Effects of Modified Thoracoabdominal Nerve Block Through Perichondrial Approach on Postoperative Pain and Analgaesic Consumption in Patients Undergoing Laparoscopic Cholecystectomy
By Onur Avci, Oguz Gundogdu, Fatih Balci, Muhammed Nail TekcanAffiliations
doi: 10.29271/jcpsp.2024.01.05ABSTRACT
Objective: To investigate postoperative analgaesic efficacy of modified thoracoabdominal nerve block through perichondrial approach (M-TAPA) and its effect on opioid consumption in patients undergoing laparoscopic cholecystectomy (LC) surgery.
Study Design: Randomised, controlled trial.
Place and Duration of the Study: Department of Anaesthesiology and Reanimation, Sivas Cumhuriyet University, Sivas, Turkiye, from April to May 2023.
Methodology: The study was conducted in two randomised groups: M-TAPA (n = 21) and control group (CG) (no block) (n = 21). All patients had standard general anaesthesia. M-TAPA patients had bilateral M-TAPA block with 0.25% bupivacaine (total volume, 40 ml) at the end of the surgery. In contrast, CG patients had only tramadol for postoperative pain. A numerical rating scale (NRS) and visual analogue scale (VAS) were used for postoperative pain assessment. Total tramadol consumption was calculated.
Results: M-TAPA's NRS and VAS scores were lower in postoperative 24 hours (p<0.05). Total tramadol consumption was 116.67 ± 32.91 mg in CG and 35.71 ± 39.19 mg in M-TAPA (p<0.001).
Conclusion: Bilateral M-TAPA block for postoperative pain control after LC surgery provided effective analgaesia for up to 24 hours and reduced total opioid consumption. Although the M-TAPA block is a novel approach, it will be a part of multimodal analgaesia for routine postoperative pain management in abdominal surgeries. However, more studies with higher numbers of patients will be needed.
Key Words: Analgaesia, Bupivacaine, Laparoscopic cholecystectomy, Nerve block, Pain management.
INTRODUCTION
Laparoscopic cholecystectomy (LC) surgery is one of the most common general surgery operations in the western countries. In approximately 30 years since its description in the literature, LC has rapidly developed to become the most commonly performed general surgical procedure in the USA.1 LC procedure's invasion is minimal, providing various advantages such as less pain, a smaller scar, and an earlier return to preoperative activity.2 For this reason, it is seen as an ideal treatment method for gallbladder diseases.
Despite the minimal invasion of LC, postoperative pain lasting for the first 24-72 hours causes significant discomfort in some patients.3 Effective postoperative pain management after LC provides comfort to patients and help them to return to their daily routines faster.
In the literature, various regional anaesthesia techniques, infiltration anaesthesia, and intraperitoneal bupivacaine administrations had been tried to decrease systemic analgaesic consumption in pain control after LC.4-8 Tulgar et al. applied bupivacaine 0.25% (40 ml in total) to the costochondral cartilage angle's upper and lower surfaces to provide analgaesia in the abdomen with thoracoabdominal nerve block with perichondrial approach (TAPA) described in 2019.9 Later, Tulgar et al. described the modified thoracoabdominal nerve block through perichondrial approval (M-TAPA) by applying local anaesthetic (LA) only to the lower surface of the chondrium.10 The application of the M-TAPA block for analgaesia after LC was reported in a limited number of studies in the literature, and it drew attention as a block that had the potential to gain popularity in the future. It was previously reported that M-TAPA block provides a sensory block between T5-T12 dermatomes.10 This study was conducted on LC cases to maintain postoperative analgaesia because of this extended dermatomal coverage.
This randomised, controlled trial aimed to investigate the M-TAPA block's postoperative analgaesic efficacy and its effect on opioid consumption in patients undergoing LC surgery.
METHODOLOGY
This unicentric study is a double-blind, prospective, and rando-mised-controlled trial; it was conducted at Sivas Cumhuriyet University from April to May 2023 after obtaining the local ethics committee's approval, with decision number 2023- 04/03. The study included 42 adult patients (>18 years) who underwent elective LC under general anaesthesia. Only ASA I-II-III class patients according to the ASA (American Society of Anaesthesiologists) risk classification were included. The detailed information about the study and the M-TAPA block was given to participating patients verbally and in writing before the surgery, and their written informed consents were obtained.
Patients who did not give consent, patients with coagulopathy, patients with signs of infection at the block application site, patients using anticoagulants, patients with LA drug allergies, patients undergoing open surgery, patients with unstable haemodynamics, patients weighing <50 kg, and patients who could not cooperate during postoperative pain assessment were excluded from the study.
The patients were divided into two groups according to their ASA values, age, gender, body mass index (BMI), and surgical time: M-TAPA (n = 21) and CG (no block) (n = 21). The patients were randomised preoperatively using the Research Rando-mizer programme, so they did not know their group. The postoperative assessment was performed by a blinded second anaesthesiologist.
