Effectiveness of Using a Gelatine-Based Model in Ultrasonography-guided Jugular Central venous Catheter Placement Training: A Randomised Clinical Trial
By Zeki Tuncel Tekgul, Cagri Yesilnacar, Mehmet Ugur Bilgin, Huseyin OzkarakasAffiliations
doi: 10.29271/jcpsp.2022.05.596ABSTRACT
Objective: To evaluate the effectiveness of using a gelatine-based model, that can be prepared easily and at a low cost, compared to training without a model in ultrasonography (USG) guided internal jugular venous catheter placement training.
Study Design: An open-label, randomised clinical trial.
Place and Duration of Study: (UHS) Izmir Bozyaka Training and Research Hospital, Izmir, Turkey, from 1st to 30th July 2019.
Methodology: Analysis was conducted with the data of 48 participants (resident physicians-RP). Group 1 (n: 26) received imaging training with USG on a human subject and then performed needle insertion training on the gelatine-based jugular vein model with the USG guidance. Group 2 (n: 22) received the same imaging training with USG but did not perform needle insertion training. Evaluation of the participants included successful long axis vein imaging time, successful in-plane needle imaging, number of changes in needle angles, the total number of punctures, successful vein puncture time, successful catheterization time, and catheterization success with the gelatine-based test manikin.
Results: Comparison of the rates of successful in-plane needle imaging (Group 1: 92.3%, Group 2: 59.1%; p = 0.006), catheterization success (Group 1: 92.3%, Group 2: 59.1%; p = 0.006), successful catheterisation time (Group 1: 77.5 sec, Group 2: 152.5 sec; p = 0.026), and total complications (Group 1: 3.8%, Group 2: 31.8%; p = 0.010) demonstrated that the model-trained RPs were significantly more successful.
Conclusion: The study results suggest that the use of a gelatine-based model in USG-guided central jugular vein catheterisation training can be an effective method to reduce complications.
Key Words: Central venous catheter, Medical training, Simulation-based training, Ultrasonography.
INTRODUCTION
Central venous catheterisation (CVC) is a surgical procedure that requires educated health care providers. Most healthcare centres adopt standardised protocols to reduce the complications associated with the procedure. Nevertheless, although the need for it is obvious, such standardisation has not emerged for the training of resident physicians (RPs) for CVC placement.1,2
CVC training programs of RPs who work in related specialties should be thoroughly and carefully considered and the current tendencies in the field should be closely monitored. The USG guidance for CVC, particularly for the jugular vein, has recently been recognised as the gold standard and as a result, USG guidance in the CVC training -both theoretical and practical- is now more essential than ever.3-7 Training (e.g. of an RP inexperienced in USG guided needle manipulation) on an actual patient might increase complications and without a doubt, it is unethical.8,9
In particular, the ability to properly visualise the target tissue and the needle, and steer the needle to the target tissue with the guidance of the USG are skills that need hours of practice. Different moulds and animal, cadaver, or virtual reality models have been developed to help with USG-guided needle manipulation training.10-12 All these options have their trade-offs. For institutions with limited resources, such as ours, options are quite limited. Models with proven efficacy cannot be purchased due to high prices and those that can be built at a low cost are not preferred by some because of doubts about their effectiveness. Controversial opinions do exist: some studies state that the gelatine-based USG training models -such as used in this study- are unreliable because, compared to the actual tissue, they may facilitate the needle visualisation, and they have a lower resistance for needle manipulation. Other studies claim that these disadvantages can be eliminated with the right temperature and correct gelatine and psyllium dung concentrations in the mixture.13-16
This study was planned to evaluate the effectiveness of using a low-cost, easy to prepare, gelatine-based model, compared to training without a model in USG guided internal jugular vein catheter placement training by performing post-training assessment.
METHODOLOGY
The study was planned and conducted as prospective randomised and open-label trail. (Local Ethics Committee decision No. 12-06-2019/06; ClinicalTrial.gov register number: NCT03996 733). A total of 173 RPs in Izmir Bozyaka Training and Research Hospital were informed about the study. Of these, 112 physicians volunteered for the study and signed the voluntary consent form. 51 RPs were excluded due to prior training or experience with the procedure. As a result, the study proceeded with the randomisation of 61 RPs into two groups. 13 physicians failed to participate or complete the study for various reasons and the analysis of the data was completed with 48 participants (Figure 1).
