Diffusion-weighted Imaging in the Benign-malignant Differentiation of Endometrial Pathologies; Effectiveness of Visual Evaluation
By Ismail Basaran, Ferhat Cengel, Aylin Hasanefendioglu BayrakAffiliations
doi: 10.29271/jcpsp.2023.01.77ABSTRACT
Objective: To investigate the effectiveness of diffusion-weighted imaging (DWI) in the differentiation of benign and malignant endometrial pathologies by measuring the apparent diffusion coefficient (ADC) values and performing a visual evaluation.
Study Design: Cross-sectional study.
Place and Duration of Study: Department of Radiology, Gaziosmanpasa Training and Research Hospital, Istanbul, Turkey, from January 2017 to September 2019.
Methodology: The inclusion criteria were women over 45 years of age with availability of the pelvic MRI in the PACS and the presence of pathological diagnosis by endometrial D and C or hysterectomy. Exclusion criteria were patients under 45 years of age, absence of histopathological results, hematoma or intrauterine device in the endometrial cavity, and endometrial thickness less than 5 mm. Quantitative ADC values were measured on ADC maps created automatically based on DWI data. DWI and ADC maps were also evaluated visually to differentiate between benign and malignant pathologies.
Results: Endometrial pathology was detected in a total of 88 patients, 36 of which were malignant and 52 benign lesions. The mean ADC values for both observers and the sensitivity and specificity in the differentiation of benign and malignant endometrial lesions were 81% - 75% and 88% - 90%, respectively (p<0.001 for both observers). The visual evaluation of b values and ADC map on DWI was also performed together, and the sensitivity and specificity in the differentiation of benign and malignant endometrial lesions were 81% - 86% and 69% - 56% for both observers, respectively (p <0.001 for both observers).
Conclusion: ADC measurements are useful in differentiating benign and malignant endometrial pathologies, and visual evaluation of the ADC map and b values in DWI together also provides positive results.
Key Words: Diffusion-weighted imaging, Endometrial pathologies, ADC, Visual evaluation.
INTRODUCTION
Uterine cavity tumours include a wide range of benign and malignant tumours, such as endometrial hyperplasia, submucosal fibroids, endometrial polyps, and endometrial cancer, causing pathological appearance in the uterus. These pathologies, and sometimes even the normal endometrium, may display features that can be confused with each other.1
It has been claimed that the diagnosis of endometrial carcinoma can be made with sonographic findings such as increased endometrial thickness and contour irregularity.
However, these findings may be insufficient to distinguish endometrial carcinoma from some benign pathologies such as physiological proliferation, hyperplasia, and polyps.2 Magnetic resonance imaging (MRI) is more sensitive than ultrasonography (USG), and become a popular method in evaluating the endometrial cavity and soft tissue, yet it also has limitations in separating benign and malignant lesions.3 Endometrial biopsy and curettage are seen as the most reliable diagnostic methods, it may result in complications and patient non-compliance due to this being an invasive intervention. It was reported 2-28% of the cases cannot be diagnosed for these reasons.4
Recently, many studies using diffusion-weighted imaging (DWI) in the differentiation of benign and malignant endometrial pathologies have been conducted and positive results have been reported in this regard. It has been reported that the apparent diffusion coefficient (ADC) values measured in these studies can be useful in the differentiation of benign and malignant lesions.1,5-8 Similar studies have been conducted in the literature in which DWI signals and ADC maps were visually evaluated in the differentiation of benign and malignant liver masses.9,10 To the best of the authors’ knowledge, this is the first study to utilise visual evaluation with the combined use of the multi- b values of DWI and the signal in the ADC map in the characterisation of endometrial pathologies. The aim of this study was to investigate whether endometrial benign-malignant pathologies can be differentiated by visual evaluation of diffusion-weighted MRI and ADC map, as well as by measuring ADC.
METHODOLOGY
After obtaining approval from the hospital ethics committee for this study (Decision No: 104, dated: 24/07/2019), patients who underwent pelvic MRI between January 2017 and September 2019 in the Health Sciences University, Radiology Clinic of Gaziosmanpaşa Training and Research Hospital, were scanned retrospectively. Among the perimenopausal-postmenopausal (over 45 years old) patients with a lesion in the endometrium or with an endometrial wall thickness greater than 5 mm in pelvic MRI examinations, a total of 101 patients with pathology results in the hospital system were included in the study. Of these patients, 6 patients were excluded from the study because the biopsy results were non-diagnostic, and 4 patients were excluded because DWI was of insufficient quality. In addition, 3 patients with hematoma in the endometrial cavity or intrauterine device were excluded from the study. The evaluation started with a total of 88 patients who met the inclusion criteria. Patients with a maximum of 2 months between the imaging dates and the biopsy dates were included in the study. According to the pathology results, the diagnosis was made by hysterectomy in 25 patients and by endometrial dilatation/curettage (D and C) biopsy in the remaining 63 patients.
