Efficacy of Ganciclovir in Cytomegalovirus Retinitis via Intravitreal Route in AIDS Patients
By Uzma Naz1, Saliha Naz2, Hadees Murad Fahad3, Syed Fawad Rizvi2Affiliations
doi: 10.29271/jcpsp.2022.12.1597ABSTRACT
A series of nine eyes of five patients at LRBT Tertiary Teaching Eye Hospital, from July 2020 to June 2021 is reported. Five patients with high clinical suspicion of CMV Retinitis were treated with weekly intravitreal injections of 2.0 mg ganciclovir for 3 weeks. Pre- and post-injection visual acuity and clinical findings were noted. After 3 injections, clinical signs of CMV retinitis started to resolve in all eyes (100%). Visual acuity improved in seven eyes (77.7%) and remained stable in two eyes (22.2%). Two of the seven eyes which initially showed improvement, later on developed retinal detachment. Patients were followed up for a minimum duration of 1 year. Intravitreal ganciclovir is effective for CMV retinitis but follow- up for a longer duration is needed as these patients are prone to develop peripheral retinal tractions and detachments.
Key Words: Ganciclovir, CMV retinitis, AIDS.
INTRODUCTION
The commonest ocular opportunistic infection found in AIDS patients is Cytomegalovirus (CMV) retinitis, accounting for 30-40% of cases.1,2 The risk of CMV retinitis is higher when the CD4 count falls below 50 cells per cubic millimetre. It is frequently bilateral and progressive and if not treated timely leads to blindness. In developed countries, the frequency of CMV retinitis has decreased due to the wide use of highly active antiretroviral therapy (HAART).3 In Asian countries, data on preponderance and impact of CMV retinitis is limited.
Generally, treatment for CMV retinitis focuses on systemic antiviral medications and intravitreal antiviral injections. The intravitreal route provides a higher concentration of antiviral drugs to control intraocular infection without increasing systemic toxicity and side effects.4 Commonly used antiviral agents are ganciclovir, valganciclovir, foscarnet, cidofovir and fomivirsen.
With limited access to foscarnet, cidofovir and fomivirsen , intravitreal ganciclovir was used as a financially acceptable therapeutic alternative.
This study was done to evaluate the efficacy of ganciclovir via intravitreal route in the Pakistani population for CMV retinitis in AIDS patients.
METHODOLOGY
This study was conducted at LRBT Tertiary Teaching Eye Hospital Karachi, Pakistan, from July 2020 to June 2021. Five patients with high clinical suspicion of CMV retinitis presented in the Uveitis clinic were included in this study. All five patients presented with decreased vision in both eyes associated with floaters. Two of the five patients were already diagnosed cases of HIV with CD-4 count less than 50/mm3 and receiving HAART treatment. All patients underwent measurement of best corrected visual acuity with Snellen’s chart, slit lamp biomicroscopy of anterior and posterior segments with 90D lens. Intraocular pressure was measured with Goldmann’s applanation tonometer. Heidelberg Spectralis (Heidelberg Engineering, Inc, Heidelberg, Germany) was used for Colored Fundus Photos, Fundus Fluorescein Angiography, and Optical Coherence Tomography. All the patients were sent to the Infectious Diseases Department for high suspicion of CMV retinitis. Three patients also tested positive for HIV with less than 50/mm3 CD-4 count and their HAART regimen was commenced. HAART regimen included minimum three drugs: one protease inhibitor, non-nucleoside reverse transcriptase inhibitor, or integrase inhibitor and two nucleotide or nucleoside reverse transcriptase inhibitors along with oral valganciclovir.
Nine affected eyes (one of the five patients was only eyed) were treated with weekly Intravitreal injections of 2.0 mg/0.1ml ganciclovir for 3 weeks after approval from the ethical review board.
Patients were called for follow-up every week for one month, then monthly for the next 3 months and then quarterly for one year. In every follow-up, visual acuity was measured and fundoscopy was done to monitor treatment response and development of complications.
