Distant Metastasis Patterns of Lung Cancer on Positron Emission Tomography/Computed Tomography Association with Age and Histological Subtype
By Sibel Goksel1, Neslihan Ozcelik2Affiliations
doi: 10.29271/jcpsp.2021.12.1438ABSTRACT
Objective: To investigate whether age or other factors are determinants of distant metastasis in patients with lung cancer.
Study Design: Observational study.
Place and Duration of Study: Department of Nuclear Medicine, Recep Tayyip Erdogan University, Rize, Turkey between December, 2018 and February, 2019.
Methodology: A total of 152 patients with lung cancer, who underwent positron emission tomography/computed tomography (PET/CT) for staging, were included in this study. Patients were grouped according to age (>65 and <65 years) and distant metastasis status. Metastasis localisation of patients was evaluated by PET/CT. Univariate/multivariate regression analyses were performed to determine risk factors for distant metastasis.
Results: No significant difference was found when the relation of distant metastasis with stage distribution was examined in both age groups. Distant metastasis rates were significantly higher in female patients than in male patients (p = 0.019) and in patients with small-cell lung carcinoma (SCLC)-adenocarcinoma than in those with other histopathological subtypes (p <0.001). Most of the patients in both groups had a stage 4 disease, and bone distant metastasis was the most common in both age groups. Univariate/multivariate analyses identified that female gender (p = 0.017/p = 0.003), SCLC subtype (p = 0.013/p = 0.008), T3/T4 tumour (p <0.001/p <0.001), and smoking history of >66 pack-years (p = 0.047/p = 0.047) were independent factors for the presence of distant metastasis.
Conclusion: Although age is not a risk factor for distant metastasis in lung cancer, female gender, T3/4 tumour, SCLC subtype, and smoking history of >66 pack-years are high-risk factors. PET/CT is recommended as the first-choice imaging technique in patients with lung cancer indicated for distant metastasis scanning.
Key Word: Lung cancer, PET/CT, Metastases, Histological subtype, T-stage.
INTRODUCTION
Lung cancer causes more than 1 million deaths each year, and is the leading cause of cancer-related death.1 In recent years, the prolongation of human life expectancy has led to an increase in cancer cases. Although, approximately half of the patients with cancer are seen in older people aged >65 years, adenocarcinoma histological subtype has begun to be seen frequently in young women, recently.2
Approximately 70% of patients have advanced disease stage at the time of diagnosis, and the 5-year survival rate of patients with lung cancer is 15%.3,4 Identifying most patients with metastatic-advanced stage is still the most important reason for the recent high mortality rate. The National Comprehensive Cancer Network 2017 guidelines recommended that patients who had risk factors such as being >55 years and had a smoking history of ≥30 pack-years should be screened by thoracic computed tomography (CT) with low-dose radiation for early diagnosis.5
In past years, some traditional imaging methods such as CT, magnetic resonance imaging, and scintigraphy were used for staging of patients with lung cancer. Recently, F-18 fluoro-deoxyglucose (FDG) positron emission tomography (PET)/CT method allows staging of the whole body simultaneously, and reflects both the anatomical and metabolic status of the tumour; and its metastasis, which has been increasingly used. Accordingly, it has become possible to easily detect metastatic diseases by PET/CT, which cannot be detected by standard methods.6,7 Thus, the detection of the distant metastases and metastatic lymph nodes by PET/CT increased, and unnecessary thoracotomies decreased.8
More than 80% of lung cancer cases are non-small cell lung cancer (NSCLC), and the rest are small cell lung cancer (SCLC). Smoking history is the most crucial risk factor in the development of lung cancer9. This risk varies depending on the duration and amount of cigarettes smoked.
The patient should be encouraged to quit smoking at any age or stage because the clinical course of those who quit smoking is better than those who continue to smoke.10,11
Knowledge of the distribution and localisation of the metastasis contributes significantly to the evaluation of clinical findings of uncertain cause and the choice of treatment, as well as of the treatment algorithms.12 Few studies have shown the distribution and frequency of distant metastasis at the time of diagnosis in lung cancer, according to age and histopathological subtypes.
Thus, this study aimed to evaluate the localisation and rate of distant metastasis by age and histopathological subtype in patients with newly diagnosed lung cancer.