MRI examination of all patients was performed using a 1.5T MRI device GE Signa Explorer 1.5 Tesla (GE Medical System, Milwaukee, WI, USA) and GE 1.5 T HD 8 channel Body array coil. MRI protocol included routine pelvic images before intravenous (IV) contrast: T2W fr-FSE without fat suppression in axial, sagittal, and coronal planes, T2W fr-FSE FAT with fat suppression in axial plane, T1W FSE-ARC without fat suppression in axial plane, LAVA without fat suppression in axial plane, LAVA with fat suppression in axial, sagittal, and coronal planes, and DWI images of the pelvis. DWIs were obtained in the axial plane by applying diffusion sensitive gradients in all three directions (x, y, z) using the breath-hold SSh-TSE-EPI sequence, with 4 different b values (b=0, 50, 800, and 1000 s/mm2). ADC maps of isotropic images were created automatically by the device. The ADC values of the masses were created on the second console of the MRI using the ADC maps created with the GE Signa Explorer 24 software.
Two radiologists with 8 years of experience in abdominal radiology (F.C) and 4 years of radiology experience (I.B) who were blinded to the pathology results independently evaluated the images. Images were transferred to the GE Signa Explorer software workstation for evaluation. ADC measurement was made from the hyperintense area of the DWI at high b values (b=1000 s/mm2) and the corresponding hypointense area in the ADC map in malignant lesions. ADC measurement was made from the hyperintense area in the ADC map in benign lesions (Figures 1 and 2). During the measurement, ADC measurement was performed by placing the largest possible ROI (region of interest) from the solid areas of the lesion that did not contain the cystic-necrotic or hemorrhagic component. The localisations and structures of the masses were verified in axial, coronal, and sagittal T2W, and contrasted T1 images. ADC measurements were made by radiologists at different times, independently of each other, and averaged based on measurements from 3 different locations.