Statistical analysis was done using SPSS version 23 (IBM, Armonk, NY, USA). Patient demographics were expressed as counts and percentages and analysed. Visual acuity pre-and post-injection were also analysed and expressed into categories as counts. A p-value <0.05 was considered statistically significant. Intraocular pressure, pre-and post-injection were also tabulated.
RESULTS
Nine eyes of five patients were treated with injection ganciclovir via intavitreal route. All patients were of male gender and the mean age was 30 (22–40) years. All patients were sexually active heterosexuals. All patients had bilateral disease (100%), and symmetrical disease was present in 3 of 5 patients (60.0%). Two of five patients (40%) had asymmetrical involvement (RE>LE). The posterior segment showed mild to severe retinitis, haemorrhages, and retinal necrosis with the normal or swollen optic disc. One patient already had a tractional retinal detachment in one eye with no perception of light. Each patient received Intravitreal ganciclovir 2.0 mg/0.1ml weekly for three weeks and followed for a duration of one year.
On commencement of therapy, all eyes (100%) showed improvement in clinical signs after 3 weeks, evident by gradual resolution of haemorrhages and exudation with retinal atrophy (Figure 1a and 1b).
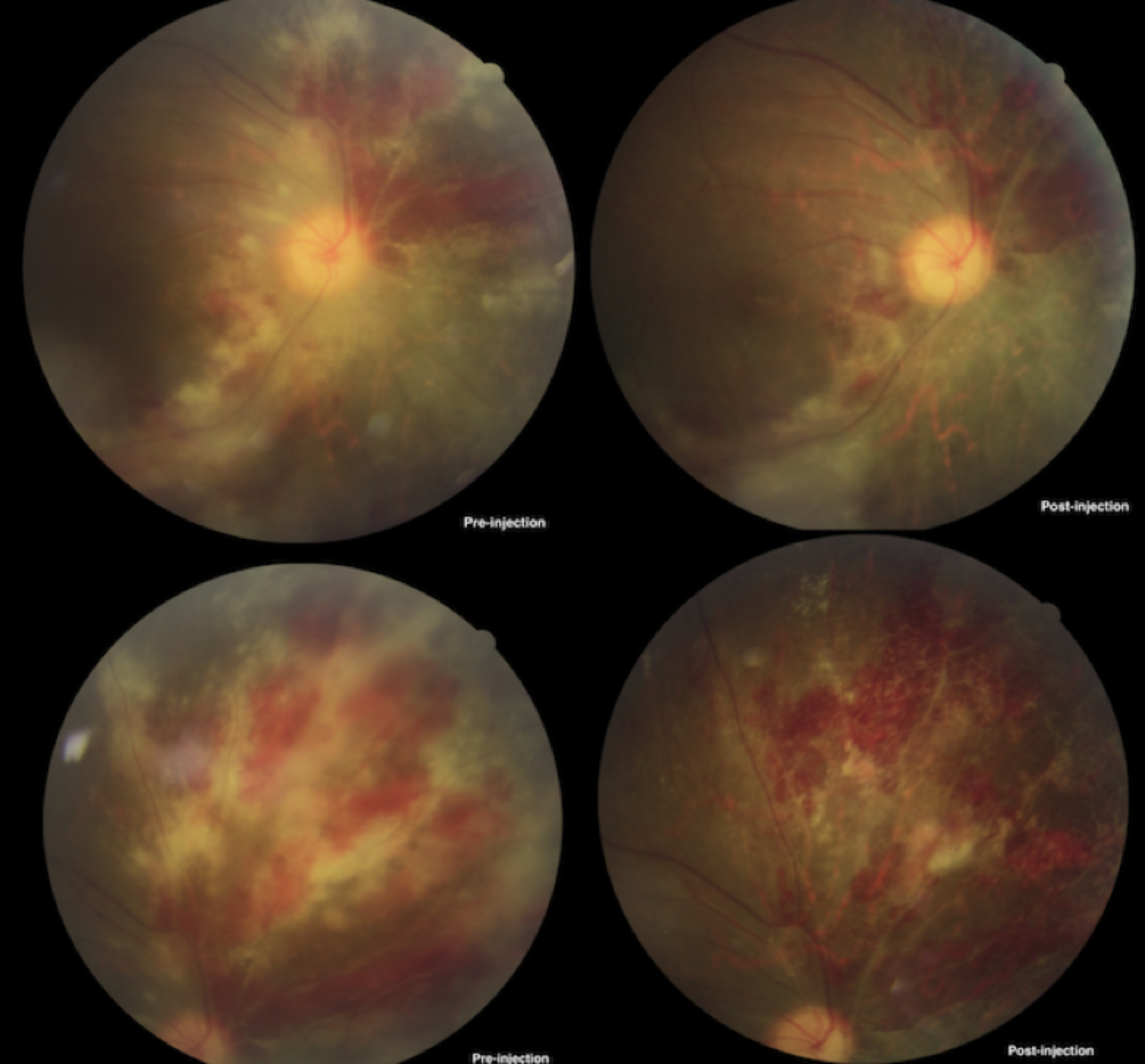 Figure 1a: Fundus photograph of patient 1, pre- and post-injection.