Software-based stratified randomisation was used to ensure homogeneity between the groups in terms of experience (as years in residency) and specialty (a consideration of manual dexterity). For this purpose, the specialty (neurosurgery, general surgery, internal medicine, otorhinolaryngology, neurology, orthopaedics, urology, infectious diseases, and anaesthesiology) and the year of residency (first 2 years as Junior and above as Senior) were used as variables. The training was carried out on four different days within 2 weeks, consisting of two stages, training and assessment. In order to standardize the process, each stage was carried out by the same investigators in a fixed period. The investigator team consisted of two attending physicians, who teach USG guided CVC placement training for at least 5 years and had received trainer training, and two resident physicians who assisted in data recording and organisation.
Training commenced with 30 minutes of theoretical training for both groups. The CVC placement algorithm and checklist of the institution were explained and participants were informed that this training aims to improve eye-hand coordination in USG guided CVC placement and does not include all the necessary training for the entire procedure.
Group 1 received a total of 120 minutes of training: 30 minutes of theoretical training, 5 minutes of USG imaging on a human being, 5 minutes of performing needle puncture on the model with USG guidance, and 80 minutes of observing the rest of the group. Group 2, same as Group 1, received a total of 120 minutes of training: 30 minutes of theoretical training, 10 minutes of USG imaging on a human being (without any intervention), and 80 minutes of observing the rest of the group.
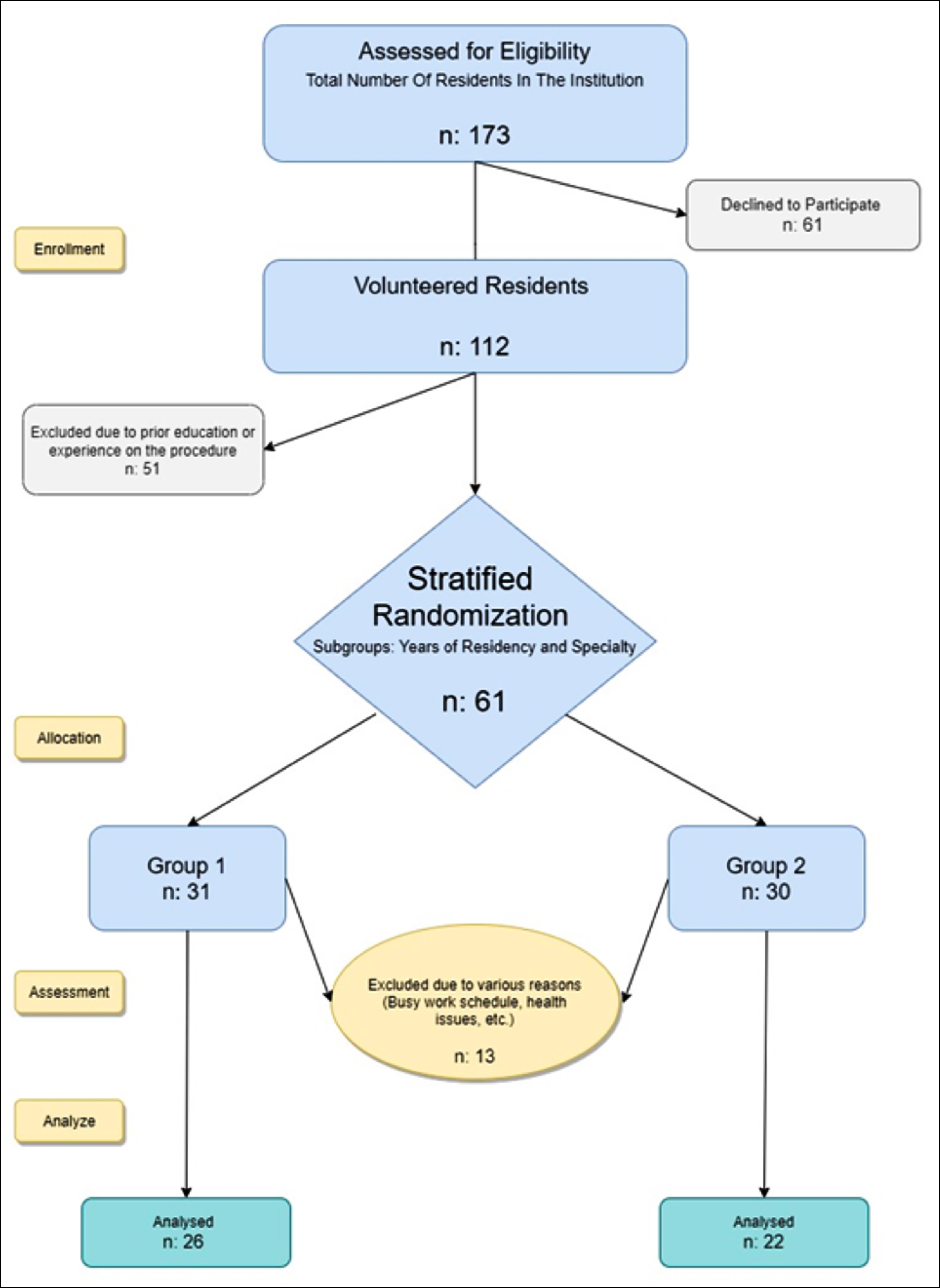 Figure 1: Flowchart (In compliance with the Consort 2010 Guideline).