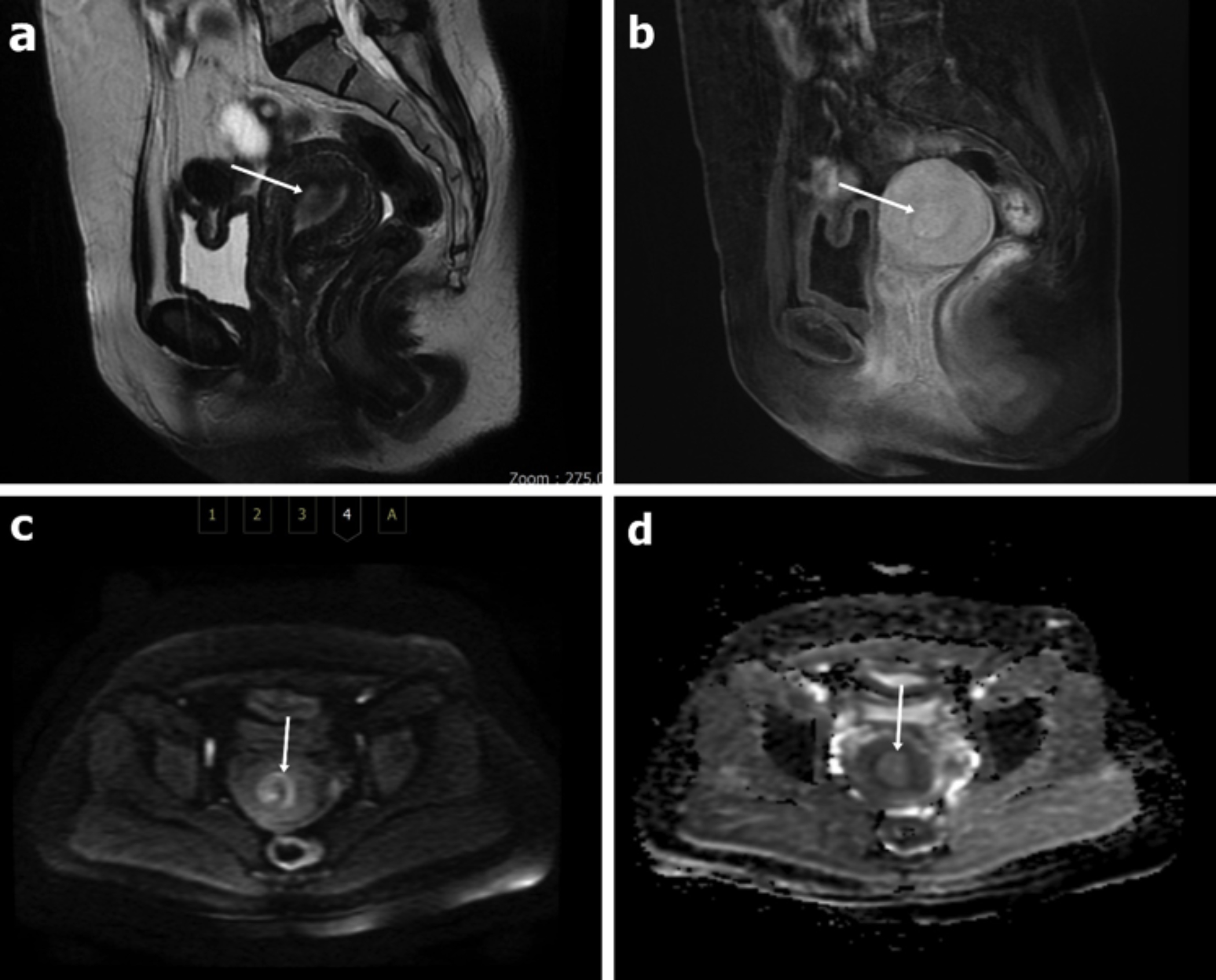 Figure 1: A 46-year-old patient with a diagnosis of an endometrial polyp. The appearance of the lesion in the sagittal plane in the T2W sequence without fat suppression (a), in the sagittal LAVA sequence with IV contrast (b). In DWI, it is observed as hypointense at high b values (b=1000 s/mm2) (c) and as hyperintense-isointense on the ADC map (d). The ADC values were measured as 1.42x10-3 mm2 /s and 1.66x10-3 mm2 /s for both observers, respectively. Both observers evaluated it as benign.
Figure 1: A 46-year-old patient with a diagnosis of an endometrial polyp. The appearance of the lesion in the sagittal plane in the T2W sequence without fat suppression (a), in the sagittal LAVA sequence with IV contrast (b). In DWI, it is observed as hypointense at high b values (b=1000 s/mm2) (c) and as hyperintense-isointense on the ADC map (d). The ADC values were measured as 1.42x10-3 mm2 /s and 1.66x10-3 mm2 /s for both observers, respectively. Both observers evaluated it as benign.
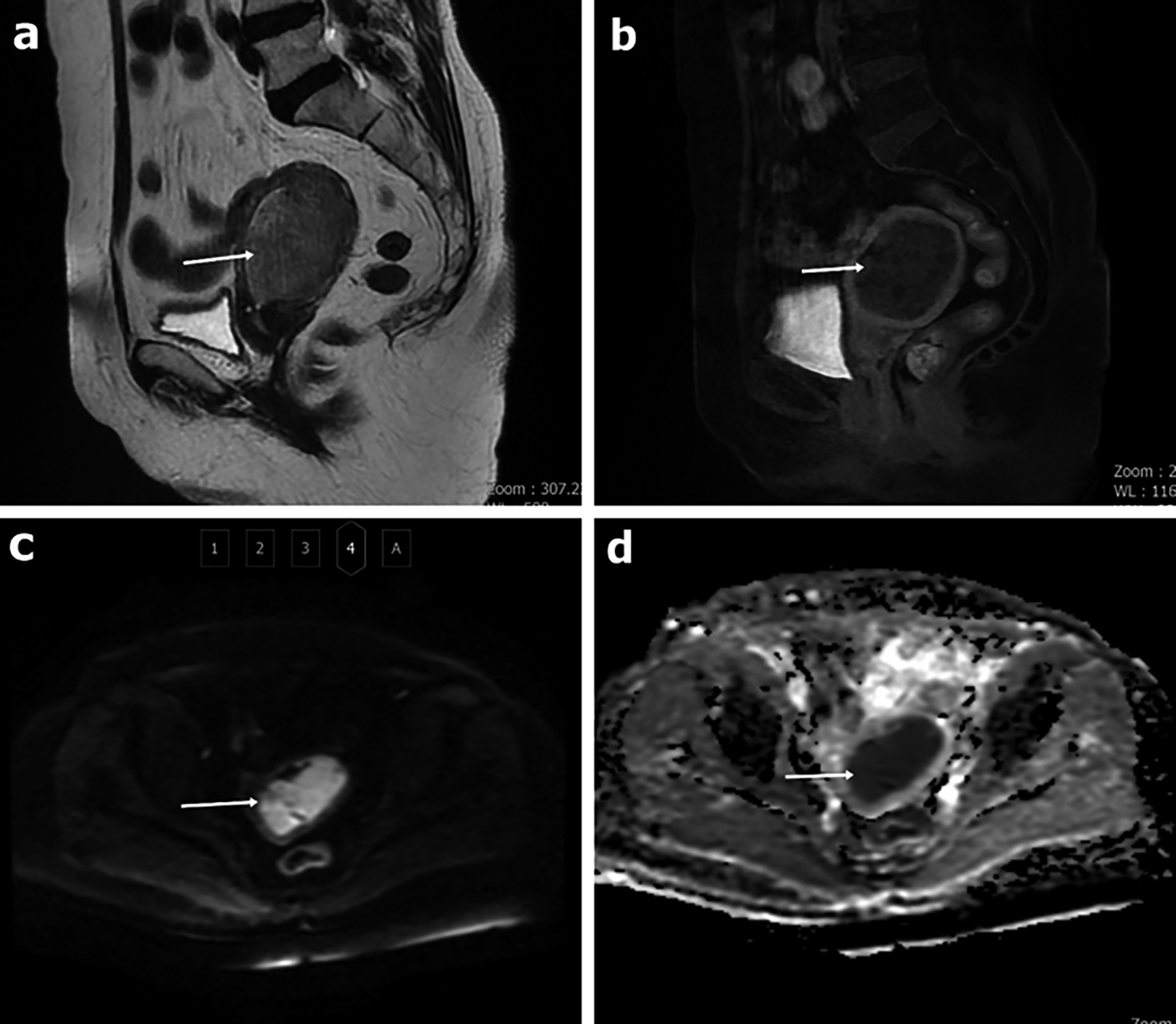 Figure 2: A 69-year-old patient with a diagnosis of endometroid-type adenocarcinoma. The appearance of the mass in the sagittal plane in the T2W sequence without fat suppression (a), in the sagittal LAVA sequence with IV contrast (b). In DWI, it is observed as hyperintense at high b values (b=1000 s/mm2) (c) and hypointense on the ADC map (d). The mean ADC value was measured as 0.73 x10-3 mm2/s for both observers. Both observers evaluated it as malignant.
Figure 2: A 69-year-old patient with a diagnosis of endometroid-type adenocarcinoma. The appearance of the mass in the sagittal plane in the T2W sequence without fat suppression (a), in the sagittal LAVA sequence with IV contrast (b). In DWI, it is observed as hyperintense at high b values (b=1000 s/mm2) (c) and hypointense on the ADC map (d). The mean ADC value was measured as 0.73 x10-3 mm2/s for both observers. Both observers evaluated it as malignant.