All patients underwent a standard general anaesthesia protocol after monitoring consisting of capnography, electrocardiography, non-invasive blood pressure, and peripheral oxygen saturation. The standard induction of general anaesthesia was performed using 1 µg/kg fentanyl, 5-7 mg/kg thiopental sodium, and 0.5 mg/kg rocuronium. Following endotracheal intubation, mechanical ventilator settings were set as tidal volume of 6-8 mL/kg, respiratory frequency at 12-16/minute; PEEP, 3-5 cmH2O; fresh gas flow, 2 L/min; gas setting of sevoflurane in a mixture of 50% air and 50% oxygen. An additional dose of fentanyl (1 µg/kg) was administered if heart rate and mean arterial pressure increased by at least 20% from the baseline during surgery. The same surgical team performed all patients' LC surgery, using the standard four-port technique where main port locations were intraumblical, infraxiphoidal, intersection of the right lateral side of the umbilicus with the anterior axillary line and an auxiliary port symmetrically, with insufflation at a pressure of 12 mmHg.
At the end of the procedure, M-TAPA patients underwent ultrasound-guided (USG) bilateral M-TAPA block by the same anaesthesiologist before waking up. CG patients were not subjected to any block or local infiltration anaesthesia. Patients in both groups received 1 g paracetamol intravenously (i.v.) and 50 mg dexketoprofen (i.v.) 10 minutes prior to the end of surgery.
Under sterile conditions for bilateral M-TAPA block application, the transducer was placed on the costal cartilage in the sagittal plane at the level of the 9-10th rib. Subsequently, a deep angle was created with the probe for visualisation of the underside of the costochondrial junction. The USG-visible needle tip was placed just below the chondrium, and saline (5 mL) was injected to confirm the location. Following confirmation, 20 mL of 0.25% bupivacaine was administered to each side, totalling 40 mL of local anaesthetic. Blocks were applied using an 80 mm USG-visible needle with a 6-10 MHz linear probe under the guidance of a portable USG (Figure 1). M-TAPA block with the same standard technique and drug dose was applied to the contralateral side for each of the M-TAPA patients. Patients were extubated with sugammadex at the end of the block application.
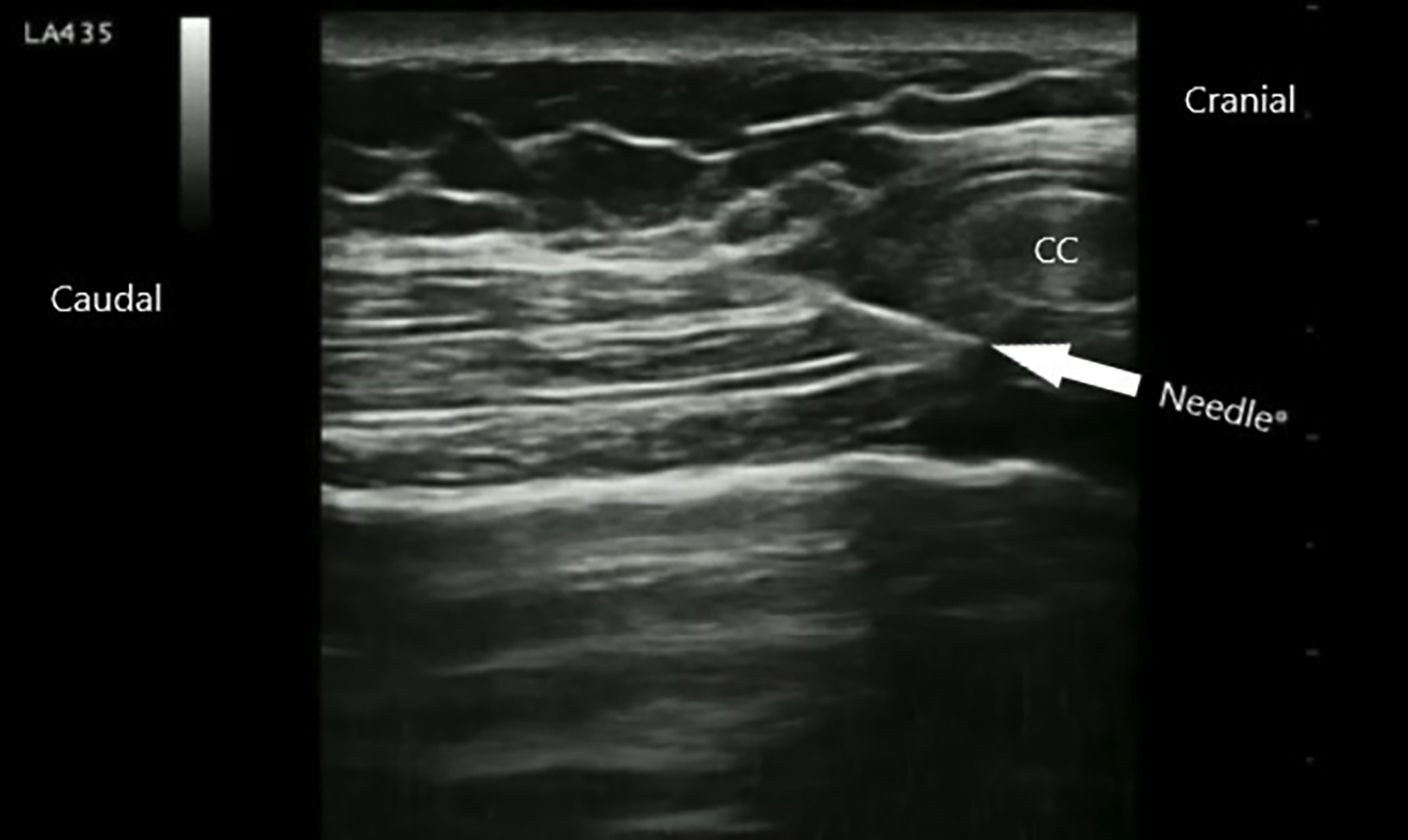 Figure 1: M-TAPA block technique.