Figure 1: Flowchart (In compliance with the Consort 2010 Guideline).
The training model was designed with the consideration of a 70 kg male patient with a height of 170 cm. 110 g of powdered bovine gelatine for viscosity, 7 g of psyllium husk for texture, and 1 g of powdered methylene blue for both opacity and preventing bacterial growth were mixed in 1 litre of tap water and then moulded in a plastic container. A 14-mm diameter silicon tube filled with a blue liquid (methylene blue solution) at a depth of 16 mm from the skin was placed to imitate the jugular vein. Another 7 mm diameter silicon tube filled with a red liquid (rifampicin solution) was placed a few millimetres medially at a depth of 21 mm from the skin to imitate the carotid artery (Figure 2 a,b).
The assessment model was built with the consideration of a 120-Kg male patient with a height of 160 cm to prevent any resemblances to the training model. Unlike the training model, a 12 mm diameter silicon tube at a depth of 32 mm from the skin for the jugular vein and a 7 mm diameter silicon tube at a depth of 37 mm for the carotid artery were placed with the same design characteristics. For a different and more challenging experience, ingredients and the design of the model were also altered. To increase the sense of reality and mimic the anatomy of the right jugular vein, we placed the assessment model in the neck of a manikin. Distilled water was used to reduce conductivity, bovine gelatine was increased to 130 g to increase viscosity and psyllium husk was increased to 10 g to increase echogenicity, thus reducing needle visibility. The front wall was covered with aluminium foil connected to a DC circuit, which activates a red light if the needle ever advances too far and completes the circuit by touching the wall (pleural simulation). Likewise, another DC circuit was placed on the bottom (at a depth of 6 cm) to activate a green light with the needle contact (bone contact simulation, Figure 2 c,d).
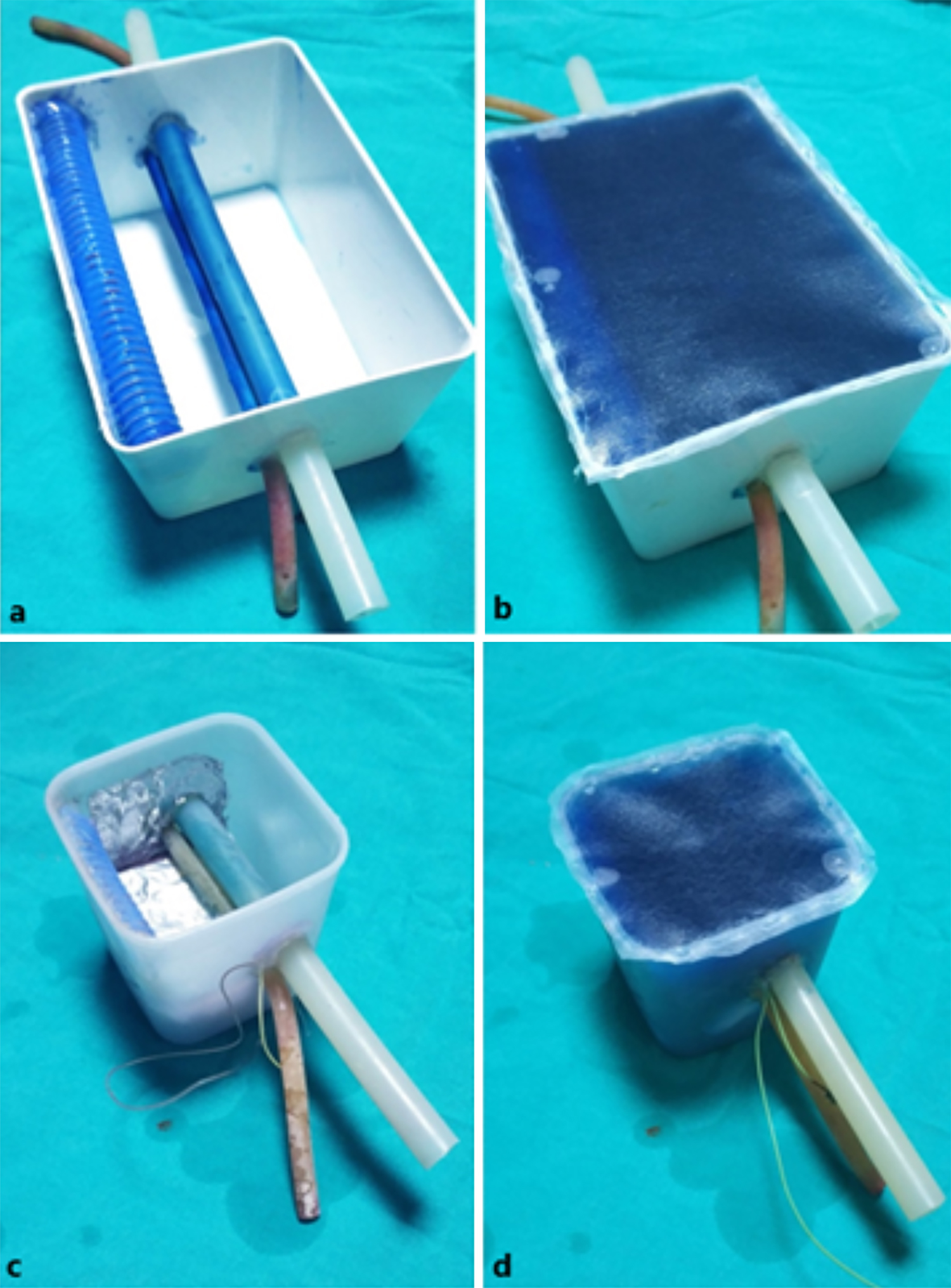 Figure 2: Training models (a,b) and assessment models (c,d).
Figure 2: Training models (a,b) and assessment models (c,d).
