecast the probability of distant metastasis in IAC patients. By using calibration plots, the predictive ability of the models in the training group and validation group was evaluated. AJCC staging system and the model's predictive performance were compared using AUC and DCA. In addition, according to the scores predicted by the nomogram, the training group was divided into three risk types. The Kaplan-Meier curve of OS was drawn for each group, which can further evaluate the clinical effectiveness of the model. The nomogram and the related calibration plots, ROC and DCA, were built using the RMS and PROC packages in R software (version 4.1.2). A p-<0.05 was statistically significant.
RESULTS
After being screened, 5742 IAC patients who satisfied the requirements were included and were assigned randomly to the training group of 4021 and the validation group of 1721. The rate of distant metastases and other factors were comparable across the two groups, with no significant difference (p>0.05, Table I). Age, gender, tumour site, histological type, T stage, N stage, surgical approach, and lymph node dissection were the factors linked to distant metastasis, according to univariate analysis (p<0.05, Table II). Six of these factors were shown to be independent predictors of distant metastasis after multivariate analysis: age (<70: OR= 2.597, 95% CI: 2.050-3.291, p<0.001), histological type (IMA: OR=1.813, 95% CI: 1.434-2.293, p<0.001), T stage (T2: OR=1.472, 95% CI: 1.119-1.936, p=0.006; T3: OR= 1.777, 95% CI: 1.237-2.553, p=0.002; T4: OR= 1.722, 95% CI: 1.171-2.534, p=0.006), N stage (N1: OR= 2.660, 95% CI: 1.789-3.955, p<0.001; N2: OR= 3.834, 95% CI: 2.921-5.032, p<0.001; N3: OR= 4.005, 95% CI: 2.671-6.006, p<0.001), surgical approach (sublobectomy: OR= 0.363, 95% CI: 0.250-0526, p<0.001; lobectomy: OR=0.125, 95% CI: 0.071-0.221, p<0.001), and lymph node dissection (yes: OR= 0.170, 95% CI: 0.071-0.221, P<0.001).
The six independent variables mentioned above were used in this research to create a nomogram that may be used to predict distant metastases in IAC patients, as shown in Figure 2. The calibration curves were almost 45° for the two sets, indicating good calibration performance (Figure 3 A-B). The prediction accuracy of the AJCC staging system and nomogram were compared using ROC curves. The nomogram's AUC value in the training set was 0.922 (95% CI: 0.912-0.932), which was much higher than the AJCC staging system's value (0.790, 95% CI: 0.770-0.810, Figure 3C). The nomogram's AUC value in the validation set was 0.919 (95% CI: 0.902-0.936), which was also much higher than the AJCC staging system's value (0.779, 95%CI: 0.748-0.810, Figure 3D). According to DCA, in the two sets, the nomogram provided a greater net benefit compared to the AJCC staging system (Figure 3 E & F). In addition, the training set was also classified into three risk groups based on the nomogram score with the cut-off value calculated using X-tile software in order to assess the clinical utility of the model. These risk groups are as follows: low-risk group: 0 to 110.54; moderate-risk group: 110.91 to 230.74; and high-risk group: 231.73 to 353.93. Based on this score, Kaplan-Meier survival curves were plotted for OS in the three risk groups, showing an obvious grading ability (Figure 4). The aforementioned findings demonstrated that the model had high predictive performance and clinical applicability.
Table I: Characteristics of patients with invasive adenocarcinoma.|
Variables |
Cohort, No. (%) |
p-value |
||
|
|
Total (n=5742) |
Training (n=4021) |
Validation (n=1721) |
|
|
Age, year, median (IQR) |
69(61-76) |
69(61-76) |
69(61-76) |
0.645 |
|
<70 |
3032(52.8) |
2115(52.6) |
917(53.3) |
0.634 |
|
≥70 |
2710(47.2) |
1906(47.4) |
804(46.7) |
|
|
Gender |
|
|
|
|
|
Female |
3414(59.5) |
2396(59.6) |
1018(60.1) |
0.758 |
|
Male |
2328(40.5) |
1625(40.4) |
703(40.8) |
|
|
Race |
|
|
|
|
|
White |
4513(78.6) |
3181(79.1) |
1332(77.4) |
0.162 |
|
Black |
578(10.1) |
405(10.1) |
173(10.1) |
|
|
Others |
651(11.3) |