Four weeks after ADC measurements were made, two radiologists independently evaluated the b values in DWI and ADC images on PACS images, without knowing the pathology results and ADC measurements. Lesions with signal loss in increasing b values (b=0, 50, 800, and 1000 s/mm2) on DWI images and hyperintense lesions relative to the myometrium on the corresponding ADC map were classified as benign, lesions with no signal loss or increased signal in increasing b values, and appeared hypointense compared to the myometrium on the corresponding ADC map were classified as malignant (Figures 1 and 2).
Normality was checked by Shapiro Wilk test, histogram, Q-Q plot, and box plot graphs. Data were presented as mean, standard deviation, median, minimum, maximum, frequency, and percentage. Variables between the two groups were analysed with the Mann-Whitney U test. A receiver operating characteristic (ROC) analysis was conducted. The area under the curves (AUCs) was measured for each observer. Using the DeLong method, it was checked whether there was a statistically significant difference between the AUC values of both observers. Diagnostic tests (sensitivity, specificity, PPD, NPD, Accuracy) and 95% confidence intervals were given. Intraclass correlation coefficients with 95% CI were calculated to assess interobserver agreement. The significance level was taken as p<0.05 and two-tailed. Analyses were performed using the NCSS 10 (2015. Kaysville, Utah, USA) software program.
RESULTS
The mean age of the patients was 59 ± 10.2 (45-89) years. Of the 88 patients included in the study, 36 (41%) had malignant and 52 (59%) had benign pathology. Of the malignant tumours, 31 (86%) were endometrial adenocarcinoma (histological grade; grade 1 in 10 patients, grade 2 in 17 patients, grade 3 in 4 patients), 2 (6%) were squamous cell carcinoma (SCC), 2 (6%) were serous adenocarcinoma, and 1(3%) was clear cell adenocarcinoma. In a total of 52 benign lesions, 23 (44%) were endometrial polyps, 15 (29%) were endometrial hyperplasia, 3 (6%) were intraepithelial neoplasia-carcinoma in situ, 9 (17%) were normal endometrial tissue in the secretory phase, and 2 (4%) were chronic endometritis.