METHODOLOGY
Patients with lung cancer, diagnosed at Recep Tayyip Erdogan University, Rize, Turkey between December, 2018 and February, 2019, were evaluated retrospectively. Patients aged >18 years and diagnosed with primary lung cancer (NSCLC and SCLC) were included in this study. The exclusion criteria were as follows: received neoadjuvant or adjuvant therapy, underwent surgery before the PET/CT scan, and had brain metastases without another distant metastasis. Brain metastases were excluded from the evaluation in distant metastasis screening because of the limitations of PET/CT in detecting brain metastases.
Patients’ files and PET/CT images available in the hospital electronic medical records were examined. Age, gender, smoking history, histopathological subtype, stage and localisation of the lymph node and distant metastasis upon diagnosis were recorded. The staging of all patients was based on the 8th Tumour-Node-Metastases (TNM) staging system, according to the initial PET/CT scan.13 All patients with distant metastasis having 1–3 metastases, were evaluated as oligometastatic. Those with more than three metastases were evaluated as having a multiple metastatic disease.
Patients were grouped according to age (>65 and <65 years) and presence of distant metastasis. The age distribution of these variables, especially the current TNM stage of patients, and distribution of metastasis patterns, were examined. The effect of demographic and histopathological subtypes of patients on predicting metastatic disease was also evaluated.
All patients fasted for at least 6 hours before PET/CT. The fasting blood glucose levels of all patients were <200 mg/dL before scanning. Moreover, 220–370 MBq18F-FDG was administered intravenously to the patients. Contrast agent was given orally to all patients. Patients underwent PET/CT (Siemens Biograph mCT/20 slices) with three-dimensional mode and time-of-flight features, following a resting period of 60–70 min in the resting room. Images were acquired from the head to the upper thigh area. Low-dose CT data were collected at an average of 120 kV–50 mAs. PET images were obtained at a rate of 2 minutes per bed position.
A nuclear medicine physician with board certification visually and semi-quantitatively evaluated PET/CT images of all patients. The primary lesion size was measured in two dimensions in the axial plane on the fused PET/CT section. PET/CT images of all patients were evaluated in detail with respect to the chest wall, bone, adjacent great vessels and pleural invasion, which are the criteria for determining the T stage. Localisations of the lymph node and distant metastasis were noted.
Data analysis was performed using the SPSS version 25.0 statistical package. Kolmogorov–Smirnov test was conducted to determine whether the quantitative variables were normally distributed in the groups. The Chi-square analysis determined the dependence between the qualitative variables. Independent sample t-test and Mann–Whitney U-test were used for comparisons between the two independent groups, such as patients’ distant metastasis status by age groups, and smoking pack-years. Qualitative variables were given as a number (n) and percentage (%). Descriptive statistics on quantitative variables were given as median (25th–75th percentile) and mean ± SD. Factors that affected distant metastasis were analysed by univariate and multivariate analyses, using a logistic regression analysis. Multivariate analysis was used to treat variables that were found significant in the univariate analysis, and p <0.05 was considered significant.
RESULTS
A total of 152 patients, diagnosed with lung cancer who underwent PET/CT for initial staging, were evaluated retrospectively. The mean age was 66.7 ± 10.3 (range, 33–90) years. Moreover, 84 (55.3%) patients were aged >65 years, and 132 (86.8%) patients were males. The demographic data of all patients according to the distant metastasis status are summarised in Table I.
Most patients had an advanced stage disease in both age groups, and distant metastasis was found in 50.7% of all patients on initial PET/CT. The distant metastasis rate was significantly higher in female patients than in male patients, at the time of diagnosis (p = 0.019). Considering the relationship between distant metastasis status with histopathological subtype, distant metastasis rates in adenocarcinoma and SCLC subtypes were significantly higher than in other subtypes. The rate of distant metastasis was significantly less in SCC than in other subtypes (p <0.001).