After the training and assessment models were prepared, the gelatine was kept in the refrigerator to set. Before each use, a small amount of USG gel was applied on the upper surface of the model and a plastic protective cover (thick-type transparent file cover) was fixed with hot silicone to prevent spillage and air entry. This layer acted as skin, preventing the needle entry point from sliding during the procedure. It also proved useful for the integrity of the gelatine when involuntary excessive pressures were applied (Figure 2).
When needle marks became visible or gelatine lose viscosity, models were reset with a hot water bath (Bain-Marie Method) and then cooled again at +4 °C so that the same model could be used again throughout the study.
The following data were measured and recorded during the evaluation: Successful long axis vein visualisation (seconds), defined as the time when the participants capture the jugular vein in the long axis by rotating the probe after capturing it in the short axis; Successful in-plane needle visualisation (yes/no), defined as full view of the needle axis under the probe just before puncturing the vein while maintaining the long axis view of the vein; The number of changes in the needle angle in one puncture (count); The total number of skin punctures (count); Successful vein puncture time (seconds), defined as the time of blood (blue liquid) aspiration from the jugular vein; Successful catheterisation time (seconds), defined as the time when the catheter guidewire was inserted into the jugular vein and the needle was removed from the skin; Complications (p: pleura contact, b: bone contact, n: no complication) and Catheterisation success (yes/no), defined as successful catheterisation within 5 minutes without any complication.
Statistical analyses were performed with Statistical Package for Social Sciences version 21 (SPSS Inc., Chicago, IL). Numerical data that fit normal distribution were expressed as mean ± SD, and numerical data that did not fit normal distribution were expressed as median (IQR, interquartile range). Categorical data were expressed as a percentage (%). The difference between mean or median values of the numerical data was analysed with a Student t-test or Mann-Whitney U-test. Significance between categorical data was assessed with a chi-squared test. P-values lower than 0.050 were interpreted as significant.
RESULTS
Table I presents the age, gender, specialty, and year of residency data of the groups and none show any significant difference as a result of successful stratified randomisation.
