Does the CDC COVID-19 Exposure Assessment Criteria for Healthcare Personnel Work in a Healthcare Setting in Pakistan?
By Imran Hassan1, Unab Inayat Khan1, Shehreen Ali1, Asif Hakim1, Asad Ali2Affiliations
doi: 10.29271/jcpsp.2023.01.57ABSTRACT
Objective: To evaluate the real-world performance of the CDC’s “Interim US guidance for risk assessment and work restriction for healthcare personnel with exposure to COVID-19” at a private healthcare system in Pakistan.
Study Design: Retrospective observational study.
Place and Duration of Study: The Aga khan University Hospital, Karachi, and its associated healthcare facilities in all four provinces of Pakistan, from February to September 2020.
Methodology: Healthcare personnel (HCPs) assessed and tested for exposures to COVID-19 were included in the study. An exposure category was assigned to each HCP presenting with exposure to COVID-19 based on the CDC criteria. Percentage positivity was recorded and compared among the different exposure categories. Logistic regression analysis was used to identify variables significantly associated with COVID-19 infection.
Results: Three thousand Six hundred and forty-seven HCPs were assessed for exposure to COVID-19 of whom 603 (16.5%) tested positive. Percent positivity was highest in high-risk symptomatic HCPs (18.2%), 15.6% in low-risk symptomatic HCPs, and 11% in high-risk asymptomatic HCPs. After controlling for age, gender, area of work, and source of exposure, compared to low-risk asymptomatic HCPs, the odds of a positive SARS-CoV-2 PCR were 2.13 (95%CI: 1.49-3.04) for high-risk symptomatic, 1.66 (95% CI: 1.12-2.46) for low-risk symptomatic, and 1.18 (95% CI: 0.83-1.68) for high-risk asymptomatic HCPs.
Conclusion: Regardless of exposure category, HCPs with symptoms consistent with COVID-19 have the highest likelihood of testing positive. The CDC exposure risk assessment criteria work best for symptomatic HCPs. Testing asymptomatic HCPs with high-risk exposures may not be necessary in low-resource settings with a limited healthcare workforce.
Key Words: COVID-19, Employee health, Occupational health and safety programs, Medical surveillance/screening, Return to work.
INTRODUCTION
The novel coronavirus SARS-COV-2, which is responsible for the COVID-19 pandemic, spreads primarily via respiratory droplets entering through the mucous membranes of the nose, mouth, and eyes of the host.1 Symptoms may occur after an incubation period of 2-14 days with a median of 5.1 days.2
Healthcare personnel (HCPs) are at a greater risk of exposure and infection due to their work environment.3 Reports suggest HCPs comprise 3% and in some cases up to 20% of the infected population,4,5 showing the marked vulnerability of HCPs due to both occupational and environmental exposures.
In Pakistan, the first COVID-19 infection was confirmed on February 26, 2020.6 Since then, there have been multiple surges in the country, with ongoing infections and deaths in HCPs. At the end of the first wave of COVID-19 in October 2020, at least 8,272 HCPs had contracted COVID-19 with 87 reported deaths.7
An objective exposure-assessment criterion is a crucial effort to direct testing and quarantine guidelines in the healthcare setting to prevent the further spread of infection. An accurate exposure risk assessment criterion has significant implications in terms of human resource optimisation through the reduction of missed workdays and mitigation of financial impact of COVID in the workplace. Identifying persons with a high pretest probability is even more important in low-resource settings where limitations in testing capacity pose a challenge to effective COVID-19 infection control.