Figure 1: M-TAPA block technique.
CC: Costal cartilage.
An NRS (score range: 0-10) and a VAS (score range: 0-5) were employed for postoperative pain assessment. A score of 0 on both scales meant no pain. In the postanaesthesia care unit (PACU) when the patient's modified Aldrete score was >9 (VAS0.min and NRS0.min), static (at rest) and dynamic (under mobilisation) NRS and VAS scores were recorded at 1, 6, 12, 18, and 24 hours, postoperatively. In patients with NRS score ≥4, 50 mg tramadol was given as an i.v. analgaesic to be administered in 10 minutes. Nausea, vomiting, pruritus, and total tramadol consumption were noted and recorded for 24 hours.
SPSS version 25.0 was used in statistical analyses. The Kolmogorov-Smirnov test and histogram plots were used to check the normality of the distribution. The results of the descriptive analysis were given as mean ± standard deviation (SD), median, and min-max values. The differentiation of categorical variables was tested using the Chi-square test, while an independent t-test was used to compare parametric variables between the two groups. The significance level was taken as p<0.05. The categorical variables were expressed as numbers and percentages.
The effect size was calculated to be d: 1.341 in the sample calculation performed with G Power 3.1.9.7 (Franz Faul, Germany) using the data from the study by Gungor et al.6 It was concluded that a minimum of 13 samples in each group and 26 samples in total would be required to reach the calculated effect size, at 95% power and 5% margin of error.
RESULTS
The study included 42 patients, 32 (76.1%) women and 10 (23.9%) men aged 19-78 years. The mean age was 50.07 ± 15.44 years, and BMI was 30.84 ± 5.74 kg/m2. The mean surgical time was 57.4 ± 9.93 minutes.
Table I: Demographic data, duration of surgery, postoperative adverse effects, and total tramadol consumption in the two groups.|
|
CG |
M-TAPA |
p-value |
||
|
n |
% |
n |
% |
||
|
Age (year) |
48.33±14.56 |
43 (30-77) |
51.81±16.44 |
53 (19-78) |
0.472 |
|
Gender |
|
|
|
|
|
|
Female |
16 |
(76.19) |
16 |
(76.19) |
>0.99 |
|
Male |
5 |
(23.81) |
5 |
(23.81) |
|
|
BMI (kg/m2) |
31.62±4.99 |
31 (24-44.7) |
30.06±6.43 |
30.8 (19-42) |
0.386 |
|
ASA |
|
|
|
|
|
|
I |
6 |
(28.57) |
2 |
(9.52) |
0.122 |
|
II |
12 |
(57.14) |
18 |
(85.71) |
|
|
III |
3 |
(14.29) |
1 |
(4.76) |
|
|
Duration of surgery (minutes) |
58.81±8.85 |
55 (45-80) |
56±10.94 |
55 (40-80) |
0.366 |
|
Nausea |
|
|
|
|
|
|
No |
15 |
(71.43) |
20 |
(95.24) |
0.038 |
|
Yes |
6 |
(28.57) |
1 |
(4.76) |
|
|
Vomiting |
|
|
|
|
|
|
No |
17 |
(80.95) |
20 |
(95.24) |
0.153 |
|
Yes |
4 |
(19.05) |
1 |
(4.76) |
|
|
Itching |
|
|
|
|
|
|
No |
21 |
(100.00) |
21 |
(100.00) |
--- |
|
Yes |
0 |
(.00) |
0 |
(.00) |
|
|
Total tramadol consumption (mg) |
116.67±32.91 |
100 (50-150) |
35.71±39.19 |
50 (0-100) |
<0.001 |
|
Independent t-test, Chi-square test; BMI: Body mass index; p<0.05: Statistically significant; ASA: American Society of Anaesthesiologists; mg: Milligram; kg: Kilogram; m2: Square meters; n: Number of patients; min-max: Minimum and maximum values. |
|||||
Table II: Comparing postoperative VAS and NRS values of the groups.