Table I: Demographic and clinical characteristics of patients with lung cancer, according to the distant metastasis status.|
|
All patients |
Patients with distant metastasis (+) |
Patients without distant metastasis (-) |
p-value |
|
All patients With oligometastasis With multipl metastasis |
152 (100%) 31 (20.4%) 46 (30.3%) |
77 (50.7%) 31 (40.3%) 46 (59.7%) |
75 (49.3%) |
|
|
Mean Age ± SD |
66.7±10.3 |
66.2±9.8 |
67.2 ±10.8 |
0.523 |
|
Age groups: ≤65 years >65 years |
68 (44.7%) 84 (55.3%) |
40 (51.9%) 37 (48.1%) |
28 (37.3%) 47(62.7%) |
0.070 |
|
Gender: Female Adenocarcinoma SCC SCLC Male Adenocarcinoma SCC SCLC NSCLC NOS |
20 (13.2%) 16 (80%) 2 (10%) 2 (10%) 132 (86.8%) 42 (31.8%) 56 (42.4%) 18 (13.7%) 9 (6.8%) 7 (5.3%) |
15 (19.5%)
62 (80.5%) |
5 (6.7%)
70 (93.3%) |
0.019 |
|
Smoking status: Yes Female Male No Female Male |
132 (86.8%) 4 (3%) 128(97%) 20 (13.2%) 16 (80%) 4 (20%) |
66 (85.7%)
11 (14.3%) |
66 (88%)
9 (12%) |
0.677 |
|
Smoking pack-years (median, 25-75th percentile) |
60 (40-100) |
70 (40-100) |
60 (36-100) |
0.434 |
|
Histopathological type: Adenocarcinoma SCC NSCLC SCLC NOS |
58 (38.2%) 58 (38.2%) 9 (5.9%) 20 (13.1%) 7 (4.6%) |
38 (49.3%) 20 (26%) 1 (1.3%) 17 (22.1%) 1 (1.3%) |
20 (26.7%) 38 (50.7%) 8 (10.6%) 3 (4%) 6 (8%) |
<0.001 |
|
*NOS: not otherwise specified, SCLC: small cell lung cancer, NSCLC: non-small cell lung cancer, SCC: squamous cell carcinoma. |
||||
Table II: Univariate or multivariate logistic regression analysis of distant metastasis.
|
Variables |
Univariate analysis |
Multivariate analysis |
||
|
OR (95% CI) |
p-values |
OR (95% CI) |
p-values |
|
|
Age (years): ≤65 >65 |
1.564 (0.699-3.497) - |
0.276 - |
- - |
- - |
|
Gender: Female Male |
21.968 (1.731-278.789) - |
0.017 - |
8.756 (2.120-36.171) - |
0.003 - |
|
Histopathological subtype: SCLC NSCLC/SCC/ Adenocarcinoma |
5.988 (1.460-24.549) - |
0.013 - |
6.380 (1.614-25.224) - |