The US Centres for Disease Control and Prevention (CDC), one of the major global organisations contributing to international health regulations,8 released its “Interim US guidance for risk assessment and work restriction for healthcare personnel with exposure to COVID-19”.9 The CDC guideline utilises 1) duration of exposure; and 2) degree of protection of mucous membranes, to define the risk-category followed by a need for testing and duration of quarantine required for each risk-category. The aim of this study was to evaluate the real-world performance of the contemporaneous version of the CDC’s “Interim US guidance for risk assessment and work restriction for healthcare personnel with exposure to COVID-19” at a private healthcare system in Pakistan during the first wave of the COVID-19 pandemic. The results of this study are expected to help inform decisions regarding the testing of HCPs presenting with exposures to COVID-19 in resource-limited settings.
METHODOLOGY
This was a retrospective observational study conducted at the Aga Khan University Hospital, Karachi and its associated healthcare facilities across Pakistan, from February to September 2020.
Through the office of Employee Health, assessment, testing, and treatment for COVID were available free of cost to all employees of the healthcare system. HCPs with a history of exposure to a PCR-confirmed case of COVID-19 were assessed by trained Employee Health physicians to determine the risk of exposure based on the CDC guidelines.9 When tested, COVID-19 reverse transcription-polymerase chain reaction (RT-PCR) was performed on a nasopharyngeal swab at a College of American Pathologist accredited (CAP-accredited) laboratory of the institution. All HCP exposure assessments and testing results were entered in a password-protected database accessible only to the Employee Health team.
For this study, deidentified data was provided to the research team after receiving an exemption from the institutional Ethics Review Committee (2020-5629-15118).
All HCPs assessed and tested for exposures to COVID-19 during the study period were included in the analysis. No HCPs were excluded from the analysis. Understanding that all employees were vital to the provision of healthcare to patients, all employees working within the healthcare system were considered HCPs.10 To recognise the additional risk of those providing direct clinical care, employees were divided into the following categories: non-clinical (including administrative and support staff), clinical non-COVID (employees working in clinical areas that were not designated for COVID-positive and COVID-suspect patients), and clinical COVID (clinical staff working in areas designated for the care of patients with suspected or confirmed COVID infection).
Using the CDC guidelines, exposure was defined as “a contact at less than six-feet distance between a COVID-positive person(s) and the HCP for a 15-minute cumulative time during a 24-hours period”. COVID-positive persons were considered infectious from 48 hours before symptom onset (or the day of positive COVID RT-PCR if asymptomatic) to 10 days after symptoms began or tested positive.
Exposure assessment included source control, personal protective equipment (PPE) worn by the exposed HCP, distance from the positive person, and duration of exposure. In addition, droplet burden, performance of an aerosol-generating procedure (AGP), and adequate ventilation of ambient air were also factored in. Based on CDC guidelines, HCPs were stratified as having a low-risk or high-risk exposure (Table I). Any exposure occurring within the household setting was considered a high-risk exposure.
Based on the assessed risk of transmission of infection during the exposure and the presence or absence of symptoms at presentation, HCPs were stratified into four exposure categories: high-risk symptomatic [n:1119 (30.7%)], high-risk asymptomatic [n:1577 (43.3%)], low- risk symptomatic [n: 539 (14.8%)], and low-risk asymptomatic [n: 408 (11.2%)].
All HCPs with high-risk exposures were tested for SARS-COV-2 using RT-PCR. If the test was negative, they were advised to quarantine till day 7 of exposure with active monitoring of symptoms. An RT-PCR was repeated on day 7. If both RT-PCR results were negative, and no symptoms had developed, HCPs were allowed to return to work with strict adherence to PPE measures and active monitoring of symptoms till day 14 of exposure.
HCPs with low-risk exposures, as defined by the CDC guideline, were tested only for the following reasons: 1) they had symptoms consistent with COVID-19 infection; 2) they were immunocompromised due to a medical condition; or 3) they were working in a clinical area with immunocompromised patients such as oncology. If not tested, they were advised to return to work with strict PPE adherence and self-reporting of symptoms till day 14 of exposure. They were advised to get tested if symptoms developed within 14 days from exposure.