|
Hour |
CG |
M-TAPA |
p-value |
||
|
Mean±SD |
Median (min-max) |
Mean±SD |
Median (min-max) |
||
|
VAS static |
|||||
|
0 |
3.05±0.67 |
3 (2-4) |
1.48±0.51 |
1 (1-2) |
<0.001 |
|
1 |
2.9±0.7 |
3 (2-4) |
1.38±0.5 |
1 (1-2) |
<0.001 |
|
6 |
2.57±0.51 |
3 (2-3) |
1.05±0.38 |
1 (0-2) |
<0.001 |
|
12 |
1.48±0.6 |
1 (1-3) |
0.71±0.64 |
1 (0-2) |
<0.001 |
|
18 |
1.14±0.79 |
1 (0-3) |
0.38±0.59 |
0 (0-2) |
0.001 |
|
24 |
0.62±0.5 |
1 (0-1) |
0.14±0.36 |
0 (0-1) |
0.001 |
|
VAS dynamic |
|||||
|
1 |
3.9±0.7 |
4 (3-5) |
2.38±0.5 |
2 (2-3) |
<0.001 |
|
6 |
3.57±0.51 |
4 (3-4) |
2.05±0.38 |
2 (1-3) |
<0.001 |
|
12 |
2.48±0.6 |
2 (2-4) |
1.71±0.64 |
2 (1-3) |
<0.001 |
|
18 |
2.1±0.89 |
2 (0-4) |
1.38±0.59 |
1 (1-3) |
0.004 |
|
24 |
1.57±0.6 |
2 (0-2) |
1.14±0.36 |
1 (1-2) |
0.008 |
|
NRS static |
|||||
|
0 |
5.76±1.22 |
5 (4-8) |
3.29±1.45 |
2 (2-6) |
<0.001 |
|
1 |
5.57±1.21 |
6 (4-8) |
3.1±1.3 |
2 (2-5) |
<0.001 |
|
6 |
4.86±0.85 |
5 (3-6) |
2.24±0.7 |
2 (1-4) |
<0.001 |
|
12 |
3.19±0.75 |
3 (2-4) |
1.9±0.94 |
2 (1-4) |
<0.001 |
|
18 |
2.67±1.15 |
2 (1-5) |
1.43±0.75 |
1 (1-4) |
<0.001 |
|
24 |
2±0.63 |
2 (1-3) |
1.14±0.57 |
1 (0-2) |
<0.001 |
|
NRS dynamic |
|||||
|
1 |
6.57±1.21 |
7 (5-9) |
4.1±1.3 |
3 (3-6) |
<0.001 |
|
6 |
5.86±0.85 |
6 (4-7) |
3.24±0.7 |
3 (2-5) |
<0.001 |
|
12 |
4.19±0.75 |
4 (3-5) |
2.9±0.94 |
3 (2-5) |
<0.001 |
|
18 |
3.67±1.15 |
3 (2-6) |
2.43±0.75 |
2 (2-5) |
<0.001 |
|
24 |
3±0.63 |
3 (2-4) |
2.14±0.57 |
2 (1-3) |
<0.001 |
|
Independent t-test was used; VAS: Visual analogue scale; p <0.05: Statistically significant; NRS: Numerical rating scale; SD: Standard deviation; Min-max: Minimum and maximum values. |
|||||
Postoperative nausea was present in 7 patients, and vomiting in 5 patients. No patient had postoperative pruritus. The mean tramadol consumption of study patients was calculated as 76.19 ± 54.37 mg.
Age, sex, BMI, ASA, duration of surgery, postoperative nausea-vomiting and pruritus, and total tramadol consumption were compared between the groups. Accordingly, M-TAPA's total tramadol consumption was lower (p <0.001). Regarding the adverse effect profile, CG had significantly more patients with nausea (p = 0.038). The number of patients with nausea in the CG was 6, while the number of patients with nausea in the M-TAPA Group was 1. There were 4 patients with postoperative vomiting in the CG and 1 in the M-TAPA (p >0.05). When groups were compared in terms of total tramadol consumption, it was found that total tramadol consumption was 116.67 ± 32.91 mg in the CG; this amount decreased to 35.71 ± 39.19 mg in M-TAPA, and the difference was statistically significant (Table I).
VAS and NRS values of the groups taken at different times were compared. M-TAPA's static and dynamic VAS and NRS values were significantly lower (p <0.05). The dynamic assessment scores measured at different postoperative times were higher than the static assessment scores (Table II, Figure 2 and 3).