Figure 1a: Fundus photograph of patient 1, pre- and post-injection.
Optic disc atrophy was observed in patients with prior disc involvement. None of the eyes showed lack of response to the treatment. Visual acuity was improved in seven out of nine eyes (77.77%) and remained stable in two eyes (22.22%). Worsening of visual acuity was not appreciated in any eye. None of the eyes showed recurrence of CMV Retinitis up to one year of follow-up.
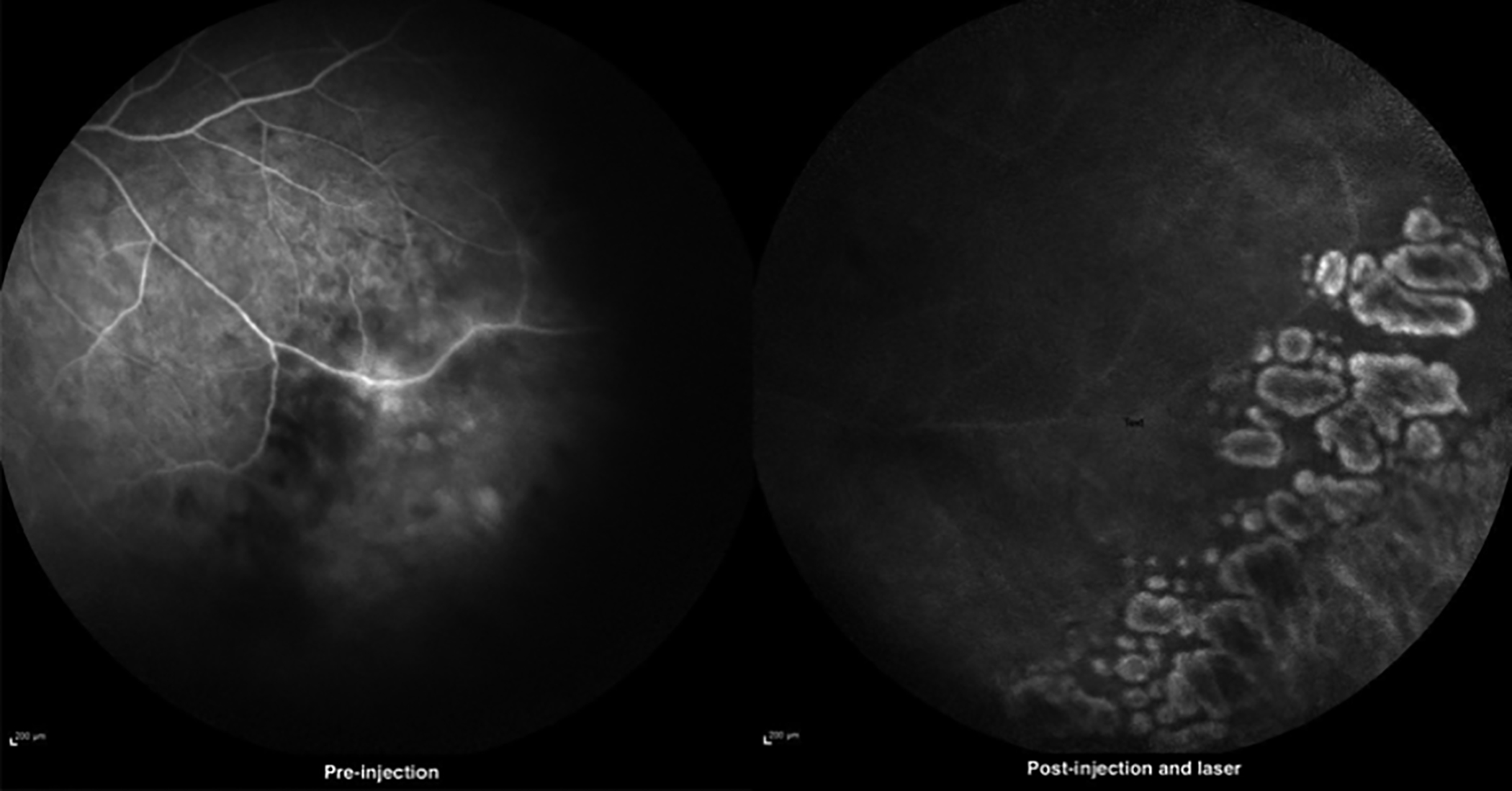 Figure 1b: Fundus fluorescein angiography of patient 2, pre- and post-injection.
Figure 1b: Fundus fluorescein angiography of patient 2, pre- and post-injection.
During the follow-up, Argon Laser was done to cordon off necrotic areas in all eyes to prevent the development of combined tractional and rhegmatogenous retinal detachment. Despite this, one of the seven eyes which initially showed visual improvement later on developed combined tractional and rhegmatogenous retinal detachment after 8 months of follow-up. One of the two eyes in which there was no visual improvement also developed combined TRD and RRD after a follow-up of two months.
DISCUSSION
In HIV patients, the most common viral opportunistic infection involving the eye is CMV retinitis which usually occurs when the CD4 count is below 50 cells per mm3.5 Around 30-40% of the patients with AIDS develop CMV retinitis.2 CMV infection is rarely encountered in immunocompetent patients. Nine cases with CMV retinitis in immunocompetent patients were reported by Gupta et al.6 Two immunocompetent patients with CMV retinitis were also reported by Radwan et al.7 In this study, all 5 patients were HIV positive and their CD-4 counts were less than 50/mm3.