In the present study, mean ADC measurement values in malignant and benign lesions were 0.98±0.3 and 1.43±0.33 x10-3 mm2/s for the 1st observer and 0.94± 0.22 and 1.46±0.3 x10-3 mm2/s for the 2nd observer, respectively (Table I). Median ADC measurement values in malignant and benign lesions were 0.88 (0.60-2.05) and 1.41 (0.73-2.16) x10-3 mm2/s for the 1st observer and 0.88 (0.68-1.66) and 1.46 (0.87-1.97) x10-3 mm2/s for the 2nd observer, respectively (Table I). The mean ADC values of malignant lesions for both observers were significantly lower than benign lesions (p<0.001 for both observers). AUCs values were measured as 0.86 (0.78-0.95) and 0.92 (0.86-0.98) for observers 1 and 2, respectively. There was no statistically significant difference between the AUCs values (p=0.12). As a result of the ROC analysis, the ADC cut-off values for the differentiation of benign and malignant lesions for both observers were found to be 1.048 and 1.046, respectively. The sensitivity, specificity, PPD, NPD, and accuracy of benign-malignant differentiation of endometrial lesions with mean ADC value was 81% (64-92)- 75% (58-88), 88% (77-96)- 90% (79-97), 83% (69-91)- 84% (70-93), 87% (77-93)- 84% (75-90) and 85% (76-92)- 84% (75-91) for observer 1 and observer 2, respectively (Table II). The intraclass correlation coefficient for the characterisation of endometrial pathologies with mean ADC measurement was 0.84 (95% CI, 0.77–0.89) showing good agreement between the observers.
Table I: Mean ADC (x10-3) values for observers.
|
Lesion |
Observer 1 |
Observer 2 |
|
Benign (n=52) |
1.43 ±0.33 1.41 (0.73-2.16) |
1.46±0.3 1.46 (0.87-1.97) |
|
Malignant (n=36) |
0.98 ±0.3 0.88 (0.60-2.05) |
0.94± 0.22 0.88 (0.68-1.66) |
|
p-value |
<0.001* |
<0.001* |
|
Data in the first line are means±standard deviations, and data in the second line are the medians (minimum-maximum). * p-value of Mann-Whitney test. |
||
Table II: The diagnostic performance of observers with ADC measure-ment.
|
Diagnostic performance (%) |
Observer 1 |
Observer 2 |
|
Sensitivity (95% CI) |
81 (64-92) |
75 (58-88) |
|
Specificity (95% CI) |
88 (77-96) |
90 (79-97) |
|
PPV (95% CI) |
83 (69-91) |
84 (70-93) |
|
NPV (95% CI) |
87 (77-93) |
84 (75-90) |
|
Accuracy (95% CI) |
85 (76-92) |
84 (75-91) |
|
ADC; apparent diffusion coefficient, CI; confidence interval, PPV; positive predictive value, NPV; negative predictive value. |
||
Table III: The diagnostic performance of observers with visual evaluation.
|
Diagnostic performance (%) |
Observer 1 |
Observer 2 |
|
Sensitivity (95% CI) |
81 (64-92) |
86 (71-95) |
|
Specificity (95% CI) |
69 (55-81) |
56 (41-70) |
|
PPV (95% CI) |
64 (54-74) |
57 (49-65) |
|
NPV (95% CI) |
84 (72-91) |
85 (71-93) |
|
Accuracy (95% CI) |
74 (63-83) |
68 (57-78) |
|
CI; confidence interval, PPV; positive predictive value, NPV; negative predictive value. |
||
The sensitivity, specificity, PPD, NPD, and accuracy of benign-malignant differentiation of endometrial lesions with DWI visual evaluation (by evaluating b values and ADC maps together in DWI) was 81% (64-92)- 86% (71-95), 69% (55-81)- 56% (41-70), 64% (54-74)- 57% (49-65), 84% (72-91) - 85% (71-93), and 74% (63-83) - 68% (57-78) for observer 1 and observer 2, respectively (Table III). True positive results for observers 1 and 2 on the visual assessment are 36/52 and 29/52 for benign and 29/36 and 31/36 for malignant pathologies respectively (all p<0.001). In the characterisation of endometrial pathologies by DWI visual evaluation without ADC measurement, the agreement between the two observers was measured as 0.7 ± 0.07 (p<0.001), and the agreement rate was found to be high.
DISCUSSION
Many promising studies have been published recently in the differentiation of benign and malignant endometrial pathologies using DWI.5-8,11-18 In the present study, the authors evaluated various endometrial pathologies quantitatively on the ADC map and visually on DWI-ADC.