Analyses of assessment reports are presented in Table II. Successful vessel imaging time, the number of changes in the needle angle, the total number of punctures, successful vein puncture time, pleural contact, and bone contact data showed no significant difference. However, in-plane needle imaging success (Group 1: 92.3%, Group 2: 59.1%; p = 0.006), catheterization success (Group 1: 92.3%, Group 2: 59.1%; p = 0.006), catheterisation time (Group 1: 77.5 sec, Group 2: 152.5 sec; p = 0.026), and the number of total complications (Group 1: 3.8%, Group 2: 31.8%; p = 0.010) data revealed that the group trained with the model was significantly more successful.
DISCUSSION
Many different moulds and animal, cadaver, manikin, or virtual reality models have been developed to help with USG-guided needle manipulation training. The main factors determining the effectiveness of these models are resemblance to human tissue in USG, the similarity of echogenicity compared to the target tissue and surroundings, feeling during the needle advance, long-term use with a low maintenance cost, and ease of use. The cost of purchase or maintenance of commercial models with proven reliability makes them highly unreachable for many institutions/training hospitals such as ours. Supervised training on an actual patient is not uncommon for many clinics and this potentially results in an increase in the complication rate. Gelatine-based models have been in circulation for a long time, but the lack of data on their effectiveness limits their broad utilisation.
Table I: Demographic data n (%), median [IQR].
|
|
Group 1 (n:26) |
Group 2 (n:22) |
p |
|
Gender |
17 (65.4%) - 9 (34.6%) |
13 (59.1%) - 9 (40.9%) |
0.654 |
|
Age (years) |
27 [2] |
28 [2] |
0.292 |
|
Specialty Neurosurgery General surgery Internal Medicine ENT Neurology Orthopaedics Urology Infectious Diseases Anaesthesiology |
1 (3.8%) 4 (15.4%) 8 (30.8%) 2 (7.7%) 3 (11.5%) 3 (11.5%) 1 (3.8%) 3 (11.5%) 1 (3.8%) |
1 (4.5%) 3 (13.6%) 9 (40.9%) 3 (13.6%) 3 (13.6%) 1 (4.5%) 0 2 (9.1%) 0 |
0.915 |
|
Year In Residency Junior (1st and 2nd year) Senior (3rd year and above) |
19 (73.1%) 7 (26.9%) |
14 (63.6%) 8 (36.4%) |
0.482 |
Table II: Assessment Reports n (%), median [IQR].
|
|
Group 1 (n:26) |
Group 2 (n:22) |
p |
|
Successful long axis vein visualisation (seconds) |
30 [15] |
33.5 [27] |
0.406 |
|
Successful in-plane needle visualisation: Y / N |
24 (92.3%) /2 (7.7%) |
13 (59.1%)/9 (40.9%) |
0.006 |
|
Number of changes in needle angle during the procedure |
2 [1] |
3 [2] |
0.072 |
|
The total number of punctures |
1 [1] |
2 [1] |
0.081 |
|
Successful vein puncture time (seconds) |
50 [29] |
83 [129] |
0.089 |
|
Catheterısatıon success: Y / N |
24 (92.3%) / 2 (7.7%) |
13 (59.1%)/ 9 (40.9%) |
0.006 |
|
Successful catheterisation time (seconds) |
77.5 [39] |
152.5 [149] |
0.026 |
|
Total complications: Y / N |
1 (3.8%) / 25 (96.2%) |
7 (31.8%)/ 15 (68.2%) |
0.010 |
|
Pleura contact: Y / N |
1 (3.8%) / 25 (96.2%) |
4 (18.2%)/ 18 (81.8%) |
0.105 |
|
Bone contact: Y / N |
0 / 26 (100%) |
3 (13.6%)/ 19 (86.4%) |
0.520 |
|
Y: Yes N: No. The power of the study was found to be 0.969 as a result of the post hoc analysis (G*Power 3.1) using total complication rates. |
|||
The need for elaborate ingredient adjustment also discourages education providers. The temperature and the ratio of gelatine affect consistency. The ratio of psyllium husk affects echogenicity and there are publications claiming that, when adjusted improperly, it might cause over-confidence due to increased needle visibility compared to actual tissue.10,15 However, the psyllium husk ratio is not clearly stated in these studies and the authors know that when kept low, the visibility of the needle in USG increases. Hofstetter et al. studied the Psyllium husk levels in the 0.5-16 g/L range and recommended that the optimum level should be in the range of 7 g to 10 g per litre for appropriate echogenicity.13 The present investigators followed the recommendation and used 110 g gelatine powder and 7 g psyllium husk in 1 L water for the training model and 130 g gelatine powder and 10 g psyllium husk in 1 L water for the assessment model (Figure 3).