0.008 - |
|
T Stage: T3-T4 T2-T1 |
0.057 (0.012-0.265) - |
<0.001 - |
0.060 (0.016-0.232) - |
<0.001 - |
|
N Stage: N(+) N0 |
1.287(0.205-8.073) - |
0.788 - |
- - |
- - |
|
Smoking status: Yes No |
0.315(0.026-3.842) - |
0.366 - |
- - |
- - |
|
Smoking pack-years: ≤66 >66 |
0.425 (0.183-0.988) - |
0.047 - |
0.440 (0.196-0.990) - |
0.047 - |
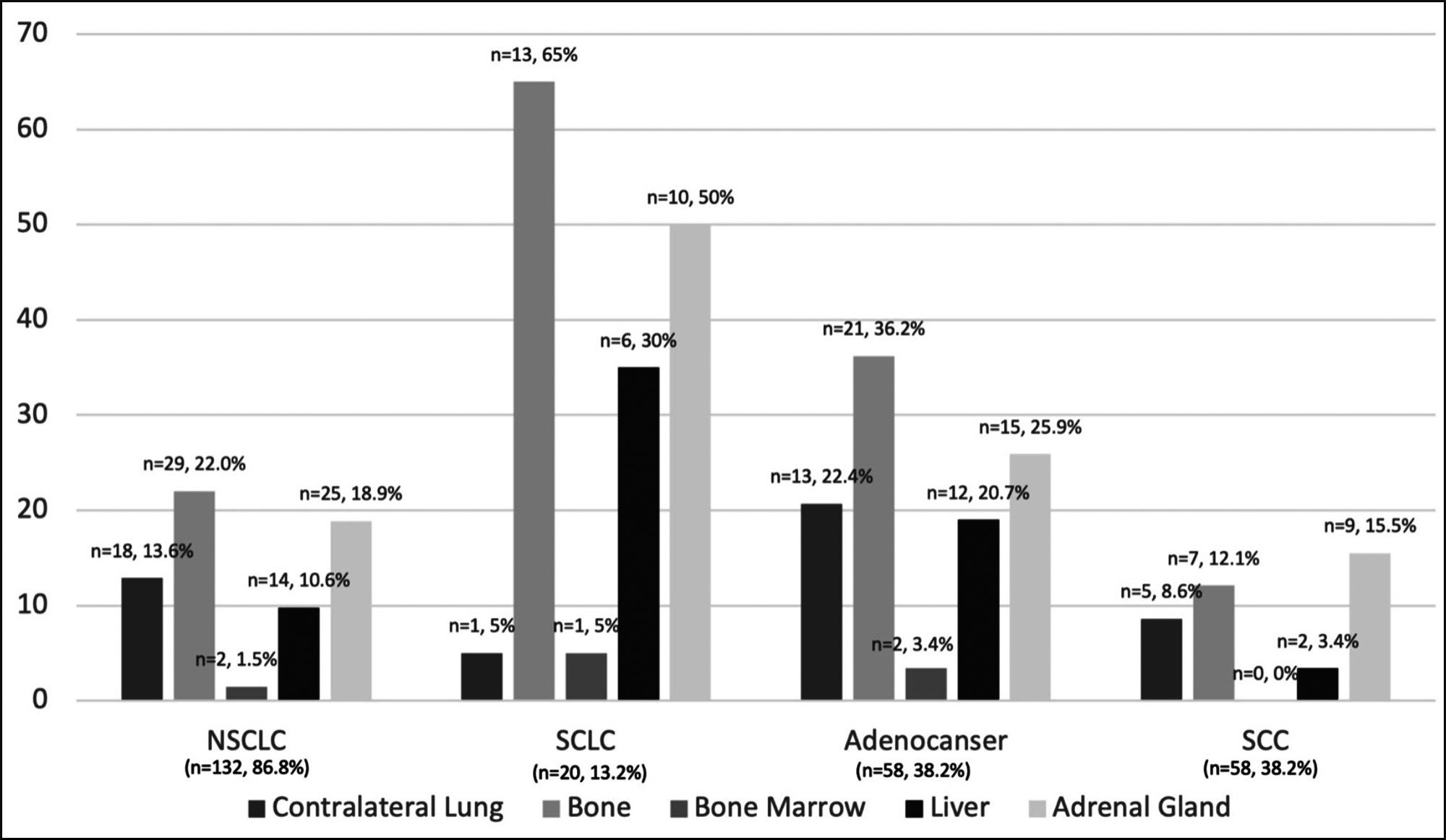 Figure 1: Distribution of distant metastasis according to histopathological subtypes.
Figure 1: Distribution of distant metastasis according to histopathological subtypes.*NSCLC includes all the histopathological subtypes of NSCLC, such as unidentified subtype, SCC, and adenocarcinoma.
In the analysis of patient’s characteristics, according to age groups, SCC was the most common subtype in patients aged >65 years (n = 36, 42.9%), and adenocarcinoma was the most common subtype in patients aged ≤65 years (n = 26, 38.2%). Although no significant difference was found between histopathological subtypes and age groups (p = 0.374), most patients with SCLC (n = 13, 65%) were diagnosed at an earlier age (≤65 years) compared with patients having other subtypes. While smoking history was similar in both age groups, smoking pack-years were significantly higher in patients aged >65 years [75 (46.3–110) vs. 48.5 (36–89), p = 0.003].
In the analysis of the distribution of TNM stages, although M1 disease was more common in the young group (n = 40, 58.8%) than in the older group (n = 37, 44%), no differences were found between TNM stages with respect to age groups (p = 0.328). Most of the patients in both age groups had stage 4 disease. According to the stages and distribution of patients by both age groups, the distribution of patients aged ≤65 years and >65 years were as follows, respectively: stage I, 3 (4.4%)–4 (4.8%); stage II, 4 (5.9%)–8 (9.5%); stage III, 21 (30.9%)–35 (41.7%); and stage IV 40 (58.8%)-37 (44%).