435(10.8) |
216(12.6) |
|
|
Tumour site |
|
|
|
|
|
Main bronchus |
29(0.5) |
20(0.5) |
9(0.5) |
0.432 |
|
Upper lobe |
2991(52.1) |
2062(51.3) |
929(54.0) |
|
|
Middle lobe |
301(5.2) |
215(5.3) |
86(5.0) |
|
|
Lower lobe |
2156(37.5) |
1539(38.3) |
617(35.9) |
|
|
Overlapping |
75(1.3) |
49(1.2) |
26(1.5) |
|
|
Others |
190(3.3) |
136(3.4) |
54(3.1) |
|
|
Histology |
|
|
|
|
|
Adenocarcinomas |
4059(70.7) |
2855(71.0) |
1204(70.0) |
0.426 |
|
Invasive mucinous adenocarcinomas |
1683(29.3) |
1166(29.0) |
517(30.0) |
|
|
Surgical approach |
|
|
|
|
|
No |
1601(27.9) |
1128(28.1) |
473(27.5) |
0.714 |
|
Segmental resection |
820(14.3) |
581(14.4) |
239(13.9) |
|
|
Lobectomy |
3321(57.8) |
2312(57.5) |
1009(58.6) |
|
|
Lymph node dissection |
|
|
|
|
|
No |
1977(34.4) |
1397(34.7) |
580(33.7) |
0.447 |
|
Yes |
3765(65.6) |
2624(65.3) |
1141(66.3) |
|
|
T stage |
|
|
|
|
|
T1 |
3493(60.8) |
2451(61.0) |
1042(60.5) |
0.607 |
|
T2 |
1309(22.8) |
906(22.6) |
403(23.4) |
|
|
T3 |
476(8.3) |
344(8.6) |
132(7.7) |
|
|
T4 |
464(8.1) |
320(8.0) |
144(8.4) |
|
|
N stage |
|
|
|
|
|
N0 |
4170(72.6) |
2919(72.6) |
1251(72.7) |
0.249 |
|
N1 |
450(7.8) |
331(8.2) |
119(6.9) |
|
|
N2 |
900(15.7) |
614(15.3) |
286(16.6) |
|
|
N3 |
222(3.9) |
157(3.9) |
65(3.8) |
|
|
Distant metastasis |
|
|
|
|
|
No |
4813(83.8) |
3365(83.7) |
1448(84.1) |
0.670 |
|
Yes |
929(16.2) |
656(16.3) |
273(15.9) |
|
|
Note: Mann-Whitney U test for continuous variables; Chi-square test for categorical variables. |
||||
DISCUSSION
Among all malignancies, lung cancer has the greatest fatality rate and continues to be the major cause of death worldwide.1 IAC is highly invasive and metastatic, with a very high possibility of distant metastasis in the advanced stage, which is an essential factor leading to poor prognosis. Therefore, early identification of high-risk groups has significant guiding implications for the treatment decision, the evaluation of long-term survival, and the formulation of follow-up frequency. Currently, diagnostic imaging techniques are still the conventional diagnostic methods for recurrence and metastasis, but they have no good predictive value. Tumour markers such as CEA, CA125, CA199, and CYFRA21-1 were also commonly used monitoring indicators. Some studies have indicated that CYFRA21-1 has a higher sensitivity in patients with metastatic NSCLC,12 but these indicators were not specific to lung cancer. In addition, some reports have revealed that the effects of several markers, excluding CYFRA21-1, on prognosis were not statistically significant.13
Therefore, to make it easier to predict the probability of distant metastases in IAC patients at the individual level and to aid physicians in screening high-risk patients for the appropriate interventions, this study developed a nomogram based on demographic and individual clinicopathological factors. The variables that make up the nomogram include age, histological type, T stage, N stage, surgical approach, and lymph node dissection, all of which are readily available in the clinic so that doctors can easily use the model. In both the groups, the nomogram exhibited outstanding concordance between the predicted value and the actual value. Notably, the greater AUC and DCA values supported the nomogram's superior predictive ability and clinical efficacy over the AJCC staging system.
Similar to Tian's findings, this study revealed that patients who are younger had a greater probability of distant metastasis.14 It might be related to young patients' infrequent examinations and cancer's advanced state at the time of its initial discovery. It may also be related to the fact that young patients have a tumour microenvironment that is more suitable for metastasis. In addition, gender significantly impacts on the occurrence and metastasis of NSCLC.15 Smoking may be a key factor in why male patients are more likely to develop distant metastases than female ones.16
Table II: Univariate and multivariate analysis of distant metastasis in invasive adenocarcinoma.