Quantitative variables were reported as means and standard deviations and categorical variables were reported as frequencies and percentages. Percentage positivity for each HCP category was calculated by dividing the number of COVID-positive tests within a category by the number of tests conducted within that category. Differences between COVID positive and negative groups were tested using Chi-square for categorical data. Continuous-level data were tested for normality using Shapiro-Wilk test, and ANOVA was used to compare the differences between the four exposure categories. Post-hoc differences between the four groups were examined using Bonferroni test.
Logistic regression was used to estimate the association of a positive SARS-CoV-2 RT-PCR test and exposure category and to identify variables associated with COVID positivity. Data were analysed using Stata/SE® V15.1. A p-value of ≤0.05 was considered statistically significant.
RESULTS
Figure 1 describes the risk stratification of HCPs whose data were analysed. Of the 3,643 HCPs assessed and tested during the study period, 947 (25.9%) had a low-risk exposure. Of these, 408 (11.2%) were asymptomatic and 539 (14.8%) had symptoms consistent with COVID-19 infection. A total of 2,696 (73.9%) HCPs were assessed to have a high-risk exposure. Of these, 1,578 (43.3%) were asymptomatic and 1119 (30.7%) had symptoms consistent with COVID-19 infection.
Table I: CDC exposure assessment criteria used during the study period.|
PPE worn by HCP |
Exposure category |
|
(a) Prolonged close contact with a person with COVID-19 who was wearing a facemask |
|
|
None |
High-risk
|
|
Not wearing the gown, gloves, eye protection, and respirator during an aerosol- generating procedure |
|
|
Wearing recommended PPE but wearing facemask instead of respirator |
Low-risk |
|
(b) Prolonged close contact with a person with COVID-19 who was not wearing a facemask |
|
|
None |
High-risk
|
|
Not wearing a facemask or respirator and eye protection |
|
|
Not wearing gown, gloves, eye protection, and respirator during AGP |
|
|
Wearing all recommended PPE but wearing facemask instead of respirator |
Low-risk |
Table II: Association between risk categories and study population characteristics.
|
Characteristics |
Total N=3643 |
Risk categories |
p-value |
|||
|
Low-risk asymptomatic, n (%) 408 (11.2%) |
Low-risk symptomatic, n (%) 539 (14.8%) |
High-risk asymptomatic, n (%) 1577 (43.3%) |
High-risk symptomatic, n (%) 1119 (30.7%) |
|||
|
Gender, n (%) |
||||||
|
Male |
1741 (48) |
210 (51.5) |
270 (50.1) |
790 (50.1) |
471 (42.1) |
<0.001* |
|
Female |
1902 (52) |
198 (48.5) |
269 (49.9) |
787 (49.9) |
648 (57.9) |
|
|
Age, years (mean ± sd)** |
32.5 + 8.5 |
34 ± 9.3 + ɤ |
32.5 ± 8.8 |
32.6 ± 8.6 + ∞ |
31.6 ± 7.8 ɤ ∞ |
<0.001** |
|
HCP work area, n (%) |
||||||
|
Non-clinical |
680 (18.7) |
73 (17.9) |
106 (19.7) |
323 (20.5) |
178 (15.9) |
0.801*** |
|
Clinical non-COVID |
2368 (65) |
250 (61.3) |
347 (64.4) |
1008 (63.9) |
763 (68.2) |
|
|
Clinical COVID |
595 (16.3) |
85 (20.8) |
86 (16) |
246 (15.6) |
178 (15.9) |
|
|
Source of exposure, n (%) |
||||||
|
HCP |
2339 (64.5) |
275 (68.6) |
391 (72.8) |
958 (60.9) |
715 (64.3) |
<0.001*** |
|
Family/community |
562 (15.5) |
14 (3.5) |
14 (2.6) |
335 (21.2) |
199 (17.9) |
|
|
Patient |
723 (20) |
112 (27.9) |
132 (24.6) |
281 (17.9) |
198 (17.8) |
|
|
COVID PCR-result |
||||||
|
Positive |
602 (16.5) |
43 (10.5) |
88 (16.3) |
229 (14.5) |
242 (21.6) |
<0.001* |
|
Negative |
3041 (83.5) |
365 (89.5) |
451 (83.7) |
1348 (85.5) |
877 (78.4) |
|
|
Reporting row percentages; * Pearson chi-square; **one-way analysis of variance (ANOVA). Similar symbols show significant differences between groups on Bonferroni post-hoc analysis; *** Cuzick nonparametric trend test across ordered groups. |
||||||
Table III: Regression analysis for association of HCP characteristics with positive SARS-COV-2 RT-PCR result.
