Fok1 VDR Gene Polymorphisms as the Risk factor for Diabetes Mellitus
By Muhammad Asif Memon1, Saeeda Baig2, Pirzada Qasim Raza Siddiqui3Affiliations
doi: 10.29271/jcpsp.2022.05.581ABSTRACT
Objective: To investigate the prevalence of single nucleotide polymorphisms of vitamin D receptor (VDR) gene, and its association with Type 2 Diabetes Mellitus.
Study Design: Cross-sectional study.
Place and Duration of Study: Ziauddin University Hospital, Karachi, from January 2018 to 2020.
Methodology: A total of 200 unrelated individuals, aged 25 to 65 years, were selected and divided into two groups, T2DM patients (100) and non-diabetic controls (100). After consent, demographics, diabetic history and related risk factors were recorded in a standard questionnaire and blood was collected. The VDR (Fok1, Taq1 and Apa1) polymorphisms were analyzed through PCR and RFLP.
Results: In T2DM patients, F/F and F/f genotypes were found in 49 (49%) and 45 (45%) patients, respectively. The ff genotype was less common in T2DM [6 (6%)], compared to healthy controls [14 (14%)], (odds ratio=0.473, 95% CI: 0.267–0.839). The frequency of T/t genotype was 36% and 31% in the control and diabetic group respectively while for A/a genotype was 38% and 34% respectively, the results were not statistically significant.
Conclusion: T2DM was found significantly associated with Fok1 Polymorphisms of VDR gene. The study found a protective role of ff of Fok1 in diabetic patients. Further studies in larger cohorts are required for validation.
Key Words: Polymorphism, Restriction fragment length, Receptor, Vitamin D3 receptor, Type 2 diabetes mellitus.
INTRODUCTION
Type 2 diabetes mellitus (T2DM), is a multi-factorial syndrome and its etiology involves a complex interplay between genetics, epigenetics, and environmental factors that affect beta-cell function and insulin sensitivity of various tissues. In recent years, Vitamin D3, through correlation of the above factors, has been found to be involved in causing T2DM. Vitamin D influences body through multiple metabolisms. The synthesis and bioavailability of Vitamin D are dependent on socioeconomic (D-rich food), environmental (sunny areas) and genetic factors (receptor genes, binding protein genes).
The studies around the world have proved that vitamin D improves the function of β-cells of islets of Langerhans in the pancreas and protect them from calamitous immune attack.1 It improves the sensitivity of insulin hormone with its receptor and reduces hormone resistance. Active vitamin D is required to carry out these functions by binding to its intracellular receptor (VDR).1
Vitamin D receptor (VDR), belongs to the receptor family of steroid hormones and is present inside the cell. After the binding of vitamin D3 there is a conformational change in the VDR which assist in its binding to the DNA and activate the transcription of genes on the DNA for the synthesis of many proteins. The vitamin D receptor gene is present on the long arm of chromosome number 12 (12q13.1) and is composed of 14 exons. It has a promoter region that is very extensive and generates different tissue-specific transcripts.2 Vitamin D receptor gene polymorphisms has been found to be of several types with diverse physiological and pathological phenotypes. The four allelic variants of the VDR gene are described as: Fok1, Apa1, Bsm 1,and Taq1.3 Out of the above four alleles, three alleles of the Bsm1, Apa1, and Taq1 polymorphisms are located at intron 8 and exon 9, near the 3' end of the VDR gene and are genetically linked, while Fok1 is present at exon 2.4
Pakistan is a sunny country, yet the majority (73%) of the population is vitamin D deficient.5 Secondly, type 2 diabetes mellitus is also very common (13.7%),6 hence, one can contemplate that VDR gene polymorphism may be one of the factors involved in the prevalence of type 2 diabetes mellitus.
The objective of this study was to investigate the prevalence of three (SNP) single nucleotide polymorphisms; Fok1, Apa1 and Taq1 of the VDR gene, and find out its correlation with the occurrence of T2DM.