|
Variables |
Univariate analysis |
|
Multivariate analysis |
||
|
OR (95% CI) |
p-value |
|
OR (95% CI) |
p-value |
|
|
Age, year |
|
|
|
|
|
|
≥70 |
1[Reference] |
NA |
|
1[Reference] |
NA |
|
<70 |
1.228(1.037-1.454) |
0.017 |
|
2.597(2.050-3.291) |
<0.001 |
|
Gender |
|
|
|
|
|
|
Female |
1[Reference] |
NA |
|
1[Reference] |
NA |
|
Male |
1.278(1.080-1.513) |
0.004 |
|
0.911(0.724-1.147) |
0.417 |
|
Race |
|
|
|
|
|
|
White |
1[Reference] |
NA |
|
|
|
|
Black |
1.222(0.933-1.600) |
0.145 |
|
|
|
|
Others |
1.426(1.109-1.833) |
0.006 |
|
|
|
|
Tumour site |
|
|
|
|
|
|
Main bronchus |
1[Reference] |
NA |
|
1[Reference] |
NA |
|
Upper lobe |
0.077(0.029-0.201) |
<0.001 |
|
0.341(0.1021.139) |
0.080 |
|
Middle lobe |
0.081(0.029-0.224) |
<0.001 |
|
0.392(0.107-1.435) |
0.157 |
|
Lower lobe |
0.075(0.029-0.198) |
<0.001 |
|
0.323(0.097-1.080) |
0.067 |
|
Overlapping |
0.139(-0.044-0.442) |
0.001 |
|
0.612(0.131-2.867) |
0.533 |
|
Others |
0.274(0.099-0.756) |
0.012 |
|
0.639(0.178-2.288) |
0.491 |
|
Histology |
|
|
|
|
|
|
Invasive non-mucinous adenocarcinomas |
1[Reference] |
NA |
|
1[Reference] |
NA |
|
Invasive mucinous adenocarcinomas. |
2.527(2.128-3.001) |
<0.001 |
|
1.813(1.434-2.293) |
<0.001 |
|
Surgical approach |
|
|
|
|