|
Variables |
Univariate analysis OR (95%CI) |
Multivariable analysis a OR (95%CI) P for model <0.001 N= 3623 |
|
Gender |
|
|
|
Female |
1 |
1 |
|
Male |
1.43 (1.20- 1.70); p <0.001 |
1.44 (1.19-1.73) |
|
Age (years) |
1.02 (1.01 – 1.03); p<0.001 |
1.01(1.00-1.02) |
|
Area of work |
|
|
|
Non-clinical (reference) |
1 |
1 |
|
Clinical Non-COVID |
0.72 (0.58- 0.89); p 0.003 |
0.85 (0.68 – 1.08); p: 0.184 |
|
Clinical COVID |
0.52 (0.38 – 0.70); p <0.001 |
0.61 (0.44 – 0.85); p<0.003 |
|
Source of exposure |
|
|
|
Patient (reference) |
1 |
1 |
|
HCP |
0.88 (0.69 -1.11); p:0.271 |
0.76 (0.60 – 0.97); p: 0.025 |
|
Family |
2.17 (1.65 – 2.85); p<0.001 |
1.89 (1.42 – 2.53); p<0.001 |
|
Risk category |
|
|
|
Low-risk asymptomatic |
1 |
1 |
|
Low-risk symptomatic |
1.65 (1.12 – 2.44); p:0.01 |
1.66 (1.12 – 2.46); p: 0.012 |
|
High-risk asymptomatic |
1.44 (1.02 – 2.04); p: 0.038 |
1.18 (0.83 – 1.68); p: 0.362 |
|
High-risk symptomatic |
2.34 (1.66 – 3.31); p <0.001 |
2.13 (1.49 – 3.04); p <0.001 |
Table II describes the differences among the four groups based on risk stratification. There was a significant age difference among the four groups with low-risk asymptomatic HCPs being older than both high-risk asymptomatic and high-risk symptomatic HCPs. There was no difference in exposure based on the area of work. However, a significant trend of high-risk exposures was seen in those exposed to other HCPs and family members as compared to those exposed to patients. Of all HCPs tested, 602 (16.5%) tested positive for COVID RT-PCR. There was a significant trend in percent positivity, with the lowest percent positivity seen in low-risk asymptomatic group [43/408 (10.5%)] and the highest percent positivity seen in a high-risk symptomatic group [242/1119 (21.6%)] (p for trend <0.001). Interestingly, HCPs with symptoms had a higher rate of testing positive compared to those without symptoms, regardless of level of exposure. In addition, a higher rate of positivity on repeat testing on day 7 of exposure was seen in those with high-risk exposures. Of the high-risk asymptomatic group, 48 (3.4%) new positives were identified on repeat PCR testing on day 7 of exposure.