METHODOLOGY
Unrelated individuals (200), from Ziauddin University Hospital, Karachi, were included in this study. Prior to the start of the study, ethical approval was obtained from Ethics Review Committee, Ziauddin University. The study subjects were divided into two groups. Group one comprised 100 subjects with type 2 diabetes, (diagnosed at least two years back) and group 2 with 100 without diabetes, as non-diabetic controls. A standard questionnaire recorded history of diseases including diabetes mellitus, chronic obstructive pulmonary disease (COPD), hypertension, smoking habits, and medications, of the patients. A basic general physical examination recorded weight, height, waist-hip circumference and blood pressure. Patients with type 1 diabetes mellitus (T1DM) or receiving insulin or any other treatment for hypercholesterolemia or hypertension were excluded from the study.
After informed consent from subjects, 5 ml of blood, in tubes containing ethylenediaminetetra acetic acid, was collected. Isolation of DNA was done by using a DNA purification kit by Fermentas (Gene Jet Genomic kit). Extracted DNA was divided into aliquots and stored at 4°C till further use.
Polymerase chain reaction (PCR) was performed in the total volume of 25µl of reaction mixture consisting of 4.5µl of DNA, 12.5 µl of Master Mix (having MgCl2, PCR buffer, dNTPs and Taq DNA polymerase), 2 µl of each primer, Forward (AGCTGGCCCTGGCACTGACTCTGCTCT) and Reverse (ATGGAAACACCTTGCTTCTTCTCCCTC) and 4 µl of nuclease-free water. The PCR tubes were placed in the thermocycler for amplification. The cycling temperatures for Fok1, Taq1 and Apa1 polymorphism were: initial denaturation at 95°C for 5 min, 35 cycles at 95°C for 30 sec, 63°C for 30 sec and 72°C for 1 min and finally one final cycle of extension at 72°C for 5 min.
The PCR products were then digested by 1.0 unit of Fok1, 3.0 unit of Taq1and 3.0 units of Apa1 restriction enzyme (Fast Digest-Fermentas) at 37°C for 4 hours, 65°C for 3 hours and 65°C for 1 hour respectively. For electrophoresis 5 µl of the digested reaction mixture was then loaded into 2% agarose gel containing ethidium bromide and run for 1 hour and then visualized under the UV light. DNA ladder of 100 bp was used for the determination of digested fragments. Fok1 digested product genotyped as homozygote FF (265 bp) and ff (169 bp and 96 bp),whereas, heterozygotes genotypes included, Ff (265, 169 and 96 bp). The Taq1 digestion revealed homozygote genotype TT (245 and 495 bp), tt homozygotes (205, 245 and 290 bp) and the heterozygotes genotype Tt (495, 205, 290 and 245 bp) fragments. The Apa1 digestion revealed genotype AA homozygote (740 bp), genotype aa homozygotes (530 and 210) and genotype Aa heterozygote (740, 530 and 210 bp) fragments.
Descriptive statistics were calculated for both clinical and biological data. Mean and standard deviation were calculated for continuous variables and student t-test was performed to analyze them. Chi-square test of association was performed for categorical variables which were presented as frequency and percentages. The p-value less than 0.05 was considered as significant.
RESULTS
The mean age of participants was 52.3±9.6 years, ranged from 25 to 65 years. Statistical significant difference was observed among patients and controls regarding weight and height (p=0.013 and 0.01 respectively). However, the difference in relation to age and BMI was not significant (p value= 0.131 and 0.119 respectively). Significant difference was observed in diabetic groups compared to healthy controls regarding fasting blood glucose (p<0.001), HbA1C (p<0.001) as well as cholesterol (p<0.001), LDL (p<0.001) and triglyceride (p<0.001).
The difference in Vitamin D levels in patients with T2DM (23.09±4.1 ng/ml) compared to the controls (29.33±2.5 ng/ml) was statistically significant (p<0.001). HbA1c was also significantly higher (p<0.001) in diabetic subjects (7.5±0.5) than in the control (5.8±0.7, Table I).