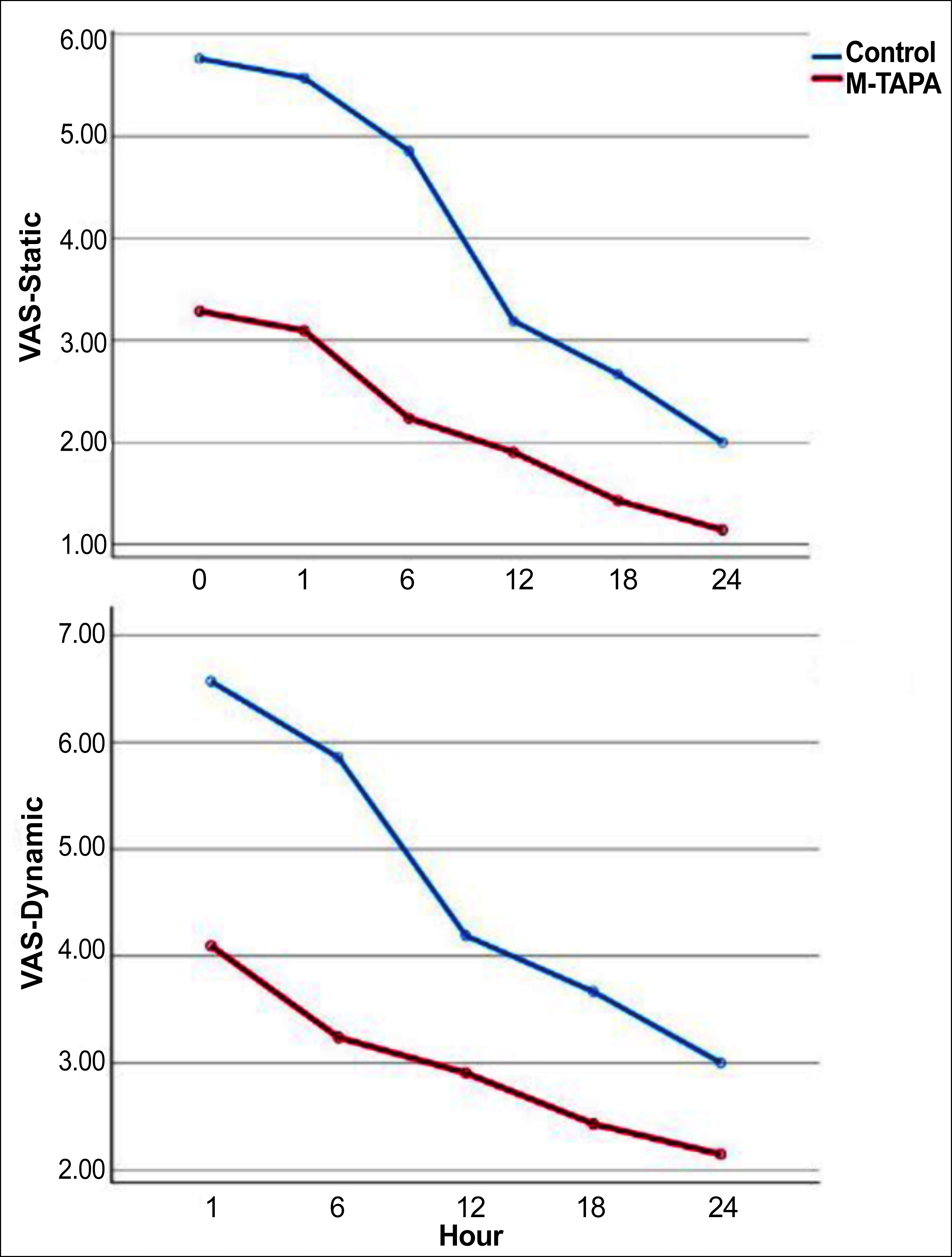 Figure 2: Visual analogue scale (VAS) scores of the groups.
Figure 2: Visual analogue scale (VAS) scores of the groups.
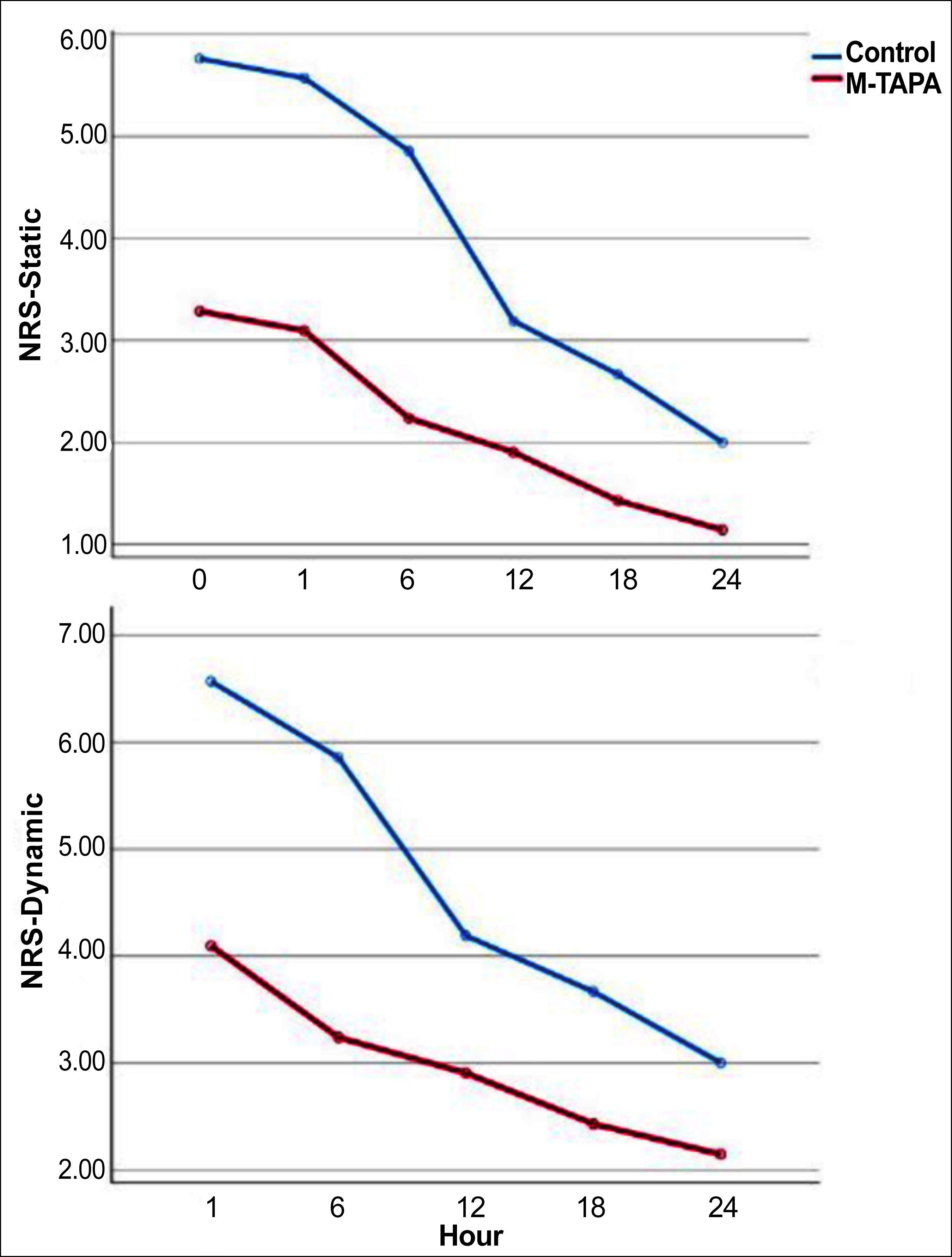 Figure 3: Numerical rating scale (NRS) scores of the groups.
Figure 3: Numerical rating scale (NRS) scores of the groups.
DISCUSSION
The current study demonstrated that bilateral M-TAPA application at the end of LC operation can provide effective analgaesia and reduce opioid consumption in postoperative pain management. The limited number of studies in the literature involving M-TAPA technique after LC made this study significant.
There are three mechanisms by which the pain originates after LC surgery: tissue trauma due to incision, phrenic nerve irritation due to pneumoperitoneum; and trauma due to gallbladder removal.11 An efficient postoperative pain management which covers these different causes is essential. However, opioids, which are among the group of effective analgaesic drugs, frequently have undesirable effects that may impair patient comfort. M-TAPA block attracted attention in recent years among the plane and nerve blocks applied to reduce these adverse effects of opioids and total opioid consumption.
Current regional anaesthesia techniques used after LC include subcostal transversus abdominis plane (subcostal TAP) block, oblique subcostal transversus abdominis plane block (OSTAP), and erector spine plane (ESP) block.6,7,12 Various studies showed that the M-TAPA block can provide adequate analgaesia in the T7-T11, T5-T10, and T3-T12 dermatome range.10,13,14 Therefore, the authors investigated the M-TAPA block's efficacy in providing abdominal wall analgaesia in LC operations.
As a result of this study, M-TAPA's NRS static and dynamic scores were significantly lower than CG at all times, from the 0th min to the 24th hour. In the literature, cases had been reported where the duration of the M-TAPA block's analgaesic effect may be prolonged up to 24 hours or even longer.10,13-15 The probable reason for this prolonged duration of analgaesic effect may be the low vascularity of the LA injected site in