|
|
No |
1[Reference] |
NA |
|
1[Reference] |
NA |
|
Segmental resection |
0.112(0.083-0.151) |
<0.001 |
|
0.363(0.250-0.526) |
<0.001 |
|
Lobectomy |
0.019(0.014-0.027) |
<0.001 |
|
0.125(0.071-0.221) |
<0.001 |
|
Lymph node dissection |
|
|
|
|
|
|
No |
1[Reference] |
NA |
|
1[Reference] |
NA |
|
Yes |
0.031(0.024-0.041) |
<0.001 |
|
0.170(0.103-0.279) |
<0.001 |
|
T stage |
|
|
|
|
|
|
T1 |
1[Reference] |
NA |
|
1[Reference] |
NA |
|
T2 |
3.030(2.467-3.720) |
<0.001 |
|
1.472(1.119-1.936) |
0.006 |
|
T3 |
4.985(3.836-6.477) |
<0.001 |
|
1.777(1.237-2.553) |
0.002 |
|
T4 |
4.389(3.338-5.770) |
<0.001 |
|
1.722(1.171-2.534) |
0.006 |
|
N stage |
|
|
|
|
|
|
N0 |
1[Reference] |
NA |
|
1[Reference] |
NA |
|
N1 |
2.898(2.140-3.924) |
<0.001 |
|
2.660(1.789-3.955) |
<0.001 |
|
N2 |
8.945(7.255-11.029) |
<0.001 |
|
3.834(2.921-5.032) |
<0.001 |
|
N3 |
20.805(14.627-29.594) |
<0.001 |
|
4.005(2.671-6.006) |
<0.001 |
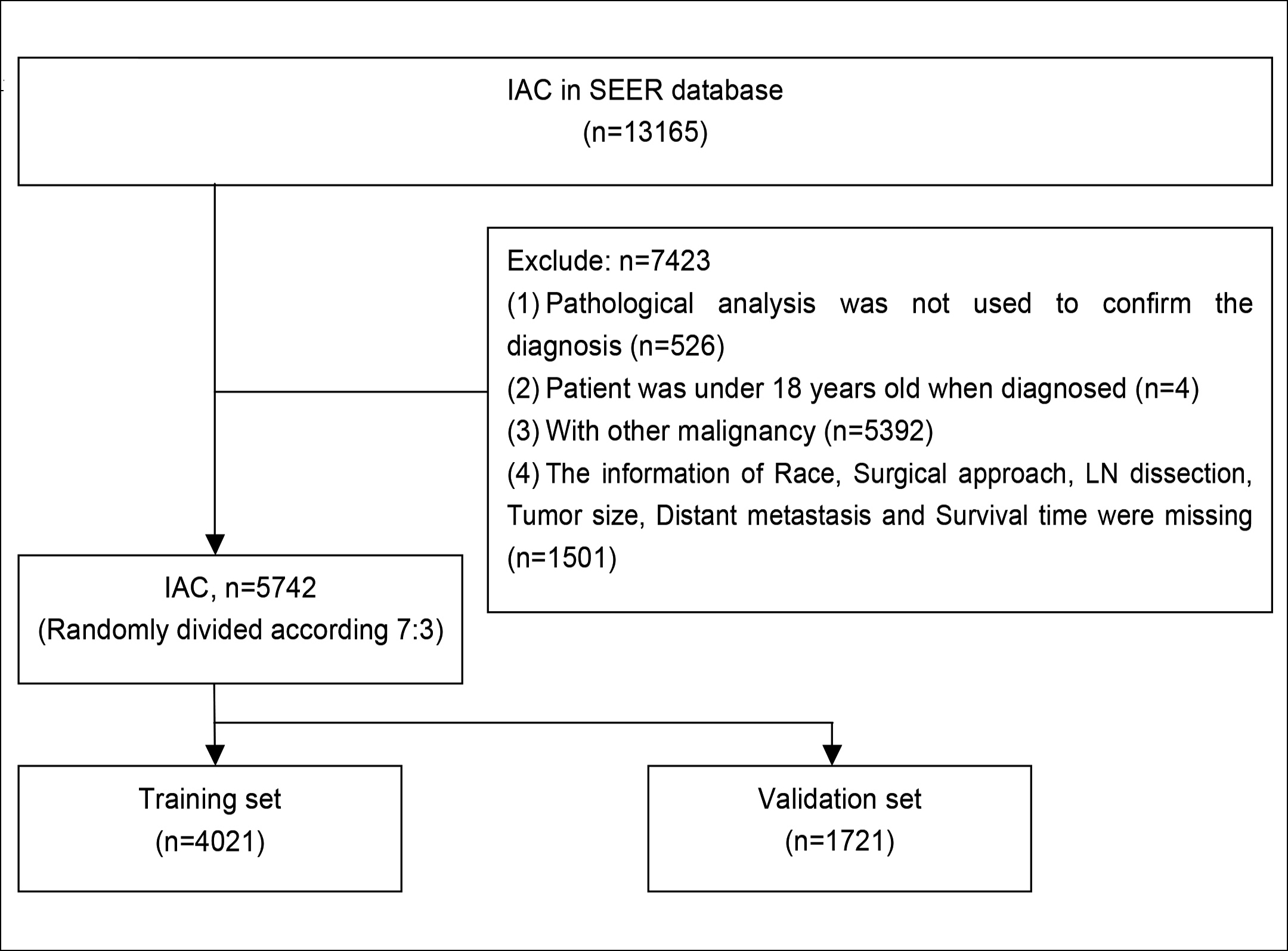 Figure 1: Patient selection flowchart.
Figure 1: Patient selection flowchart.
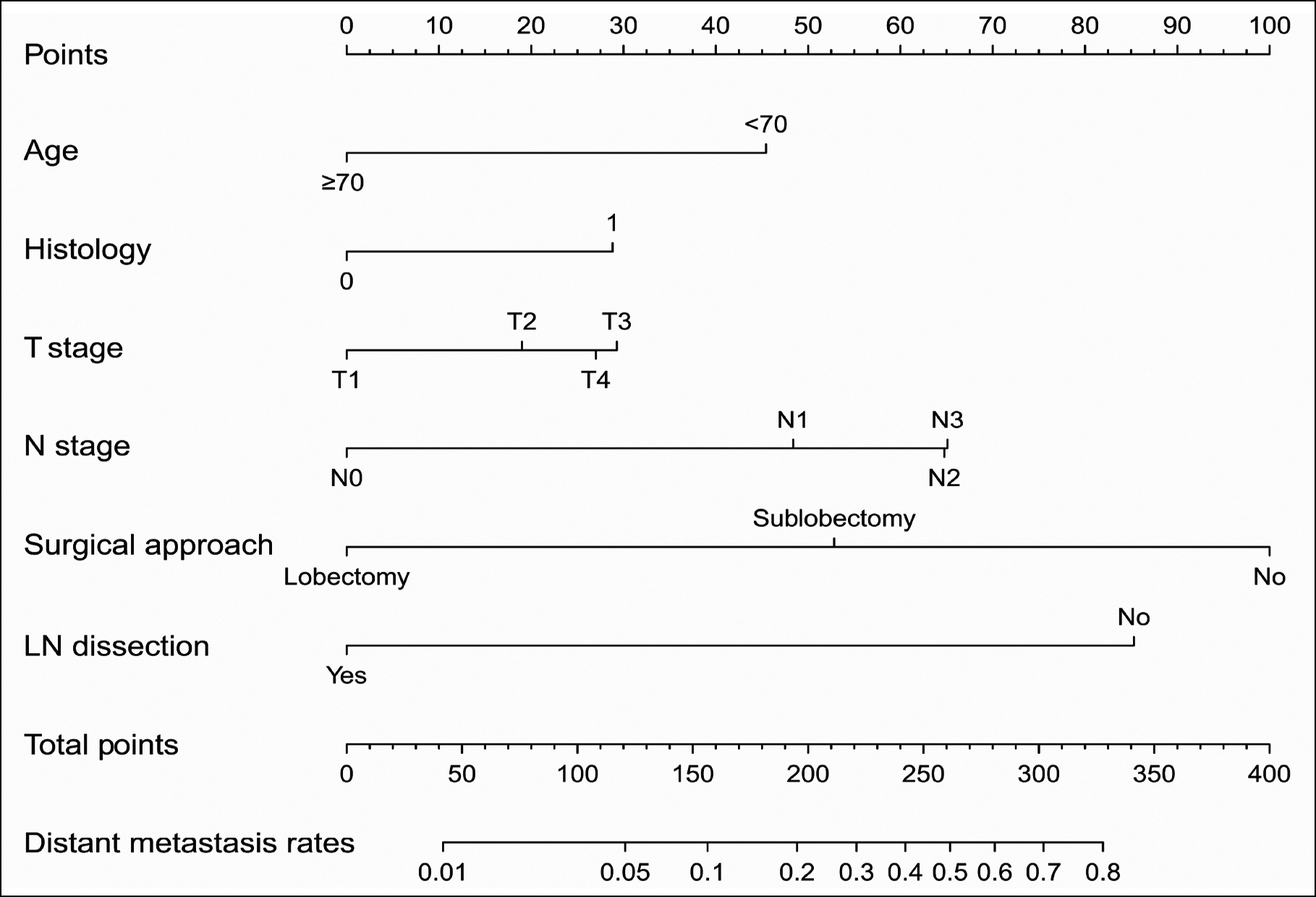 Figure 2: Nomogram for predicting distant metastasis in IAC patients. 0 replaces Invasive non-mucinous adenocarcinomas; 1 replaces Invasive mucinous adenocarcinomas.