Table I: Demographic and biochemical characteristic comparison between the diabetic and non-diabetic group.
|
Variable |
Diabetic mean + sd |
Non-diabetic mean + sd |
p-value |
|
Age (years) |
52.3±9.6 |
50.11±10.8 |
0.131 |
|
Weight (kg) |
77.3±11.5 |
72.5±15.3 |
0.013 |
|
Height (cm) |
164.5±7.0 |
161.7±7.7 |
0.01* |
|
BMI (kg/m2) |
28.6±4.2 |
27.7±3.8 |
0.119 |
|
FBS (mg/dl) |
140.2±13.7 |
91.9±9.2 |
<0.001* |
|
HbA1C % |
7.5±0.5 |
5.8±0.7 |
<0.001* |
|
Cholesterol (mg/dl) |
183.9±20.9 |
140.1±11.7 |
<0.001* |
|
LDL (mg/dl) |
141.06±7.7 |
114.1±7.3 |
<0.001* |
|
HDL (mg/dl) |
38.5±4.0 |
43.5±3.5 |
<0.001* |
|
Triglyceride (mg/dl) |
209.2±30.3 |
120.7±9.7 |
<0.001* |
|
Vitamin D (ng/ml) |
23.09±4.1 |
29.3±2.5 |
<0.001* |
|
*Highly significant. BMI: body mass index. Data expressed as the means ± SD. P-values were obtained by the Student's t-test. SD: Standard deviation. FBS: Fasting blood sugar, TG: Triglycerides, TC: Total cholesterol, HDL: High-density lipoprotein, LDL: Low-density lipoprotein. |
|||
Analysis of VDR gene of Fok1 polymorphism revealed the prevalence of F/F genotype in the diabetic group as 49 (49%) and in controls 45 (45%). The results also affirm that the frequency of genotype F/f as 45 (45%) and 41 (41%) in diabetes and controls respectively. Levels of f/f genotype were 6 (6%) in diabetics and 14 (14%) in control subjects as shown in (Table II). There was less ff genotype in diabetic group compared to the healthy controls (odds ratio = 0.473, 95% CI: 0.267–0.839). Interestingly control subjects had higher frequency of f allele (51% vs. 33%) suggesting that f allele may have a protective