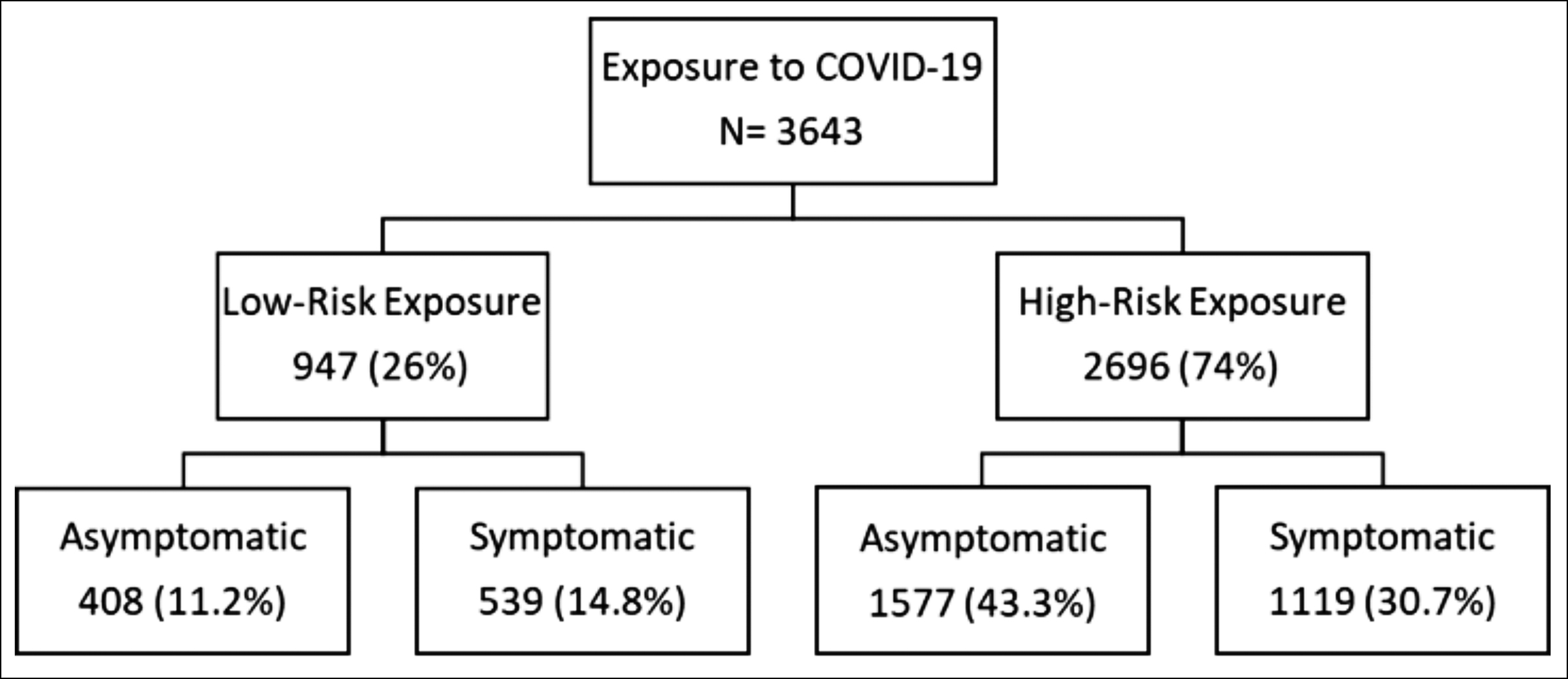 Figure 1: Healthcare Personnel stratification based on exposure risk and symptoms.
Figure 1: Healthcare Personnel stratification based on exposure risk and symptoms.
To examine the association between different risk factors and a positive COVID-PCR result, univariate and multivariable logistic regression was used (Table III). After adjusting for other variables of interest, compared to female HCPs, male HCPs had 1.44 times higher odds of COVID positivity (aOR: 1.44, CI: 1.19-1.73; p <0.001). Interestingly compared to those working in non-clinical areas, HCPs working in clinical COVID areas had the lowest odds of COVID positivity (aOR: 0.61, CI: 0.44-0.85; p= 0.003).