Figure 2: Nomogram for predicting distant metastasis in IAC patients. 0 replaces Invasive non-mucinous adenocarcinomas; 1 replaces Invasive mucinous adenocarcinomas.
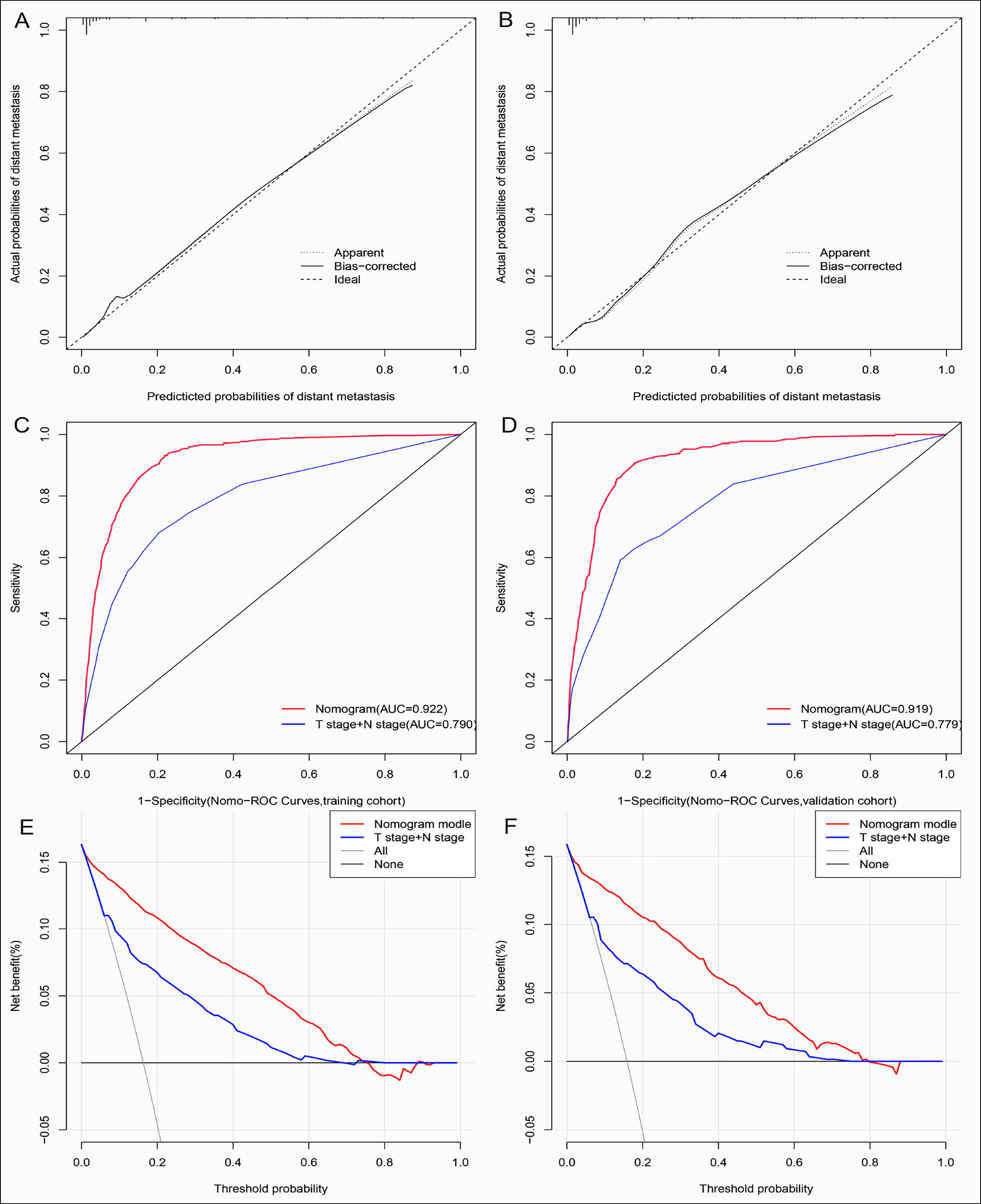 Figure 3: (A, B) Calibration graphs in the training and validation sets. (C, D) ROC curves of the nomogram and AJCC staging system (T stage + N stage) in training and validation sets. (E, F) DCA of the nomogram and AJCC staging system (T stage + N stage) in training and validation sets.