role (p= 0.01), whereas, the F allele maybe a predisposing factor of T2DM in the Pakistani population (Figure 1). There was a significant association observed regarding Fok1 alleles in their possible interactions in the diabetic group when compared with healthy controls (Table II).
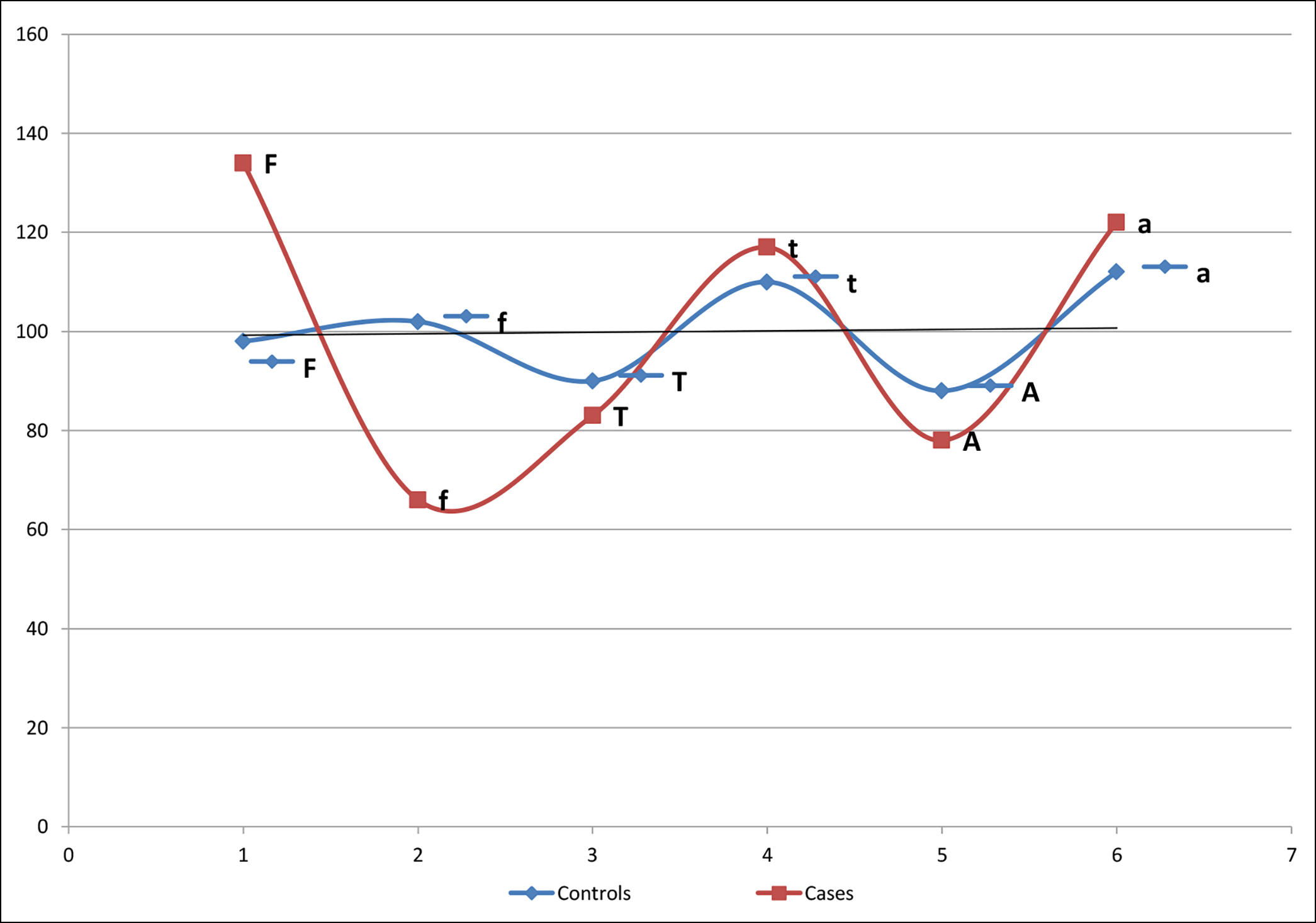 Figure 1: Allele frequency of Fok1, Taq1 and Apa1polymorphism in T2DM.
Figure 1: Allele frequency of Fok1, Taq1 and Apa1polymorphism in T2DM.
Table II: Frequency of polymorphism within exon 2 and 9 of VDR gene in diabetic patients and controls.
|
Genotype |
Controls |
Diabetic |
p-value |
|
Fok1 (Exon 2) |
|||
|
F/F |
45 (45%) |
49 (49%) |
0.169 |
|
F/f |
41 (41%) |
45 (45%) |
|
|
f/f |
14 (14%) |
6 (6%) |
|
|
Taq1 (Exon 9) |
|||
|
T/T |
28 (28%) |
31 (31%) |
0.748 |
|
T/t |
36 (36%) |
31 (31%) |
|
|
t/t |
36 (36%) |
38 (38 %) |
|
|
Apa1 (Intron 8) |
|||
|
A/A |
27 (27%) |
31 (31%) |
0.780 |
|
A/a |
38 (38%) |
34 (34%) |
|
|
a/a |
35 (35%) |
35 (35%) |
|
|
Alleles |
Controls |
Diabetic patients |
p-value |
|
FF allele |
|
|
|
|
Yes |
49 |
67 |
0.010* |
|
No |
51 |
33 |
|
|
ff allele |
|
|
|
|
Yes |
51 |
33 |
0.010* |
|
No |
49 |
67 |
|
|
*Highly significant. Chi-square test used. |
|||
However, regarding the frequency of T/T, T/t and t/t genotypes no remarkable difference was seen between the cases and controls. Similarly, differences in alleles between both groups related to the frequency of T and t were also not consequential. Furthermore, Apa1 polymorphism (frequency of, A/a, A/A and a/a genotype and alleles A and a) did not show any differences between healthy controls and cases.