When distant metastasis patterns were examined, bone metastasis was the most common (n=42, 27.6%) in all patients. Only three patients (15%) with SCLC did not have distant metastasis, and 14 of the patients with distant metastasis (70%) had multiple metastatic disease. Of the patients with multiple metastatic diseases (n=46), 24 of them (52.2%) were diagnosed with adenocarcinoma, and 14 of them (30.4%) were diagnosed with SCLC. Looking at the relationship between localisations of distant metastasis with age groups, the authors found that the only contralateral lung metastasis was statistically different between the two age groups. Other organ metastases were similar in young and elderly patients. Contralateral lung metastasis was found to be significantly higher in young patients than in elderly [12 (17.6) vs 6 (7.1%), p=0.046]. A total of 31 (40.3%) patients presented with oligometastatic disease. The most common histopathological subtype was adenocarcinoma (n=14, 45.2%); and the secondly was SCC (n=13, 41.9%) in patients with oligometastasis. Distant metastasis patterns and distribution metastases are as follows: Adrenal gland 35 (23%), contraleteral lung 18 (11.8%), pleura 14 (9.2%), bone 42 (27.6%), liver 20 (13.1%), bone marrow 3 (2%), soft tissue 4 (2.6%), pancreas 3 (2%), periton 4 (2.6%), others. Twenty-one (13.8%) patients had distant metastasis with atypical localisation such as skin, testis, nasal cavity, abdominal, cervical, and axillary lymph node detected on PET/CT scan.
The histopathological subtype with the highest rate of distant metastasis was SCLC, and the histopathological subtype with the least distant metastasis was SCC (p<0.001). While the most common distant organ metastasis was the adrenal gland in the SCC subtype, the most common distant organ metastasis was the bone in the other three histopathological subtypes. Figute-1 shows the distribution of distant metastasis according to histopathological subtypes.
In the univariate and multivariate regression analyses of factors affecting distant metastasis, female gender (p = 0.017, p = 0.003), SCLC histopathological subtype (p = 0.013, p = 0.008), T3–4 disease (all p <0.001) and smoking history >66 pack-years (all p = 0.047) were found to be factors affecting distant metastasis. No relationship was found between age and distant metastasis. The univariate and multivariate analyses of factors affecting distant metastasis are given in Table II.
DISCUSSION
In this analysis of patients diagnosed with stage I–IV lung cancer, most patients had advanced stage disease confirmed by PET/CT. 18F-FDG PET/CT was performed to detect the presence of distant metastasis in all patients.
Most patients had adenocarcinoma and SCC, and 55.3% of the patients were diagnosed at age >65 years, and rates were similar to those in the literature.13 SCC was the more common subtype among older and male patients. By contrast, adenocarcinoma was more common among female patients, and SCLC was more common in patients aged ≤65 years, similar to that reported in the literature.14
In this study, differences were found between the histopathological subgroups in the distribution pattern of distant metastasis. In contrast to the study conducted by Torok et al., the most common subtype with distant metastasis was SCLC, including adenocarcinoma.15 As possible reason for this difference, while Torok et al. included only the histopathological subtypes of NSCLC, patients with both NSCLC and SCLC were included in this study.
The present results showed that T4, N2, M1 and stage 4 diseases were more common in patients with lung cancer diagnosed at age ≤ 65 years, but no difference was found. Although no difference was noted between age groups with distant metastasis, distant metastasis detection rate was more common in patients aged ≤ 65 years at the time of diagnosis, similar to literatures.14,15 The lack of difference in this study may be related to the cut-off value, as patiewwwwnts were divided into two age groups. If the cut-off value was 60 years of age instead of 65 years, perhaps, there would be a significant difference between the two age groups.