plane blocks and, consequently, decreased LA absorption.16 In a prospective, randomised controlled study, Bilge et al. investigated the M-TAPA block's analgaesic efficacy after LC surgeries in 68 patients. A bilateral M-TAPA block was performed using 25 ml of 0.25% bupivacaine (total LA dose, 50 ml) on each side in the M-TAPA group. M-TAPA's NRS scores were lower than CG's in the 24-hour follow-up.17 In the current study, a total LA dose of 40 ml was administered, and similar NRS results to those were obtained by Bilge et al. in terms of NRS scores obtained during 24-hour follow-ups. In the study of Erturk et al. comparing unilateral M-TAPA and TAPA block after LC surgery, which consisted of 56 patients and included 12 hours of postoperative follow-up, the TAPA group's static NRS scores were found to be significantly lower at 1st and 12th hours.18 In that study, the reason for the superiority of the TAPA block over the M-TAPA block in terms of NRS scores may be that 35 ml of 0.25% bupivacaine was used in the TAPA block. In comparison, 20 ml of 0.25% bupivacaine was used in the M-TAPA block. In a study of 60 patients by Gungor et al. comparing M-TAPA block and local anaesthetic infiltration (LI) after LC surgery, the M-TAPA group's dynamic NRS scores were lower during the first 16 hours.6 In the study of Bilge et al. comparing M-TAPA block and OSTAP block after LC surgery in 76 patients, dynamic NRS scores at the 12th hour were lower in the M-TAPA group.7 Both studies differed from this study because they did not include a CG, and LA was applied to all patients. The fact that NRS scores were lower for a longer time in patients who underwent M-TAPA block for the first 24 hours postoperatively than CG to whom no block was applied in the current study was not a surprising result compared to the studies of Gungor et al. and Bilge et al.6,7 Regarding the studies in the literature on M-TAPA block's analgaesic efficacy after LC, pain assessments were made only with the NRS scale. In contrast, in this study, NRS and VAS scales were used for pain assessment, and the score changes in both scales were found to be parallel.