The source of exposure was significantly associated with the likelihood of infection. As compared to HCPs that were exposed to a patient with COVID infection, HCPs exposed to family members had almost two times higher odds (aOR: 1.89, CI: 1.42-2.53; p: <0.001) of testing positive for COVID-19. While it was the most common source of exposure (64.5%), exposure to a COVID-positive HCP did not increase likelihood of infection (aOR: 0.76, CI: 0.60-0.97; p= 0.025), regardless of the area of work or the exposure category.
Of the four exposure categories, compared to low-risk asymptomatic, HCPs with a high-risk exposure and symptoms were twice as likely to have COVID infection (aOR: 2.13, CI: 1.49-3.04; p <0.001); followed by HCPs with low-risk exposures and symptoms (aOR:1.66, CI:1.12-2.46; 0.012).
DISCUSSION
Infections in healthcare personnel pose a double challenge during the COVID-19 pandemic. Not only are HCPs at a higher risk of getting infected due to their nature of work, but their absence from work due to quarantine and isolation puts an added strain on overstretched healthcare system resources. Classification of exposure risk category provides an objective means of making an informed decision regarding testing and quarantine of HCPs. This holds especially true in low resource settings, where availability and cost of testing are additional limiting factors.
The results of this study show that HCPs with symptoms have a higher likelihood of being COVID-positive regardless of the exposure-risk. Similar results were seen in a study among 592 HCPs in an academic hospital in USA,11 citing an almost doubling of the likelihood of a COVID positivity in the presence of 3 or more COVID-compatible symptoms (OR = 1.95 (95% CI: 1.10–3.64). Multiple other studies have also reported the value of individual symptoms in predicting COVID positivity among HCPs.12-14
In the present study, HCPs with a high-risk exposure had a higher likelihood of testing positive compared to those with a low-risk exposure. A prospective study of 667 HCPs from USA using the CDC exposure assessment guideline reported percent positivity among HCPs of 9.2% (CI: 4.3%–16.7%), 4.7% (CI: 2.2%–8.7%), and 1.6% (CI: 0.6%–3.4%), in high-risk, medium-risk and low-risk exposures respectively (p<0.01).15 Using criteria similar to the CDC’s, data based on 3398 HCPs from Greece showed that HCPs with high-risk exposures had a higher percentage positivity compared to moderate and low-risk exposure (5%, 1%, and 1%, respectively; p < .001).16 These studies looking at the performance of COVID exposure assessment protocols did not stratify exposure risk categories by symptomatic status. In the current study, this relationship between intensity of exposure and COVID positivity did not hold true in the case of asymptomatic HCPs with high-risk exposures. Low-risk symptomatic HCPs had a higher likelihood (aOR: 1.66, CI: 1.12 – 2.46) of testing positive compared to high-risk asymptomatic (aOR: 1.18, CI: 0.83-1.68) suggesting that being symptomatic is a stronger predictor of COVID positivity compared to the intensity of exposure.
While these findings make it easier to decide on testing and isolation of symptomatic HCPs, it is also known that people with asymptomatic infections with SARS-CoV-2 can carry a similar viral load as symptomatic infections and are infectious from 2-3 days before symptom onset.17 These HCPs can be a source of outbreaks in close clinical settings and can put vulnerable patients at potential risk. A positive yield of 3.8% and 3.4% on day-7 repeat testing was seen among symptomatic and asymptomatic HCPs with high-risk exposures respectively. This highlights the utility of the 7-day exclusion from work recommendation for HCPs with high-risk exposure regardless of symptoms.
Most of the reported exposures were to other HCPs within the healthcare setting however such exposures were not associated with an increased likelihood of COVID positivity compared to exposure to patients. Closer proximity and a higher burden of respiratory secretions in patient-related exposures might account for this observation. In contrast, the likelihood of getting infected was highest when the known exposure was to a family member. Exposures in the household setting where routine observation of effective protective measures is not usually possible are expected to involve longer durations of unprotected contact with positive household members.