DISCUSSION
The study showed thatT2DM was significantly associated with Fok1 Polymorphisms compared to other genotypes. Majority of studies around the world on association of VDR gene polymorphisms with diabetes mellitus have found a significant association with Fok1 Polymorphisms. A study conducted in Santiago de Chile on type 2 diabetic community also concluded VDR Fok1 gene polymorphism as a possible risk factor for type 2 diabetes mellitus especially in elderly population.7 Northeastern Indian population also found a close association of type II diabetes mellitus with VDR polymorphisms of Fok1 as well as Bsm1, and Taq1.8
The present study observed an association of F/F genotype of VDR gene with diabetes depicting F allele as a highly significant (p <0.01) risk predisposing factor of T2DM in the Pakistani population. A study conducted in Saudi Arabia showed a statistically significant frequency of Ff heterozygous genotype in patients compared to controls (33% vs. 21%, OR = 1.9, 95% CI = 1.006-3.587, p = 0.04).9 Among VDR SNPs, Fok1 has been revealed as functional polymorphism through extensive studies. This restriction site, Fok1 is located near the promoter region within the 5′ end, resulting in different translation initiation sites on VDR.10
The f allele was significantly lower (p<0.01) in patients with T2DM, compared to healthy subjects which is similar with the study conducted on Moroccan population.11 The present study observed that f allele of Fok1 can be considered as a protective shield against vitamin D deficiency. Previously a study also showed that this allele could affect the circulating levels of vitamin D and induce cardiovascular risk.12
A meta-analysis investigated the association of Fok1 VDR gene polymorphism with T2DM and reported that the Fok1 polymorphism of the VDR gene was related considerably with T2DM risk in the Chinese population, but not in Caucasians.13 Another meta-analysis inspected the affiliation among four well-characterised VDR polymorphisms with T2DM, and concluded that allele f and variant homozygote of ff of Fok1 were essentially related with T2DM and are the risk factors for T2DM. Taq1, Bsm1 or Apa1 SNPs are not associated with Diabetes Melitus in the Asian population.14 These inferences are in accordance with this study; which proves that Fok1 SNPs are risk factors for T2DM in other populations as well. Other VDR gene SNPs; Taq1, ApaI or BsmI were also not found associated with diabetic microvascular complications, however, Fok1 in the Caucasian population was found associated with diabetic neuropathy.13
The other SNPs, Taq1, Bsm1, and Apa1 polymorphisms, located at the 3′ untranslated region (3′ UTR) of the VDR gene, are alleged to alter VDR expression. These VDR genotypes have been reported to potentially affect the individual’s response to treatment. This was demonstrated in Bsm1 CC genotype women, who compared to TT genotype women showed greater improvement in bone mineral density after treatment.22 In agreement with our outcomes a recent meta-analysis did not discover any affiliation of the other three genotypes (Bsm 1, Apa1 and specially Taq1) with expanded T2DM risk in overall and subgroup analysis.13
Regarding Taq1 and Apa1 genotypes, our study did not show any statistically significant differences among cases and controls. The frequency of T and t alleles of Taq1 and ‘A’ and ‘a’ allele of Apa1were not significantly different in cases and controls. A study in the Pakistani population from Lahore also confirmed the same as our results regarding the association of Apa1 polymorphism with T2DM.15 The same type of study was conducted in Saudi Arabia in 2017 by A. P Iyer et al.16 and their results showed a higher frequency of homozygous recessive genotype aa in the diabetic population but no association was found for Taq1 polymorphism.
Vitamin D is reported to be engaged in several biological processes. Deficiency in vitamin D levels due to polymorphism or any other reason can disrupt the vitamin D related endocrine system and can lead to several common diseases which may progress with time, such as cardiovascular disorders, diabetes mellitus, cancer, tuberculosis and osteoarthrosis.12 In this study the deficiency of vitamin D was significantly higher in type II diabetes mellitus patients (26.87 ng/ml) compared to controls (29. 28 ng/ml). Similar to our study, Dipti Sarma8 also demonstrated that patients with type 2 diabetes had significantly higher levels of deficiency of Vitamin D compared to controls.