In the study by Bilge et al., a postoperative analgaesia proto-col consisting of 1 g paracetamol for every eight hours and 50 mg tramadol as a rescue analgaesic was applied to all patients. M-TAPA's rescue tramadol consumption was 100 mg (0-200), and CG's was 200 mg (100-300).17 Unlike the study of Bilge et al., the routine postoperative analgaesia protocol was arranged to administer 50 mg tramadol (i.v.) when the patient's NRS score was ≥4. CG received no analgaesic other than tramadol in the postoperative period. In both studies, intraoperative non-opioid (i.v.) analgaesics were administered to all patients. In this study, M-TAPA's total tramadol consumption was statistically lower than CG's at the end of the 24-hour follow-up. While the tramadol consumption in the study by Bilge et al. reflected the rescue opioid dose, the total tramadol consumption in the current study was obtained by comparing the total tramadol consumption with that of a group of patients who had received tramadol alone for postoperative analgaesia. Another study by Bilge et al. comparing M-TAPA and OSTAP block and showing that M-TAPA block reduces tramadol consumption after LC surgery, determined 1 g paracetamol for every 6 hours and 50 mg dexketoprofen for every 12 hours as routine postoperative analgaesia protocol and 50 mg tramadol was administered as rescue analgaesic.7 Total rescue tramadol consumption was also calculated in Bilge et al.'s study and it was revealed that the M-TAPA group decreased tramadol consumption more than OSTAP at the end of 24 hours of monitoring.7
Any adverse effects such as nausea and vomiting may be expected in cases with high total opioid consumption; therefore, the current study also examined these adverse effects. Nausea was statistically less frequent in M-TAPA, where total tramadol consumption was lower. Gungor et al. did not calculate total tramadol consumption in their study in which nausea frequency was lower in the M-TAPA group than the LI group. This finding regarding nausea may be because of the lower tramadol consumption in the M-TAPA group.6
The current study had some limitations: 20 ml of local anaesthetic was administered per group in this study. NRS and VAS scores could have been obtained using higher volumes, increasing the sample size, and testing different tramadol consumptions. Only the VAS score, NRS score, and total tramadol consumptions were analysed in the current study to assess the M-TAPA block's postoperative analgaesic efficacy. On the other hand, patient satisfaction, sensory dermatome site, and postoperative recovery time were not analysed. If the study had administered the M-TAPA block before the incision instead of at the end of surgery and before awakening the patient, intraoperative opioid consumption could have been examined, and postoperative opioid consumption may have differed.
CONCLUSION
In conclusion, bilateral M-TAPA block in postoperative pain control after LC surgery provided effective analgaesia for up to 24 hours and reduced total opioid consumption. M-TAPA block, which is still a novel approach, is useful to be a part of multimodal analgaesia for routine postoperative pain management in abdominal surgeries.
ETHICAL APPROVAL:
The study was approved by the Institutional Review Board of Sivas Cumhuriyet University (No. 2023-04/03 dated: 11.04. 2023).
CLINICAL TRIAL REGISTRATION:
This research was registered to clinicaltrials.gov.tr with the clinical trial number NCT05891652.
PATIENTS' CONSENT:
The consent for publication was obtained from the patients whose data are included in this manuscript.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS' CONTRIBUTION:
OA: Conception and designing of the study, and wrote the manuscript.
OG: Selected patients, analysed the data, and critically reviewed for important intellectual content.
MNT, FB: Selected patients, did literature research, and wrote the manuscript.
All authors agreed on the final version of the manuscript for publication.
REFERENCES
- Centers for Disease Control and Prevention. National Center for Health Statistics (2016); Health data interactive. Available from: www.cdc.gov/nchs/hdi.htm [cited 8 May 2023].
- Kapoor T, Wrenn SM, Callas PW, Abu-Jaish W. Cost analysis and supply utilization of LC. Minim Invasive Surg 2018; 10(2018):7838103. doi: 10.1155/2018/7838103.
- Toleska M, Dimitrovski A. Is opioid-free general anesthesia more superior for postoperative pain versus opioid general anaesthesia in laparoscopic cholecystectomy? Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2019; 40(2): 81-7. doi: 10.2478/prilozi-2019-0018.