Similar to previous studies, this study showed that the most common extranodal metastatic localisation is the bone (27.6%) in all patients.14,15 According to Riihimäki et al., the most common distant organ metastasis was brain, followed by the bone.14 These results are quite comparable because the authors excluded brain metastasis.15-18 While SCC is the most common subtype that causes adrenal gland metastases, the other three subtypes cause bone metastasis most frequently in the present study. Unlike the literature, patients with SCC had more adrenal gland metastases, whereas patients with other histopathological subtypes had more bone metastases. This result, which is different from the literature, is possibly due to the difference in the distribution of the ratio of histopathological subtypes and the exclusion of central nervous system involvement in distant metastasis evaluation.14
The results of this study showed that the histological subtype, smoking intensity, T stage of the primary tumour, and gender of the patients have a significant effect on distant metastasis in lung cancer. Female gender, advanced T stage (T3–T4), high smoking pack-years (>66), presence of SCLC and adenocarcinoma histopathological subtype were found to be high-risk factors of distant metastasis. The histopathological subtype with the highest rate of distant metastasis was SCLC, and the histopathological subtype with the least distant metastasis was SCC in this study, which is the same as that in the literature13. The distribution patterns of metastases by histopathological subtypes justify the seed and soil hypothesis mentioned by Riihimäki et al.14
With improved treatment modalities and early diagnosis of patients, data on the increased cancer survival and development of distant metastasis have gained importance in recent years. Recognising patients at high risk of developing distant metastasis, especially those with lung cancer, where cancer-related deaths are the most common, the time of diagnosis is important. These patients should be screened for distant metastasis before starting treatment or surgery. PET/CT is recommended in many studies to detect occult locoregional metastasis and distant metastasis in patients with NSCLC.19 Many studies have demonstrated the success of FDG PET/CT in both NSCLC and SCLC in showing hidden metastases and in patient management.20-24 FDG PET/CT is the most appropriate imaging method for scanning the whole body at one time.
This study has a few limitations. Firstly, this study has a retrospective design, and the number of patients is limited. Thus, we could not evaluate different histopathological subtypes in separate groups. Secondly, although brain metastasis is quite common in lung cancer, the authors excluded the presence of brain metastasis because of the low sensitivity of PET/CT to detect brain metastasis. Finally, oligometastatic or multiple metastatic diseases were judged according to PET/CT findings, and histopathological sampling from metastatic foci was not performed.
CONCLUSION
Distant metastasis is frequently seen at the time of diagnosis in patients with lung cancer. In this study, age is not a risk factor in the development of distant metastasis. Therefore, in all patients with lung cancer, who are at high risk of developing distant metastasis, PET/CT should be considered, regardless of patients’ age. This study is valuable as results can contribute to the literature of knowing the distant metastasis pattern of patients with stage 4 through PET/CT, evaluating the approach to patients with multiple oligometastases and enabling systemic treatments for distant metastasis.
ETHICAL APPROVAL:
The protocol for the current study was approved by the Ethics Committee of the Recep Tayyip Erdogan University (Approval No. 2021/16), and adhered to the principles of the Declaration of Helsinki.
PATIENTS' CONSENT:
Informed consents were taken from all patients before writing the article.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
SG: Study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, reviewing the paper, advices, and final approval.
NO: Study conception and design, interpretation of data, reviewing the paper, drafting of manuscript, advices, and final approval.
REFERENCES
- Saito S, Espinoza-Mercado F, Liu H, Sata N, Cui X, Soukiasian HJ. Current status of research and treatment for non-small cell lung cancer in never-smoking females. Cancer Biol Therapy 2017; 18(6):359-68. doi: 10.1080/15384047. 2017.1323580.
- Fidler-Benaoudia MM, Torre LA, Bray F, Ferlay J, Jemal A. Lung cancer incidence in young women vs. young men: A systematic analysis in 40 countries. Int J Cancer 2020; 147(3):811-9. doi: 10.1002/ijc.32809.
- Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clinicians 2018; 68(6):394-424. doi: 10.3322/caac.21492.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: A Cancer J Clinicians 2016; 66(1):7-30. doi: 10.3322/caac.21332.
- Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, et al. Non–small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J National Comprehensive Cancer Network 2017; 15(4): 504-35. doi: 10.6004/jnccn.2017.0500.
- Coleman RE. PET in lung cancer. Journal of Nuclear Medicine. 1999; 40(5):814-20.
- Sheikhbahaei S, Mena E, Yanamadala A, Reddy S, Solnes LB, Wachsmann J, et al. The value of FDG PET/CT in treatment response assessment, follow-up, and surveillance of lung cancer. Am J Roentgenol 2017; 208(2):420-33. doi: 10.2214/AJR.16.16532.