Contrary to expectation, HCPs working in clinical COVID areas had a lower likelihood of COVID positivity compared to those working in non-clinical areas. Other studies have reported similar findings.18,19 The heightened alertness when working with known COVID-positive patients is likely to contribute to stricter observation of protective measures and thus fewer infections.
This study has some limitations. As HCPs were self-reporting symptoms and exposures or were being identified on contact tracing after a COVID-positive case was identified in the healthcare system, it is possible that few asymptomatic employees may have been missed. Resources were not sufficient to allow performing regular mandatory testing for all employees. However, given the robust mechanism of contact tracing and free access to assessment and testing, this is not expected to markedly affect the results of the current study.
Despite these limitations, this study has several strengths. The results show that there was adequate utilisation of the services. Exposure risk was determined by employee health physicians who were trained to use the CDC criteria uniformly. Refreshers were arranged on a regular basis as new information became available. Data entry was done by trained clinical staff contributing to the accuracy of data. Identical data were collected on all HCPs who were assessed in a prospective manner, removing recall bias.
CONCLUSION
While risk stratification of HCP exposures to COVID-19 is useful in distinguishing those at a higher risk of infection, the CDC’s “Interim US guidance for risk assessment and work restriction for healthcare personnel with exposure to COVID-19” performed best in those with symptoms. Thus, in resource-limited settings, for asymptomatic HCPs with high-risk exposure to COVID-19, it may be reasonable to forego testing in the continued absence of symptoms.
ACKNOWLEDGEMENT:
The authors would like to thank the COVID command (Dean Professor Adil Haider and CEO Ms. Shagufta Hassan) for setting up the employee health system during COVID. The authors would like to acknowledge the tireless work of the Employee Health team for maintaining the Employee Health database that was used for this study, and the faculty and staff of Department of Family Medicine at the Aga Khan University Hospital, Karachi.
ETHICAL APPROVAL:
This retrospective observational study was reviewed and approved by the Ethical Review Committee at Aga Khan University (2020-5629-15118).
PATIENTS’ CONSENT:
As this was an evaluation of anonymised retrospective data, there was an exemption from ERC for informed consents from participants.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
UIK, AA, IH: Conceptualisation, design, methodology, literature review, writing, and revision of the manuscript.
IH, UIK, SA, AH: Curation of data and literature review.
IH, UIK: Data analysis.
All authors have reviewed and approved the final version of the manuscript.
REFERENCES
- Ochani R, Asad A, Yasmin F, Shaikh S, Khalid H, Batra S, et al. COVID-19 pandemic: From origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez Med 2021; 29(1):20-36. doi: 10.53854/liim-2903-3.
- McAloon C, Collins A, Hunt K, Barber A, Byrne AW, Butler F, et al. Incubation period of COVID-19: A rapid systematic review and meta-analysis of observational research. BMJ Open 2020; 10(8):e039652. doi: 10.1136/bmjopen-2020- 039652.
- Zabarsky TF, Bhullar D, Silva SY, Mana TSC, Ertle MT, Navas ME, et al. What are the sources of exposure in healthcare personnel with coronavirus disease 2019 infection? Am J Infect Control 2021; 49(3):392-5. doi: 10.1016/j.ajic. 2020.08.004.
- COVID C, Team R, COVID C, Team R, COVID C, Team R, et al. Characteristics of healthcare personnel with COVID-19—United States, February 12–April 9, 2020. Morbidity Mortality Weekly Report 2020; 69(15):477. doi: 10.15585/mmwr.mm6915e6.
- Calo F, Russo A, Camaioni C, De Pascalis S, Coppola N. Burden, risk assessment, surveillance and management of SARS-CoV-2 infection in health workers: A scoping review. Infect Dis Poverty 2020; 9(1):139. doi: 10.1186/s40249- 020-00756-6.
- Abid K, Bari YA, Younas M, Tahir Javaid S, Imran A. Progress of COVID-19 epidemic in Pakistan. Asia Pac J Public Health 2020:154-6. doi: 10.1177/1010539520927259.