- Manan A, Khan AA, Ahmad I, Usman M, Jamil T, Sajid MA. Intraperitoneal bupivacaine as post-laparoscopic cholecystectomy analgesia. J Coll Physicians Surg Pak 2020; 30(1):9-12. doi: 10.29271/jcpsp.2020.01.09.
- Grape S, Kirkham KR, Akiki L, Albrecht E. Transversus abdominis plane block versus local anesthetic wound infiltration for optimal analgesia after laparoscopic cholecystectomy: A systematic review and meta-analysis with trial sequential analysis. J Clin Anesth 2021; 75:110450. doi: 10.1016/j.jclinane. 2021.110450.
- Gungor H, Ciftci B, Alver S, Golboyu BE, Ozdenkaya Y, Tulgar S. Modified thoracoabdominal nerve block through perichondrial approach (M-TAPA) vs. local infiltration for pain management after laparoscopic cholecystectomy surgery: A randomized study. J Anesth 2023; 37:254–260. doi: 10.1007/ s00540-022-03158-0.
- Bilge A, Basaran B, Altiparmak B, Et T, Korkusuz M, Yarımoglu R. Comparing ultrasound-guided modified thoracoabdominal nerves block through perichondrial approach with oblique subcostal transversus abdominis plane block for patients undergoing laparoscopic cholecystectomy: A randomized, controlled trial. BMC Anesthesiol 2023; 23:139. doi: 10.1186/ s12871-023-02106-z.
- De Cassai A, Sella N, Geraldini F, Tulgar S, Ahiskalioglu A, Dost B, et al. Single-shot regional anesthesia for laparoscopic cholecystectomies: A systematic review and network meta-analysis. Korean J Anesthesiol 2023; 76(1): 34-46. doi: 10. 4097/kja.22366.
- Tulgar S, Senturk O, Selvi O, Balaban O, Ahiskalioglu A, Thomas DT, et al. Perichondral approach for blockage of thoracoabdominal nerves: Anatomical basis and clinical experience in three cases. J Clin Anesth 2019; 54:8-10. doi: 10.1016/j.jclinane.2018.10.015.
- Tulgar S, Selvi O, Thomas DT, Deveci U, Ozer Z. Modified thoracoabdominal nerves block through perichondrial approach (M-TAPA) provides effective analgesia in abdominal surgery and is a choice for opioid sparing anesthesia. J Clin Anesth 2019; 55:109. doi: 10.1016/j.jclinane.2019.01.003.
- Donatsky AM, Bjerrum F, Gögenur I. Surgical techniques to minimize shoulder pain after LC. A systematic review. Surg Endosc 2013; 27:2275–82. doi: 10.1007/s00464-012-2759-5.
- Daghmouri MA, Akremi S, Chaouch MA, Mesbahi M, Amouri N, Jaoua H, et al. Bilateral erector spinae plane block for postoperative analgesia in laparoscopic cholecystectomy: A systematic review and meta-analysis of randomized controlled trials. Pain Pract 2021; 21(3):357-65. doi: 10. 1111/papr.12953.
- Aikawa K, Tanaka N, Morimoto Y. Modified thoracoabdominal nerves block through perichondrial approach (M-TAPA) provides a sufficient postoperative analgesia for laparoscopic sleeve gastrectomy. J Clin Anesth 2020; 59:44-5. doi: 10. 1016/j.jclinane.2019.06.020.
- Altiparmak B, Korkmaz Toker M, Uysal Aİ, Turan M, Gumus Demirbilek S. The successful usage of modified thoracoabdominal nerves block through perichondrial approach (M-TAPA) for analgesia of laparoscopic ventral hernia repair. J Clin Anesth 2019; 57:1-2. doi: 10.1016/j.jclinane.2019.02. 016.
- Matsuura H, Terada Y, Rokkaku Y, Tamagawa H, Taniguchi E, Saito Y, et al. Analgesic efficacy of modified thoracoabdominal nerves block through the perichondrial approach in laparoscopic cholecystectomy: A retrospective study with propensity analysis. Asian J Endosc Surg 2023; 1‐5. doi:10. 1111/ases.13183.
- Suseela I, Anandan K, Aravind A, Kaniyil S. Comparison of ultrasound-guided bilateral subcostal transversus abdominis plane block and port-site infiltration with bupivacaine in laparoscopic cholecystectomy. Indian J Anaesth 2018; 62(7): 497-501. doi: 10.4103/ija.IJA_55_18.
- Bilge A, Başaran B, Et T, Korkusuz M, Yarımoğlu R, Toprak H, et al. Ultrasound-guided bilateral modified-thoracoabdominal nerve block through a perichondrial approach (M-TAPA) in patients undergoing laparoscopic cholecystectomy: A randomized double-blind controlled trial. BMC Anesthesiol 2022; 22(1):329. doi: 10.1186/s12871-022-01866-4.
- Ertürk T, Ersoy A. Postoperative analgesic efficacy of the thoracoabdominal nerves block through perichondrial approach (TAPA) and modified-TAPA for laparoscopic cholecystectomy: A randomized controlled study. Signa Vitae 2022; 18(2):114-20. doi:10.22514/sv.2022.004.