The quantitative results with ADC measurement were similar to the previously published studies.1,6-8 In the study of Bakır et al. with 140 patients, 61 of whom were malignant and 79 were benign, the mean ADC values were found to be 0.8±0.1 x10-3 mm2/s in the malignant group and 1.4±0.2 x10-3 mm2/s in the benign group.6 In the study of Tamai et al. with 18 patients with endometrial cancer (histological grades; 10 patients grade 1, 4 patients grade 2, 4 patients grade 3), mean ADC values were measured as 0.88±0.16 x10-3 mm2/s in the malignant group and 1.53±0.1 x10-3 mm2/s in the benign group.19 It was suggested that there may be a correlation between low ADC values and increasing histological grade in endometrial cancer. In addition, in the study by Yan et al. with 98 patients with endometrial cancer (80 patients with endometrial type, 18 patients with non-endometroid type), 67 of the patients had high-grade and 31 patients had low-grade tumours.20 The mean ADC values of low-grade tumours were reported as 0.96 ± 0.23, and that of high-grade tumours as 0.8±0.11. In the present study, the mean ADC values measured in the malignant patient group were relatively higher compared to the literature, and the authors think that this is due to the high ratio of low-grade lesions in this study.
In the study by Takeuchi et al. with 67 patients, 45 of whom had malignant and 22 of whom had benign lesions, ADC values were 0.84 ± 0.19 and 1.58 ± 0.36 x10-3 mm2/s for endometrial malignant and benign lesions, respectively (p <0.001).1 In their study, a cut-off value of 1.2 x10-3 mm2/s for malignant lesions, had the sensitivity, specificity, PPD, and NPD values to be 96%, 95%, 98%, and 91%, respectively. In the study by Karakaş et al. with 32 patients, 10 of whom had malignant and 22 of whom had benign lesions, the ADC values in malignant and benign lesions were 0.73±0.15 x10-3 mm2/s and 1.28±0.37 x10-3 mm2/s, respectively.8 Cut-off value of 0.91 x10-3 mm2/s for malignant lesions found a sensitivity value of 90% and a specificity value of 82% in lesion characterisation.8 In the study by Keçeci et al., the mean ADC value in malignant and benign lesions was 0.94±0.18 x10-3 mm2/s and 1.45±0.22 x10-3 mm2/s, respectively, and the sensitivity and specificity values were 86% and 93%, respectively at the cut-off value of 1.1 x10-3 mm2/s for malignant lesions.21 In the study by Fujii et al., ADC values were measured as 0.94±0.19 x10-3 mm2/s and 1.44±0.34 x10-3 mm2/s for malignant and benign lesions, respectively, and when they took the cut-off value of 1.15 x10-3 mm2/s for malignant lesions, the sensitivity, specificity and accuracy levels were found as 85%, 100%, and 92%, respectively.7 In the present study, when the ADC cut-off values were taken as 1.048 and 1.046 for both observers, respectively, the sensitivity, specificity, PPD, NPD, and accuracy of the ADC values for the two observers in the differentiation of benign and malignant endometrial lesions was 81% - 75%, 88% - 90%, 83% - 84%, 87 - 84%, and 85% - 84%, respectively (p <0.001 for both observers). Except for Karakaş et al.,8 the cut-off value is consistent with other studies in the literature. In the study by Karakaş et al., the cut-off value was determined as 0.91 x10-3 mm2/s,8 which differs from the present and other studies in the literature. This may have resulted from the submucosal myoma patients included in the benign group, or the ratio of high-grade lesions in the malignant patient group in the study of Karakaş et al.8 In the present study, ADC cut-off value (except for Karakaş et al.8) and sensitivity, specificity, PPD, NPD, accuracy rates in the characterisation of endometrial lesions using ADC measurements were found to be similar and statistically significant.