- Smoragiewicz M, Laskin J, Wilson D, Ramsden K, Yee J, Lam S, et al. Using pet-ct to reduce futile thoracotomy rates in non-small-cell lung cancer: A population-based review. Current Oncol 2014; 21(6):e768-74. doi: 10.3747/co.21.2125.
- Hurria A, Kris MG. Management of lung cancer in older adults. CA: A Cancer J Clinicians 2003; 53(6):325-41. doi: 10.3322/canjclin.53.6.325.
- Bach PB, Kattan MW, Thornquist MD, Kris MG, Tate RC, Barnett MJ, et al. Variations in lung cancer risk among smokers. J National Cancer Institute 2003; 95(6):470-8. doi: 10.1093/jnci/95.6.470.
- Woloshin S, Schwartz LM, Welch HG. Risk charts: Putting cancer in context. J National Cancer Institute 2002; 94(11):799-804. doi: 10.1093/jnci/94.11.799.
- Milovanovic IS, Stjepanovic M, Mitrovic D. Distribution patterns of the metastases of the lung carcinoma in relation to histological type of the primary tumor: An autopsy study. Annals Thoracic Medicine 2017; 12(3):191-8. doi: 10.4103/atm.ATM_276_16.
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thoracic Oncol 2016; 11(1):39-51. doi: 10.1016/j.jtho.2015.09.009.
- Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, et al. Metastatic sites and survival in lung cancer. Lung Cancer 2014; 86(1):78-84. doi: 10.1016/j.lungcan.2014.07.020.
- Torok JA, Gu L, Tandberg DJ, Wang X, Harpole DH, Kelsey CR, et al. Patterns of distant metastases after surgical management of non–small-cell lung cancer. Clinical Lung Cancer 2017; 18(1):e57-e70. doi: 10.1016/j.cllc.2016.06. 011.
- Oikawa A, Takahashi H, Ishikawa H, Kurishima K, Kagohashi K, Satoh H. Application of conditional probability analysis to distant metastases from lung cancer. Oncol Letters 2012; 3(3):629-34. doi: 10.3892/ol.2011.535.
- Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, et al. Metastatic patterns in adenocarcinoma. Cancer 2006; 106(7):1624-33. doi: 10.1002/cncr.21778.
- Quint LE, Tummala S, Brisson LJ, Francis IR, Krupnick AS, Kazerooni EA, et al. Distribution of distant metastases from newly diagnosed non-small cell lung cancer. Ann Thoracic Surg 1996; 62(1):246-50. doi: 10.1016/0003- 4975(96) 00220-2.
- Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Bakeret S, et al. American Society of Clinical Oncology treatment of unresectable non–small-cell lung cancer guideline: Update 2003. J Clinical Oncol 2004; 22(2):330-53. doi: 10.1200/JCO.2004.09.053.
- Eschmann S, Friedel G, Paulsen F, Reimold M, Hehr T, Scheiderbauer J, et al. Impact of staging with 18 F-FDG-PET on outcome of patients with stage III non-small cell lung cancer: PET identifies potential survivors. European J Nuclear Med Molecular Imaging 2007; 34(1):54-59. doi: 10.1007/s00259-006-0197-0.
- Takahashi Y, Suzuki S, Matsutani N, Kawamura M. 18F‐fluorodeoxyglucose positron emission tomography/computed tomography in the evaluation of clinically node‐negative non‐small cell lung cancer. Thoracic Cancer 2019; 10(3): 413-20. doi: 10.1111/1759-7714.12978.
- Liu L, Ruan M, Xie W. Using F-FDG PET/CT to Diagnose and treat non-small cell lung cancer. In: Huang G Eds. Nuclear medicine in oncology. Singapore; Springer; 2019: p.25-45.
- Xu T, Zhang X, Zhang S, Liu C, Fu W, Zeng C, et al. Imaging features and prognostic value of 18F-FDG PET/CT detection of soft-tissue metastasis from lung cancer: A retrospective study. BMC Cancer 2020; 20(1):596.doi: 10.1186/s12885- 020-07080-0.
- Yu B, Zhu X, Liang Z, Sun Y, Zhao W, Chen K. Clinical usefulness of F-FDG PET/CT for the detection of distant metastases in patients with non-small cell lung cancer at initial staging: A meta-analysis. Cancer Manag Res 2018; 10:1859-64. doi: 10.2147/CMAR.S155542.