Treatment of CMV retinitis consists of antiviral therapy and antiretro viral therapy (HAART). Antiviral agents include ganciclovir, cidofovir, foscarnet, fomivirsen, and valganciclovir. These agents can be administered by various routes including oral, intravenous and intravitreal.
Ganciclovir, cidofovir, and foscarnet are effective when given intravenously but due to systemic side effects, their use is limited. Valganciclovir via oral route is effective with less side effects. The higher targeted concentrations of drugs can be achieved by therapy via the intravitreal route without increasing systemic side effects and toxicity.8 Ganciclovir is a cheaper and equally efficacious alternative as compared to foscarnet, cidofovir, and fomivirsen.9
This regime for CMV retinitis via intravitreal route was effective, as all patients (100%) showed improvement in clinical signs of CMV retinitis with improvement in VA in seven out of nine eyes (77.7%) and stability in two eyes (22.2%). Worsening of visual acuity was not appreciated in any eye. Similar outcomes were noticed in other studies. A study conducted by Stephen et al. showed clinical resolution in 88% cases. Similarly, the study conducted by Montero et al. also showed 75% response rate with stability or improvement in vision after intravitreal injections.9
Different authors have reported variable rates of relapse of CMV retinitis ranging from 33% to 53%.10 In this study, no relapse was encountered over a follow-up of one year. This might be due to the reason that patients were on HAART and maintenance dose valganciclovir and their CD4 counts were stable throughout.