In the double-blinded study by Kierans et al.,5 endometrial pathologies were visually evaluated according to whether there was an increase in signal at high b values in DWI. For observer 1, 20% of benign lesions and 82% of malignant lesions, and for observer 2, 20% of benign lesions and 94% of malignant lesions showed an increased signal of high b values. With these data, sensitivity, specificity, PPD, NPD, and accuracy values for both observers in the differentiation of benign and malignant endometrial lesions using increased b values were 82%-94%, 80%-80%, 67-70%, 90-97%, and 81%-85%, respectively (p<0.001 for both observers). In the same study, a separate visual evaluation was conducted according to whether there was a signal loss in the lesion by taking the normal myometrium as a reference in the ADC map. For observer 1, 40% of benign lesions and 82% of malignant lesions, and for observer 2, 20% of benign lesions and 94% of malignant lesions showed a signal loss in the ADC map. The sensitivity, specificity, PPD, NPD, and accuracy values for both observers in the differentiation of benign and malignant endometrial lesions with signal loss in the ADC map were 88% - 94%, 60% - 80%, 52-70%, 91-97%, and 69%-85%, respectively (p <0.001 for both observers). Bakir et al. evaluated endometrial lesions visually only on the basis of high b-value (b=1000 s/mm2) signal features and evaluated 69.8% of polyps as hypointense-isointense, and 100% of malignant and other benign groups as hyperintense.6 In addition, Tamai et al. reported in their study that malignant lesions and normal endometrial tissue were observed as hyperintense at high b (b=1000 s/mm2) values, and therefore they could not be used in lesion characterisation.19 These data contradict the high sensitivity, specificity, PPD, NPD, and accuracy rates by Kierans et al.5 in the differentiation of malignant-benign lesions using high b values. The authors also have the opinion that using b values alone in DWI is not sufficient for lesion characterisation, and it would be more appropriate to visually evaluate ADC values in combination with b values. In the characterisation of endometrial pathologies, b values on DWI and signals on the ADC map were visually evaluated qualitatively and quantitatively with signal measurement on the ADC map, and benign-malignant differentiation could be made with both methods (p<0.001 for both observers and both evaluations).
There are certain limitations of the present study. First, the study design was retrospective and was conducted in a single centre. Subtypes other than endometrioid-type adenocarcinoma were very few in malignant lesions, which prevented us from comparing malignant lesions among themselves. Among the benign lesions, we had patients who were pathologically diagnosed with D&C. This biopsy method is prone to sampling errors.4,22
CONCLUSION
According to the results of the present study, benign-malignant differentiation of endometrial pathologies can be made with ADC measurements. Similarly, visual evaluation of b values in DWI and of the ADC map gave positive results in the differentiation of benign and malignant lesions. Characterisation of endometrial pathologies can be performed by visual evaluation of DWI without ADC measurement.
ACKNOWLEDGEMENT:
The authors would like to thank Sevim Purisa, PhD, for statistical data support.
ETHICAL APPROVAL:
The study was approved by the Institutional Ethics Committee of Gaziosmanpasa Training and Research Hospital (Decision No. 104, dated: 24/07/2019), and ethical approvals were obtained prior to initiation of the research work.
PATIENTS’ CONSENT:
The need for signed informed consent was waived due to the retrospective nature of the study.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
IB: Study design, data collection, literature review, and writing.
FC: Study design, data collection, data analysis, statistical analysis, literature review, and discussion.
AHB: Data collection, data analysis, literature review, and discussion.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Takeuchi M, Matsuzaki K, Nishitani H. Diffusion-weighted magnetic resonance imaging of endometrial cancer: Differentiation from benign endometrial lesions and pre-operative assessment of myometrial invasion. Acta Radiol 2009; 50(8):947-53. doi:10.1080/028418509 03099981.
- Grasel RP, Outwater EK, Siegelman ES, Capuzzi D, Parker L, Hussain SM. Endometrial polyps: MR imaging features and distinction from endometrial carcinoma. Radiology 2000; 214(1):47-52. doi:10.1148/radiology.214.1.r00ja3647.
- Shen SH, Chiou YY, Wang JH, Yen MS, Lee RC, Lai CR, et al. Diffusion-weighted single-shot echo-planar imaging with parallel technique in assessment of endometrial cancer. Am J Roentgenol 2008; 190(2):481-8. doi:10.2214/AJR. 07.2155.
- Gurkan Zorlu C, Omer C, Isik AZ, Kutluay L, Kuscu E. Accuracy of pipelle endometrial sampling in endometrial carcinoma. Gynecol Obstet Invest 1994; 38(4):272-5. doi:10.1159/000292495.
- Kierans AS, Bennett GL, Haghighi M, Rosenkrantz AB. Utility of conventional and diffusion-weighted MRI features in distinguishing benign from malignant endometrial lesions. Eur J Radiol 2014; 83(4):726-32. doi:10.1016/j.ejrad. 2013.11.030.
- Bakir B, Sanli S, Bakir VL, Ayas S, Yildiz SO, Iyibozkurt AC, et al. Role of diffusion weighted MRI in the differential diagnosis of endometrial cancer, polyp, hyperplasia, and physiological thickening. Clin Imaging 2017; 41:86-94. doi:10.1016/j.clinimag.2016.10.016.
- Fujii S, Matsusue E, Kigawa J, Sato S, Kanasaki Y, Nakanishi J, et al. Diagnostic accuracy of the apparent diffusion coefficient in differentiating benign from malignant uterine endometrial cavity lesions: Initial results. Eur Radiol 2008; 18(2):384-9. doi:10.1007/s00330-007-0769-9.