- Coronavirus has infected 8,000 healthcare workers in Pakistan so far 2020 [updated 10/21/2020; cited 2022 03/14/2022]. Available from: https://www.geo.tv/latest/ 314386-coronavirus-has-infected-8000-healthcare-workers-in-pakistan-so-far.
- Tappero JW, Cassell CH, Bunnell RE, Angulo FJ, Craig A, Pesik N, et al. US centers for disease control and prevention and its partners' contributions to global health security. Emerg Infect Dis 2017; 23(13):S5-S14. doi: 10.3201/eid2313.170946.
- Interim guidance for managing healthcare personnel with SARS-CoV-2 infection or exposure to SARS-CoV-2 2019 [updated 21 January 2022; cited 2022 13 January 2022]. Available from: www.cdc.gov/coronavirus/2019-ncov/hcp/ guidance-risk-assesment-hcp.html.
- Kuhar DT, Carrico R, Cox K, de Perio MA. Infection control in healthcare personnel. Appendix 2: Terminology: U.S. Department of Health and Human Services; 2019 [updated October 2, 2019.
- Lan FY, Filler R, Mathew S, Buley J, Iliaki E, Bruno-Murtha LA, et al. COVID-19 symptoms predictive of healthcare workers' SARS-CoV-2 PCR results. PLoS One 2020; 15(6):e0235460. doi: 10.1371/journal.pone.0235460.
- Sacks CA, Dougan M, McCoy Jr TH, Zheng A, Buonomo G, North CM, et al. The association between symptoms and COVID-19 test results among healthcare workers. Annals Surgery 2020; 272(6):e329. doi: 10.1097/SLA.000000 0000004483.
- Iversen K, Bundgaard H, Hasselbalch RB, Kristensen JH, Nielsen PB, Pries-Heje M, et al. Risk of COVID-19 in health-care workers in Denmark: An observational cohort study. Lancet Infect Dis 2020; 20(12):1401-8. doi: 10.1016/ S1473-3099(20)30589-2.
- Tostmann A, Bradley J, Bousema T, Yiek W-K, Holwerda M, Bleeker-Rovers C, et al. Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, the Netherlands, March 2020. Eurosurveillance 2020; 25(16):2000508. doi: 10.2807/ 1560-7917.ES.2020.25.16.2000508.
- Gragnani CM, Fernandes P, Waxman DA. Validation of centers for disease control and prevention level 3 risk classification for healthcare workers exposed to severe acute respiratory coronavirus virus 2 (SARS-CoV-2). Infect Control Hosp Epidemiol 2021; 42(4):483-5. doi: 10.1017/ice. 2020.1353.
- Maltezou HC, Dedoukou X, Tseroni M, Tsonou P, Raftopoulos V, Papadima K, et al. SARS-CoV-2 Infection in healthcare personnel with high-risk occupational exposure: Evaluation of 7-day exclusion from work policy. Clin Infect Dis 2020; 71(12):3182-7. doi: 10.1093/cid/ciaa888.
- Ra SH, Lim JS, Kim GU, Kim MJ, Jung J, Kim SH. Upper respiratory viral load in asymptomatic individuals and mildly symptomatic patients with SARS-CoV-2 infection. Thorax 2021; 76(1):61-3. doi: 10.1136/thoraxjnl-2020-215042.
- Eyre DW, Lumley SF, O'Donnell D, Campbell M, Sims E, Lawson E, et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. Elife 2020; 9:e60675. doi: 10.7554/ eLife.60675.
- Mandic-Rajcevic S, Masci F, Crespi E, Franchetti S, Longo A, Bollina I, et al. Source and symptoms of COVID-19 among hospital workers in Milan. Occup Med (Lond) 2020; 70(9): 672-9. doi: 10.1093/occmed/kqaa201.