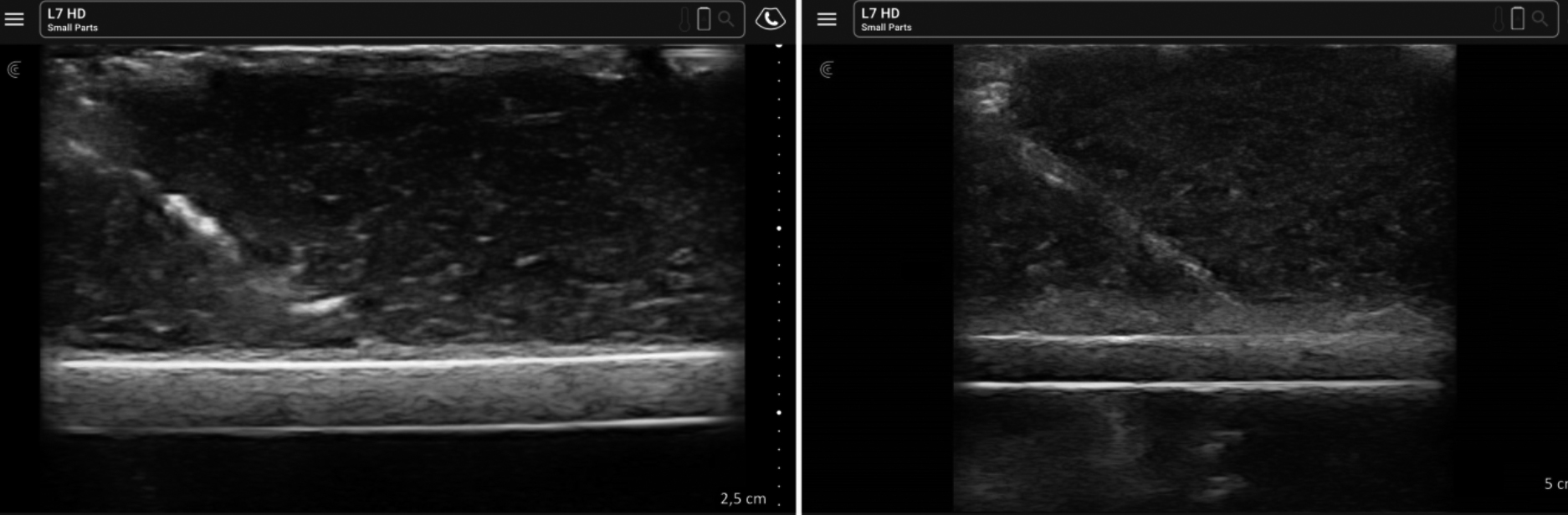 Figure 3: USG images of training (110 g gelatine powder and 7 g psyllium husk in 1 L water) and assessment (130 g gelatine powder and 10 g psyllium husk in 1 L water) models.
Figure 3: USG images of training (110 g gelatine powder and 7 g psyllium husk in 1 L water) and assessment (130 g gelatine powder and 10 g psyllium husk in 1 L water) models.
Due to a better learning curve for needle manipulation and fewer complication rates, the authors decided to use the long axis in-plane technique for the USG guided CVC placement training.3,6 Stratified randomisation with the year of residency and speciality subgroups ensured that there was no difference between the groups regarding experience or skills that could affect the study results (p = 0.482 and p = 0.915, respectively).
The first measured data in the assessment was the time of successful long axis vein imaging. Although jugular vein catheterisation can be performed with the short, oblique, or long axis image, the authors preferred the long axis since it is more educative to maintain the image on the screen during needle manipulation. Both groups were trained about capturing the jugular vein in the long axis by capturing it in the short axis and then rotating the probe. Group 1 was trained on the model for 5 minutes and then on a human being for another 5 minutes, 10 minutes in total. Group 2 was trained on a human being for 10 minutes. Thus, all participants received this training for the same duration. In the assessment, they were asked to repeat this on the manikin model. As all physicians received this training for the same amount of time and by similar standards, there was no significant difference between the two groups, as expected (p = 0.406).