The prevalence of vitamin D deficiency was the same in our cohort as in a study conducted in Caribbean subjects with T2DM who also found it to be 42.6%.12 Low vitamin D levels in the Kashmiri diabetic population observed an inverse relationship between glycosylated hemoglobin levels and vitamin D levels.17 Lipid profile parameters (TG, TC and LDL-C) were significantly high with lower HDL-C in diabetic patients when compared with the control population.17 Results of our study corroborates with study of Mackawy,18 who recommended that VDR gene polymorphism is affiliated with low vitamin D level and may also be associated with lipid profile parameters and lower HDL-C in diabetics. The mechanism that how vitamin D could alter the lipid profile can be hypothesised through induction of suppression of PTH by vitamin D which in turn reduces the lipolysis19 or increase in intestinal absorption of calcium by vitamin D may lead to a decrease in serum triglyceride levels by reducing hepatic triglyceride formation and secretion.20 Vitamin D also plays an important role in improving the secretion of insulin as well as in insulin sensitivity, hence in this manner indirectly influences lipid metabolism.21
Large number of studies explored the possibility of VDR polymorphism involvement in the pathogenesis of type 2 diabetes.22 Several genes involved in metabolic process of T2DM were considered candidate for the pathogenesis of the disease.23 Among them VDR gene can be considered the excellent candidate for susceptibility to disease. On the other hand, in the case of Apa I and Taq I polymorphism, significant differences were not found between cases and controls with respect to any of the genotypes.
CONCLUSION
A significant relationship between Fok1 gene polymorphism of VDR and susceptibility of T2DM was found in the studied individuals. The role of f allele was more protective in contrast to F allele. No important relationship was found between Taq1 and Apa1 polymorphism and T2DM. A larger study with more parameters is needed to assess the independent role of each factor and its relationship with the disease.
ETHICAL APPROVAL:
The study was approved by Ziauddin Ethics Review Committee (ERC) of Ziauddin University (reference code: 0070617AMPHY).
PATIENTS’ CONSENT:
Written informed consent was obtained from the patients prior to sample collection.
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
AM: Conceptualised and designed the study/article, did sample collection, lab work, and acquisition of data. Data entry, analysis and interpretation of data. Drafted the primary draft of the manuscript.
SB: Revised the data and design of the study. Also revised and critically analysed the draft of the whole article and added intellectual content into it. Also checked the grammar and paraphrasing of the manuscript. Help in lab work and sample collection also.
PQRS: Helped in the conception of the study, drafting, and editing of the article.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Ortlepp JR, Metrikat J, Albrecht M, von Korff A, Hanrath P, Hoffmann R. The vitamin D receptor gene variant and physical activity predicts fasting glucose levels in healthy young men. Diabet Med 2003; 20(6):451-4. doi: 10.1046/j. 1464-5491.2003.00971.x.
- Ogunkolade BW, Boucher BJ, Prahl JM, Bustin SA, Burrin JM, Noonan K, et al. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes 2002; 51(7):2294-300. doi: 10.2337/ diabetes.51.7.2294.
- Zella JB, DeLuca HF. Vitamin D and autoimmune diabetes. J Cellular Biochemistry 2003; 88(2):216-22. doi: 10.1002/ jcb.10347.
- Oh JY, Barrett-Connor E. Association between vitamin D receptor polymorphism and type 2 diabetes or metabolic syndrome in community-dwelling older adults: The rancho bernardo study. Metabolism 2002; 51(3):356-9. doi: 10.1053/ meta.2002.29969.
- Junaid K, Rehman A, Jolliffe DA, Wood K, Martineau AR. High prevalence of vitamin D deficiency among women of child-bearing age in Lahore Pakistan, associating with lack of sun exposure and illiteracy. BMC Women's Health 2015; 15(1):1-8. doi: 10.1186/s12905-015-0242-x.
- Adnan M, Aasim M. Prevalence of type 2 diabetes mellitus in adult population of Pakistan: A meta-analysis of prospective cross-sectional surveys. Ann Glob Health 2020; 86(1):7. doi: 10.5334/aogh.2679.
- Angel B, Lera L, Marquez C, Albala C. The association of VDR polymorphisms and type 2 diabetes in older people living in community in santiago de chile. Nutr Diabetes 2018; 8(1):31. doi: 10.1038/s41387-018-0038-9.