Figure 3: (A, B) Calibration graphs in the training and validation sets. (C, D) ROC curves of the nomogram and AJCC staging system (T stage + N stage) in training and validation sets. (E, F) DCA of the nomogram and AJCC staging system (T stage + N stage) in training and validation sets.
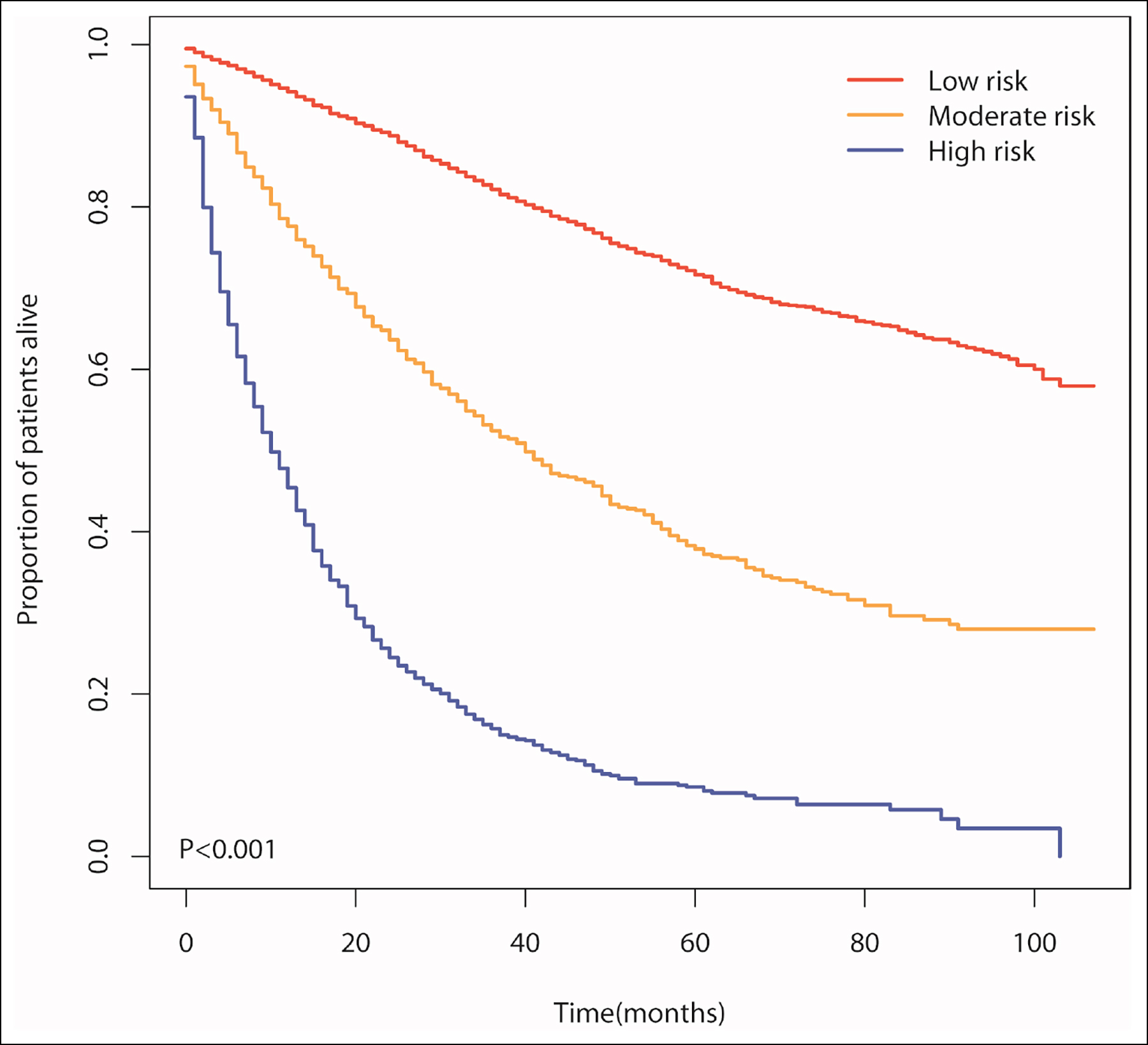 Figure 4: Kaplan-Meier curves for patients’ OS among the three risk groups based on the nomogram score in the training set.