Successfully capturing and maintaining in-plane needle image in the USG is a subtle skill. It requires eye-hand coordination that is good enough to hold the USG probe still and direct the needle with the dominant hand while following it on the screen. 92.3% of the residents who received the hands-on training on this subject were successful. This rate was only 59.1% for the residents who only received the theoretical training (p = 0.006). This study suggests that this process requires experience and a gelatine-based training model that is easy to build with low-budget such as for this study could help physicians to gain that experience in an efficient way. It is crucial to acquire this skill because following the needle on the screen at all times is one of the most important factors in reducing complication rates. Unsurprisingly, in-plane needle visualisation success overlaps with the catheterisation success as well (Group 1: 92.3% Group 2: 59.1%; p = 0.006). Residents who achieved a proper in-plane needle image on the screen also achieved catheter success. Most of the Participants’ feedback confirmed that the most difficult part was to keep the needle visible on the screen and practicing this on the model is very beneficial in this regard.
Regarding the number of changes in the needle angle, the total number of punctures, and successful vein puncture time, Group 1 obtained better scores, but this did not result in statistically significant differences between the groups (p = 0.072, p = 0.081, p = 0.089, respectively). It is believed that this is due to the small sample size of the study and a greater sample-sized study may demonstrate statistical significance.
Another notable result was decreased successful catheterisation time in Group 1 (p = 0.026). Group 2 took almost twice as long to place guidewire in vein and the authors can say that practice training on the model reduces time to complete the procedure as well. The reason the authors decided to use guidewire placement in the vein instead of the actual catheter for ‘successful catheterisation time’ is because the silicone tube used as the jugular vein is hard to dilate and requires serious physical force to do so. This process could also damage the model and prevent reuse.
USG guidance for jugular vein catheterization significantly reduced -but not eliminated- the complications such as arterial or pleural puncture.17,18 Reasons for these complications to persist include involuntary off-screen needle movements. The authors designed the assessment model with two DC circuits, one for the bone in deep and one for pleura in anterior, which flashes the indicator lights with needle contact. Even though participants were explained and warned about these traps both during training and before assessment, the authors observed that the first reaction to an indicator light was always a surprise. They did not even realise that the needle tip was off the screen and reported that this was a very instructive and memorable experience. The pleural contact on the anterior wall (red indicator) was lit by 1 (3.8%) of the residents in Group 1 and 4 (18.2%) in Group 2 (p = 0.105). The bone contact on the lower wall (green indicator), was not lit by any resident in Group 1 but lit by 3 (13.6%) of the residents in Group 2 (p = 0.520). The lack of a statistically significant difference between these rates might also be due to the small sample size. Nevertheless, the difference in the total number of complications provides statistical significance with Group 1 having fewer complications (p = 0.010). This proves the importance of the experience gained by performing needle puncture with a training model. The power of the study was therefore calculated according to the total complication rates between the groups.
The biggest limitation of this study is the fact that both test models and assessment manikins were designed by the researchers. The main reason for this is that commercial models with proven efficiency cannot be purchased for clinical use due to financial constraints. Such a risk of bias in mind, the authors tried to build the training and assessment models conveniently different from each other. Another limitation originates from the open-label nature of the study. During the assessment, the data recording team was aware of the participant’s group. However, since assessment reports consist of only objectively measurable data, the authors believe results are not affected anyhow.
CONCLUSION
Use of a gelatine-based model in USG guided central vein catheterisation training is an effective method to reduce complications, compared to training without a model. The approximate cost of the model was calculated as 5 dollars per unit (as of April 2021). This kind of USG friendly, gelatine-based intervention models are easy to build with a very low budget and they can be used for a variety of procedures during the training of residents.
ETHICAL APPROVAL:
Approval of the local ethics committee was received with the number 12.06.2019/06.
PATIENT’S CONSENT:
All volunteers participating in the study were informed and written consent was obtained.
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
ZTT: Conception and design, analysis and interpretation of data, technical procedures, and statistics analysis.
CY: Technical procedures, and statistics analysis.
MUB, HO: Manuscript writing, and critical revision.
All authors approved the final version of the manuscript to be published.
REFERENCES
- McGraw R, Chaplin T, McKaigney C, Rang L, Jaeger M, Redfearn D, et al. Development and evaluation of a simulation-based curriculum for ultrasound-guided central venous catheterisation. Can J Emerg Med 2016; 18(6):405-13. doi.org/10.1017/cem.2016.329.
- Woo MY, Frank J, Lee AC, Thompson C, Cardinal P, Yeung M, et al. Effectiveness of a novel training program for emergency medicine residents in ultrasound guided insertion of central venous catheters. Can J Emerg Med 2009; 11(4): 343-8. doi.org/10.1017/s1481803500011398
- Safety Committee of Japanese Society of Anesthesiologists. Practical guide for safe central venous catheterization and management 2017. J Anesth 2020; 34(2).167-86. doi.org/10. 1007/s00540-019-02702-9.