- Sarma D, Chauhan VS, Saikia KK, Sarma P, Nath S. Prevalence pattern of key polymorphisms in the vitamin D receptor gene among patients of type 2 diabetes mellitus in northeast India. Indian J Endocrinol Metab 2018; 22(2):229-35. doi: 10.4103/ijem.IJEM_213_17.
- Ali R, Fawzy I, Mohsen I, Settin A. Evaluation of vitamin D receptor gene polymorphisms (Fok‐I and Bsm‐I) in T1DM Saudi children. J Clin Lab Anal 2018; 32(5):1-6. doi: 10. 1002/jcla.22397.
- Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene 2004; 338(2):143-56. doi: 10. 1016/j.gene.2004.05.014.
- Errouagui A, Benrahma H, Charoute H, Ghalim N, Barakat A, Kandil M, et al. Relationship between vitamin d receptor (VDR) gene polymorphisms and susceptibility to type 2 diabetes mellitus in Moroccans population. Int J Innov Applied Stud 2014; 8(2):503-14.
- Velayoudom-Cephise FL, Larifla L, Donnet JP, Maimaitiming S, Deloumeaux J, Blanchet A, et al. Vitamin D deficiency, vitamin D receptor gene polymorphisms and cardiovascular risk factors in Caribbean patients with type 2 diabetes. Diabetes Metab 2011; 37(6):540-5. doi: 10.1016/j.diabet. 2011.05.005.
- Li L, Wu B, Liu JY, Yang LB. Vitamin D receptor gene polymorphisms and type 2 diabetes: A meta-analysis. Arch Med Res 2013; 44(3):235-41. doi: 10.1016/j.arcmed. 2013.02.002.
- Palomba S, Orio F, Russo T, Falbo A, Tolino A, Manguso F, et al. BsmI vitamin D receptor genotypes influence the efficacy of antiresorptive treatments in postmenopausal osteoporotic women. A 1-year multicenter, randomised and controlled trial. Osteoporos Int 2005; 16(8):943-52. doi: 10.1007/s00198-004-1800-5.
- Sikander M. Relationship of ApaI VDR gene polymorphism to type 2 diabetes mellitus in Pakistani population. Electronic J Biol 2017; 13(4):338-42.
- Iyer AP, New SL, Khoja S, Al-Ghamdi MA, Bahlas S. Association of Apa I polymorphism of vitamin d receptor gene with type 2 diabetes mellitus in Saudi population. J Experimental Biol 2017; 5(2):271-76.
- Malik RA, Promela M, Bhat AA, Sheikh Ishaq, Rabia Farooq, Shah PA, et al. Vitamin D receptor (VDR) gene polymorphism and type 2 diabetes mellitus (T2DM)-A case control study in ethnic population of kashmir valley. Advan Res Gastroenterol Hepatol 2017; 4(5):01-6.
- Mackawy AM, Badawi ME. Association of vitamin D and vitamin D receptor gene polymorphisms with chronic inflammation, insulin resistance and metabolic syndrome components in type 2 diabetic Egyptian patients. Meta Gene 2014; 2:540-6. doi: 10.1016/j.mgene.2014.07.002.
- Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. FASEB J 2000; 14(9): 1132-8.
- Cho HJ, Kang HC, Choi SA, Ju YC, Lee HS, Park HJ. The possible role of Ca2+ on the activation of microsomal triglyceride transfer protein in rat hepatocytes. Biol Pharm Bull 2005; 28(8):1418-23. doi: 10.1248/bpb.28.1418.
- Prokopenko I, McCarthy MI, Lindgren CM. Type 2 diabetes: New genes, new understanding. Trends Genet 2008; 24(12):613-21. doi: 10.1016/j.tig.2008.09.004.
- Hossein-nezhad A, Holick MF. Vitamin D for health: A global perspective. Mayo Clin Proc 2013; 88(7):720-55. doi: 10.1016/j.mayocp.2013.05.011.
- Barroso I. Genetics of type 2 diabetes. Diabet Med 2005; 22(5):517-35. doi: 10.1111/j.1464-5491.2005.01550.x.