Common complications encountered after intravitreal injection are cataracts, high intraocular pressure, vitreous haemorrhage, retinal detachment, and endophthalmitis.11,12 In this study, these complications were not encountered except retinal detachment in two eyes after a follow-up of more than 3 months but this detachment was not due to the intravitreal injection rather as a consequence of retinal necrosis due to CMV retinitis resulting in holes and tears formation. The small sample size is the obvious limitation of this study.
CONCLUSION
Intravitreal ganciclovir is a safe, economical, and effective treatment modality for CMV retinitis. Since these patients are prone to develop retinal necrosis and subsequently retinal detachment, long-term follow-up is required.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
UN: Study design and literature search.
SN: Literature review and initial drafting.
HMF: Statistical analyses and data analyses.
SFR: Critical review of the manuscript for final approval.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Munro M, Yadavilli T, Fonteh C, Arfeen S, Lobo-Chan AM. Cytomegalovirus Retinitis in HIV and Non-HIV Individuals. Microorganisms 2020; 8(1):55. doi: 10.3390/micro organisms8010055.
- Perello R, Vergara A, Monclus E, Jimenez S, Montero M, Saubi N, et al. Cytomegalovirus infection in HIV-infected patients in the era of combination antiretroviral therapy. BMC Infectious Diseases 2019; 19(1):1030. doi: 10.1186/ s12879-019-4643-6.
- Ocieczek P, Barnacle RJ, Gumulira J, Phiri S, Heller T, Grabska-Liberek L. Cytomegalovirus retinitis screening and treatment in human immunodeficiency virus patients in malawi: A feasibility study. Open Forum Infect Dis 2019; 6(11):ofz439. doi: 10.1093/ofid/ofz439.
- Kim MH, Woo JS. Ocular Drug Delivery to the Retina: Current innovations and future perspectives. Pharmaceutics 2021; 13(1): 108. doi: 10.3390/pharma ceutics13010108.
- Karkhaneh R, Lashay A, Ahmadraji A. Cytomegalovirus retinitis in an immunocompetent patient. A case report. J of Current Ophthalmol 2016; 28(2):93-95. doi: 10.1016/j.joco. 2015.12.003.
- Gupta S, Vemulakonda AG, Suhler EB. Cytomegalovirus retinitis in the absence of AIDS. Can J of Ophthalmol 2013; 48(2):126-9. doi: 10.1016/j.jcjo.2012.12.002.
- Radwan A, Metzinger LJ, Hinkle MD, Stephen Foster C. Cytomegalovirus Retinitis in immunocompetent patients: Case reports and literature review. Ocular Immunol and Inflam 2013; 21(4): 324-8. doi: 10.3109/09273948.2013. 786095.
- Bradley S T, Regillo D. How to treat cytomegalovirus retinitis. Eye Net Magazine 2022.
- Teoh CS, Ou X, Lim HT. Intravitreal ganciclovir maintenance injection for cytomegalovirus retinitis: Efficacy of a low-volume, intermediate-dose regimen. Ophthalmol 2012; 119(3): 588-95. doi: 10.1016/j.ophtha.2011.09.004.
- Lu LP, Fan MC, Lau JK. Long-term follow-up of cytomegalovirus retinitis in non-HIV immunocompromised patients: Clinical features and visual prognosis. Am J of Ophthalmol 2016; 165:145-53. doi: 10.1016/j.ajo.2016. 03.015.
- Sampat Kapil M, Garg Sunir J. Complications of intravitreal injections. Current Opinion Ophthalmol 2010; 21(3): 178-83. doi: 10.1097/ICU.0b013e328338679a.
- Grzybowski A, Told R, Sacu S. 2018 Update on Intravitreal Injections: Euretina expert consensus recommendations. Ophthalmologica 2018; 239(4):181–19. doi: 10.1159/000 486145.