- Karakitsos D, Labropoulos N, De Groot E, Patrianakos AP, Kouraklis G, Poularas J, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: A prospective comparison with the landmark technique in critical care patients. Crit Care 2006; 10(6):162. doi.org/10.1186/cc5101.
- Jagneaux T, Caffery TS, Musso MW, Long AC, Zatarain L, Stopa E, Freeman N, Quin CC JG. Simulation-based education enhances patient safety behaviors during central venous catheter placement. J Patient Saf 2017; doi.org/10. 1097/PTS.0000000000000425.
- Corvetto MA, Pedemonte JC, Varas D, Fuentes C, Altermatt FR. Simulation-based training program with deliberate practice for ultrasound-guided jugular central venous catheter placement. Acta Anaesthesiol Scand 2017; 61(9):1184-91. doi.org/10.1111/aas.12937.
- Martins RS, Sabzwari SR, Iqbal M. Effectiveness of simulation-based clinical skills training for medical students in respiratory medicine: A pilot study. J Coll Physicians Surg Pakistan 2021; 31(12):1468-72. doi.org/10.29271/jcpsp. 2021.12.1468.
- Griswold-Theodorson S, Hannan H, Handly N, Pugh B, Fojtik J, Saks M, et al. Improving patient safety with ultrasonography guidance during internal jugular central venous catheter placement by novice practitioners. Simul Healthc 2009; 4(4):212-6. doi.org/10.1097/SIH.0b013e3181b1b837.
- Roark AA, Ebuoma LO, Ortiz-Perez T, Sepulveda KA, Severs FJ, Wang T, et al. Impact of simulation-based training on radiology trainee education in ultrasound-guided breast biopsies. J Am Coll Radiol 2018; 15(10):1458-63. doi.org/10. 1016/j.jacr.2017.09.016.
- Kondrashova T, Coleman C. Enhancing learning experience using ultrasound simulation in undergraduate medical education: Student perception. Med Sci Educ 2017; 27(3):489-96. doi.org/10.1007/s40670-017-0416-2.
- Nestel D, Groom J, Eikeland-Husebø S, O’Donnell JM. Simulation for learning and teaching procedural skills: The state of the science. Simul Healthc 2011; 6(S10-13). doi.org/10. 1097/SIH.0b013e318227ce96.
- Richardson C, Bernard S, Dinh VA. A cost-effective, gelatin-based phantom model for learning ultrasound-guided fine-needle aspiration procedures of the head and neck. In: journal of ultrasound in medicine. J Ultrasound Med 2015; 34(8):1479-84. doi.org/10.7863/ultra.34.8.1479.
- Hofstetter LW, Fausett L, Mueller A, Odéen H, Payne A, Christensen DA, et al. Development and characterisation of a tissue mimicking psyllium husk gelatin phantom for ultrasound and magnetic resonance imaging. Int J Hyperth 2020; 37(1):283-90. doi.org/10.1080/02656736.2020.1739345.
- Kondrashova T, Canaan R, Gunn B, Pazdernik V, Houser JJ. Development of competency in needle-guided procedures through the use of soft-embalmed cadavers. Mo Med 2020; 117(5):461-8.
- 15. Hocking G, Hebard S, Mitchell CH. A review of the benefits and pitfalls of phantoms in ultrasound-guided regional anesthesia. Reg Anesth Pain Med 2011; 36(2): 162-70. doi.org/10.1097/aap.0b013e31820d4207.
- Dahmani J, Laporte C, Pereira D, Belanger P, Petit Y. Predictive model for designing soft-tissue mimicking ultrasound phantoms with adjustable elasticity. IEEE Trans Ultrason Ferroelectr Freq Control 2020; 67(4):715-26. doi.org/10. 1109/TUFFC.2019.2953190.
- Kunhahamed M, Abraham S, Palatty B, Krishnan S, Rajeev P, Gopinathan V. A comparison of internal jugular vein cannulation by ultrasound-guided and anatomical landmark technique in resource-limited emergency department setting. J Med Ultrasound 2019; 27(4):187-91. doi.org/10.4103/JMU.JMU_2_19.
- Aydın G, Akcaboy Z. Extracavally malpositioned central venous catheter. J Coll Physicians Surg Pak 2020; 30(4): 459-60. doi.org/10.29271/jcpsp.2020.04.459.