- Karakas O, Karakas E, Dogan F, Kilicaslan N, Camuzcuoglu A, Incebiyik A, et al. Diffusion-weighted MRI in the differential diagnosis of uterine endometrial cavity tumors. Wien Klin Wochenschr 2015; 127(7-8):266-73. doi:10. 1007/s00508-015-0709-7.
- Battal B, Kocaoglu M, Akgun V, Karademir I, Deveci S, Guvenc I, et al. Diffusion-weighted imaging in the characterization of focal liver lesions: Efficacy of visual assessment. J Comput Assist Tomogr 2011; 35(3):326-31. doi:10.1097/RCT.0b013e318216efeb.
- Girometti R, Del Pin M, Pullini S, Cereser L, Como G, Bazzocchi M, et al. Accuratezza dell’analisi visiva vs. la quantificazione del coefficiente di diffusione apparente nella differenziazione con imaging pesato in diffusione fra lesioni focali epatiche benigne solide e maligne. Radiol Medica 2013; 118(3):343-55. doi:10.1007/s11547-012- 0873-z.
- Liu J, Yuan F, Wang S, Chen X, Ma F, Zhang G, et al. The ability of ADC measurements in the assessment of patients with stage I endometrial carcinoma based on three risk categories. Acta radiol 2019; 60(1):120-8. doi:10.1177/ 0284185118768105.
- Çavusoğlu M, Ciliz DS, Ozsoy A, Duran S, Elverici E, Atalay CR, et al. Diffusion-weighted MRI of postmenopausal women with vaginal bleeding and endometrial thickening: Differentiation of benign and malignant lesions. J Belgian Soc Radiol 2016; 100(1). doi:10.5334/jbr-btr.1118.
- Moharamzad Y, Davarpanah AH, Yaghobi Joybari A, Shahbazi F, Esmaeilian Toosi L, Kooshkiforooshani M, et al. Diagnostic performance of apparent diffusion coefficient (ADC) for differentiating endometrial carcinoma from benign lesions: A systematic review and meta-analysis. Abdom Radiol 2021; 46(3):1115-28. doi:10.1007/s00261- 020-02734-w.
- Elsammak A, Shehata SM, Abulezz M, Gouhar G. Efficiency of diffusion weighted magnetic resonance in differentiation between benign and malignant endometrial lesions. Egypt J Radiol Nucl Med 2017; 48(3):751-9. doi:10.1016/j.ejrnm. 2017.02.008.
- Mansour TMM, Ahmed YAA aal, Ahmed GAER. The usefulness of diffusion-weighted MRI in the differentiation between focal uterine endometrial soft tissue lesions. Egypt J Radiol Nucl Med 2019; 50(1). doi:10.1186/s43055- 019-0076-x.
- Wang X, Zhao Y, Hu Y, Zhou Y, Ye X, Liu K, et al. Evaluation and validation of the diagnostic value of the apparent diffusion coefficient for differentiating early-stage endo-metrial carcinomas from benign mimickers at 3T MRI. Oncotarget 2017; 8(28):46390-7. doi:10.18632/ oncotarget.18553.
- Keriakos NN, Darwish E. Diffusion weighted imaging in suspicious uterine tumors; how efficient is it?. Egypt J Radiol Nuclear Med 2018; 49(3):838-45. doi.org/10.1016/j. ejrnm.2018.04.003.
- Shen Y, Lv F, Xiao Z, Bi Q. Utility of the relative apparent diffusion coefficient for preoperative assessment of low risk endometrial carcinoma. Clin Imaging 2019; 56:28-32. doi:10.1016/j.clinimag.2019.03.001.
- Tamai K, Koyama T, Saga T, Umeoka S, Mikami Y, Fujii S, et al. Diffusion-weighted MR imaging of uterine endometrial cancer. J Magn Reson Imaging 2007; 26(3):682-7. doi:10. 1002/jmri.20997.
- Yan B, Zhao T, Liang X, Niu C, Ding C. Can the apparent diffusion coefficient differentiate the grade of endometrioid adenocarcinoma and the histological subtype of endometrial cancer? Acta Radiol 2018; 59(3):363-70. doi:10.1177/0284185117716198.
- Kececi IS, Nural MS, Aslan K, Danacl M, Kefeli M, Tosun M. Efficacy of diffusion-weighted magnetic resonance imaging in the diagnosis and staging of endometrial tumors. Diagn Interv Imaging 2016; 97(2):177-86. doi:10.1016/j.diii.2015. 06.013.
- Ben-Baruch G, Seidman DS, Schiff E, Moran O, Menczer J. Outpatient endometrial sampling with the pipelle curette. Gynecol Obstet Invest 1994; 37(4):260-2. doi:10.1159/ 000292573.