Figure 4: Kaplan-Meier curves for patients’ OS among the three risk groups based on the nomogram score in the training set.
In this study, gender was not an independent factor affecting distant metastasis, which may be related to the differences in subjects. The study population consisted of patients with IAC, about 60% of whom were female, and who did not smoke commonly. At the same time, smoking was much less strongly associated with LUAD than squamous cell carcinoma (LUSC),15 an important component of NSCLC. Patients with IMA in this study had a greater probability of distant metastasis than those with invasive non-mucinous adenocarcinomas. Huang et al. reported that pathological subtypes of invasive non-mucinous adenocarcinoma were associated with organ-specific metastasis in patients with LUAD.17 However, the low incidence of IMA leads to less related research at present, and its relationship with distant metastasis needs further investigation.
In the nomogram, the surgical approach played an important role in predicting distant metastasis. It is generally accepted that sublobectomy has a higher recurrence rate and metastasis than lobectomy. However, with the development of examination methods and surgical techniques, this view is being challenged. Raman et al. have indicated that lobectomy significantly improved the survival rate compared with sublobectomy for LUAD patients with tumour diameter larger than 10 mm.18 Yu et al. have reported that lobectomy was linked to better cancer-specific survival (CSS) and OS in NSCLC patients with a 21–30 mm tumour size.19 In contrast, Chan et al. have shown no statistical difference between lobectomy and sublobectomy in terms of metastasis or recurrence rates, recurrence-free survival, and OS in NSCLC patients at stage T1c.20 This matter is still controversial, and patients who underwent sublobectomy in this study had a greater probability of distant metastasis than those who underwent lobectomy.
The risk of distant metastasis was greater in those without lymph node dissection, which was in line with the National Comprehensive Cancer Network (NCCN) guidelines for lymph node dissection.21 In general, the authors believe that the prognosis of lung cancer will deteriorate with the increase of tumour diameter. However, in this study, patients with IAC of 5-7 cm in diameter had the highest risk of distant metastasis, which needs to be further investigated. Similar results have not been found in other studies of NSCLC, but have been reported in studies related to SCLC.22,23
The probability of distant metastasis increased with N stage in this study's nomogram, but there was no significant difference between N2 and N3 stages. Previous studies found no statistical difference between N2 and N3 stages of OS and CSS in lung cancer. Furthermore, there were some defects in the AJCC staging system, suggesting that the number of involved lymph nodes should be added to the staging system.24,25
This study has several limitations. Firstly, a large-scale multicenter investigation should be required because this was a retrospective analysis with potential confounding biases. Secondly, a model's over-fitting may occur because it lacks external validation from other institutions. In the SEER database, the number of patients treated with ablation was tiny, making it impossible for the authors to include it in the study of the surgical approach. In addition, some essential clinicopathological factors such as tumour markers, smoking, and gene mutation status were missing from the SEER database, which needs to be further studied. Therefore, the clinical practicability of this nomogram needs to be further verified and supplemented.
CONCLUSION
Demographic and clinicopathological characteristics were combined to identify the independent predictors of distant metastases in IAC patients. A nomogram with outstanding predictive performance and clinical value was established.
FUNDING:
This work was supported by Shandong University (grant No. 26020312001907).
ETHICAL APPROVAL:
The study was approved by the Ethics Committee of Jinan Central Hospital. (No. 2021-216-01, Date: 2021.12.24).
PATIENTS' CONSENT:
The information in the SEER database was anonymous and freely available to the public. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
ZP: Design, acquisition and analysis of data, and manuscript writing.
HL, ZC: Result interpretation and discussion.
LZ: Design the study and agree to be accountable for all aspects of the work.
All authors have approved the final version of the manuscript to be published.
REFERENCES
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71(3): 209-49. doi: 10.3322/caac.21660.
- Pirker R. Conquering lung cancer: Current status and prospects for the future. Pulmonology 2020; 26(5):283-90. doi: 10.1016/j.pulmoe.2020.02.005.
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018; 553(7689):446-54. doi: 10.1038/nature25183.
- Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, et al. The 2021 WHO classification of lung tumours: Impact of advances since 2015. J Thorac Oncol 2022; 17(3):362-87. doi: 10.1016/j.jtho.2021.11.003.
- Denisenko TV, Budkevich IN, Zhivotovsky B. Cell death-based treatment of lung adenocarcinoma. Cell Death Dis 2018; 9(2):117. doi: 10.1038/s41419-017-0063-y.
- Ruiz-Cordero R, Devine WP. Targeted therapy and checkpoint immunotherapy in lung cancer. Surg Pathol Clin 2020; 13(1):17-33. doi: 10.1016/j.path.2019.11.002.
- Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016; 893:1-19. doi: 10.1007/ 978-3-319- 24223-1_1.
- Goksel S, Ozcelik N. Distant metastasis patterns of lung cancer on positron emission tomography/computed tomography association with age and histological subtype. J Coll Physicians Surg Pak 2021; 31(12):1438-44. doi: 10.29271/ jcpsp.2021.12.1438.
- Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008; 26(8):1364-70. doi: 10.1200/JCO.2007.12. 9791.
- Yang M, Li D, Jiang W, Zhu L, Ju H, Sun Y, et al. Development and external validation of a novel nomogram for screening Chinese Lynch syndrome: based on a multicenter, population study. Ther Adv Med Oncol 2021; 13:175883592 11023290. doi: 10.1177/17588359211023 290.
- Cronin KA, Ries LA, Edwards BK. The surveillance, epidemiology, and end results (SEER) program of the national cancer institute. Cancer 2014; 120 Suppl 23:3755-7.
- Ma S, Shen L, Qian N, Chen K. The prognostic values of CA125, CA19.9, NSE, and SCC for stage I NSCLC are limited. Cancer Biomark 2011; 10(3-4):155-62.
- Hoseok I, Cho JY. Lung Cancer Biomarkers. Adv Clin Chem 2015; 72:107-70. doi: 10.1016/bs.acc.2015.07.003.
- Tian Y, He Y, Li X, Liu X. Novel nomograms to predict lymph node metastasis and distant metastasis in resected patients with early-stage non-small cell lung cancer. Ann Palliat Med 2021; 10(3):2548-66. doi: 10.21037/apm-20- 1756.
- Rusmaully J, Tvardik N, Martin D, Billmann R, Cenee S, Antoine M, et al. Risk of lung cancer among women in relation to lifetime history of tobacco smoking: A population-based case-control study in France (the WELCA study). BMC Cancer 2021; 21(1):711. doi: 10.1186/ s12885-021- 08433-z.
- Stapelfeld C, Dammann C, Maser E. Sex-specificity in lung cancer risk. Int J Cancer 2020; 146(9):2376-82. doi: 10.1002/ ijc.32716.
- Hung JJ, Jeng WJ, Wu YC, Chou TY, Hsu WH. Factors predicting organ-specific distant metastasis in patients with completely resected lung adenocarcinoma. Oncotarget 2016; 7(36):58261-73. doi: 10.18632/oncotarget.11338.
- Raman V, Jawitz OK, Voigt SL, Rhodin KE, D'Amico TA, Harpole DH, et al. The effect of tumour size and histologic findings on outcomes after segmentectomy vs lobectomy for clinically node-negative non-small cell lung cancer. Chest 2021; 159(1):390-400. doi: 10.1016/j.chest.2020. 06.066.
- Yu X, Zhang R, Zhang M, Lin Y, Zhang X, Wen Y, et al. Segmental resection is associated with decreased survival in patients with stage IA non-small cell lung cancer with a tumour size of 21-30 mm. Transl Lung Cancer Res 2021; 10(2):900-13. doi: 10.21037/tlcr-20-1217.
- Chan EG, Chan PG, Mazur SN, Normolle DP, Luketich JD, Landreneau RJ, et al. Outcomes with segmentectomy versus lobectomy in patients with clinical T1cN0M0 non-small cell lung cancer. J Thorac Cardiovasc Surg 2021; 161(5): 1639-48 e2. doi: 10.1016/j.jtcvs.2020.03.041.
- Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines insights: Non-small Cell lung cancer, version 2.2021. J Natl Compr Canc Netw 2021; 19(3):254-66. doi: 10.6004/jnccn.2021.0013.
- Li J, Liu F, Yu H, Zhao C, Li Z, Wang H. Different distant metastasis patterns based on tumour size could be found in extensive-stage small cell lung cancer patients: A large, population-based SEER study. PeerJ 2019; 7:e8163. doi: 10.7717/peerj.8163.
- Lu YJ, Yang Y, Yuan YH, Wang WJ, Cui MT, Tang HY, et al. A novel nomogram based on SEER database for the prediction of liver metastasis in patients with small-cell lung cancer. Ann Palliat Med 2020; 9(5):3123-37. doi: 10.21037/apm- 20-886.
- Fan Y, Du Y, Sun W, Wang H. Including positive lymph node count in the AJCC N staging may be a better predictor of the prognosis of NSCLC patients, especially stage III patients: A large population-based study. Int J Clin Oncol 2019; 24(11):1359-66. doi: 10.1007/s10147-019-01483-1.
- Xu L, Su H, She Y, Dai C, Zhao M, Gao J, et al. Which N descriptor is more predictive of prognosis in resected non-small cell lung cancer: The number of involved nodal stations or the location-based pathological n stage? Chest 2021; 159(6):2458-69.