Differentiating Clinical Characteristics Between Necrotizing Enterocolitis and Food Protein-induced Enterocolitis When Both have Pneumatosis Intestinalis: A Single-centre Study
By Ya Hu1, Ziyu Hua2, Kaizhen Liu3, Hong Wei4Affiliations
doi: 10.29271/jcpsp.2022.05.646ABSTRACT
Objective: To compare the clinical characteristics of necrotizing enterocolitis (NEC) and food protein-induced enterocolitis (FPIES) when both have pneumatosis intestinalis (PI) and to identify them.
Study Design: Analytical study.
Place and Duration of Study: Department of Neonatology, Children’s Hospital of Chongqing Medical University, Chongqing, China, from January to December 2019.
Methodology: Medical data of neonates, who were diagnosed with NEC (Bell's Stage ≥2a) or FPIES, were retrospectively evaluated. All included infants had abdominal radiographic PI positive. According to the infants’ diagnoses, they were classified into groups NEC and FPIES. The clinical characteristics of NEC and FPIES were compared to find the differences.
Results: A total of 293 infants were included, of which 205 were diagnosed with NEC and other 88 were FPIES. NEC was characterised by low birth weight (BW), gestational age (GA) and onset time; NEC had higher rates of mother's antenatal steroid therapy, formula feeding, sepsis, and anemia. NEC and FPIES both had a set of similar signs and symptoms which varied depending on the severity of the disease, except for abdominal tenderness and absent bowel sounds only observed in NEC. The rates of continuous elevated C-reactive protein (CRP) and thrombocytopenia were also higher in NEC than in FPIES (p<0.05).
Conclusion: When PI-positive, although infants diagnosed with NEC or FPIES lack specific signs and symptoms, there are still clinical characteristics that need to be focused on: risk factors (BW, GA, onset time, mother's antenatal steroid therapy, formula feeding, sepsis, and anemia), abdominal signs (abdominal tenderness and absent bowel sounds), the results of CRP and platelet, which may help clinicians to identify them.
Key Words: Necrotizing enterocolitis, Food protein-induced enterocolitis syndrome, Pneumatosis intestinalis, Neonate.
INTRODUCTION
Necrotizing enterocolitis (NEC) is one of the most severe diseases of preterm neonates and has a high mortality rate of about 1-7.7%.1,2 In the neonatal period, pneumatosis intestinalis (PI) is one of the most important radiographic findings of NEC based on Bell's staging standards.3 But this radiological finding is not specific to NEC alone as it also occurs in other diseases.
Given the significant morbidity, and mortality associated with NEC, neonates with radiological PI-positive are occasionally misdiagnosed and treated as NEC.
In clinical practice, it is not rare that food protein-induced enterocolitis (FPIES) is misdiagnosed as NEC when having a radiologic finding of PI.4,5 The clinical presentations, laboratory findings, and abdominal imaging of NEC overlap with those of FPIES.4 Furthermore, oral food challenge, the gold standard for FPIES, is not routinely performed in neonates. Therefore, it is very difficult to completely distinguish between FPIES and NEC in the neonatal period with similar clinical manifestations and imaging results.6 Most of them are discharged with the diagnosis of suspected NEC or suspected FPIES.
However, the management, morbidity, and mortality between NEC and FPIES are vastly different. Once misdiagnosed as NEC, neonates with FPIES are given antibiotics and fasting, which may prolong the time of parenteral nutrition, increase unnecessary antibiotic use or increase the risk of catheter-related infections as well as radiation exposure for repeated abdominal radiography. It is important to discern suspected FPIES from classic NEC with similar radiographic findings especially PI positive to make a definitive diagnosis and provide correct management.
The aim of this study was to compare the clinical characteristics of necrotizing enterocolitis and food protein-induced enterocolitis when both have pneumatosis intestinalis and enable clinicians to correctly identify them and manage PI.
METHODOLOGY
This study was conducted as a retrospective, single-centre, analytical study. Inclusion involved infants who were diagnosed with NEC (Bell's Stage ≥2a) or FPIES with abdominal radiographic PI-positive admitted to the Children’s Hospital of Chongqing Medical University (CHCMU) between January and December 2019. According to the infants' diagnoses, they were classified into group NEC and FPIES. The clinical characteristics of groups NEC and FPIES were compared to find the differences in this study.
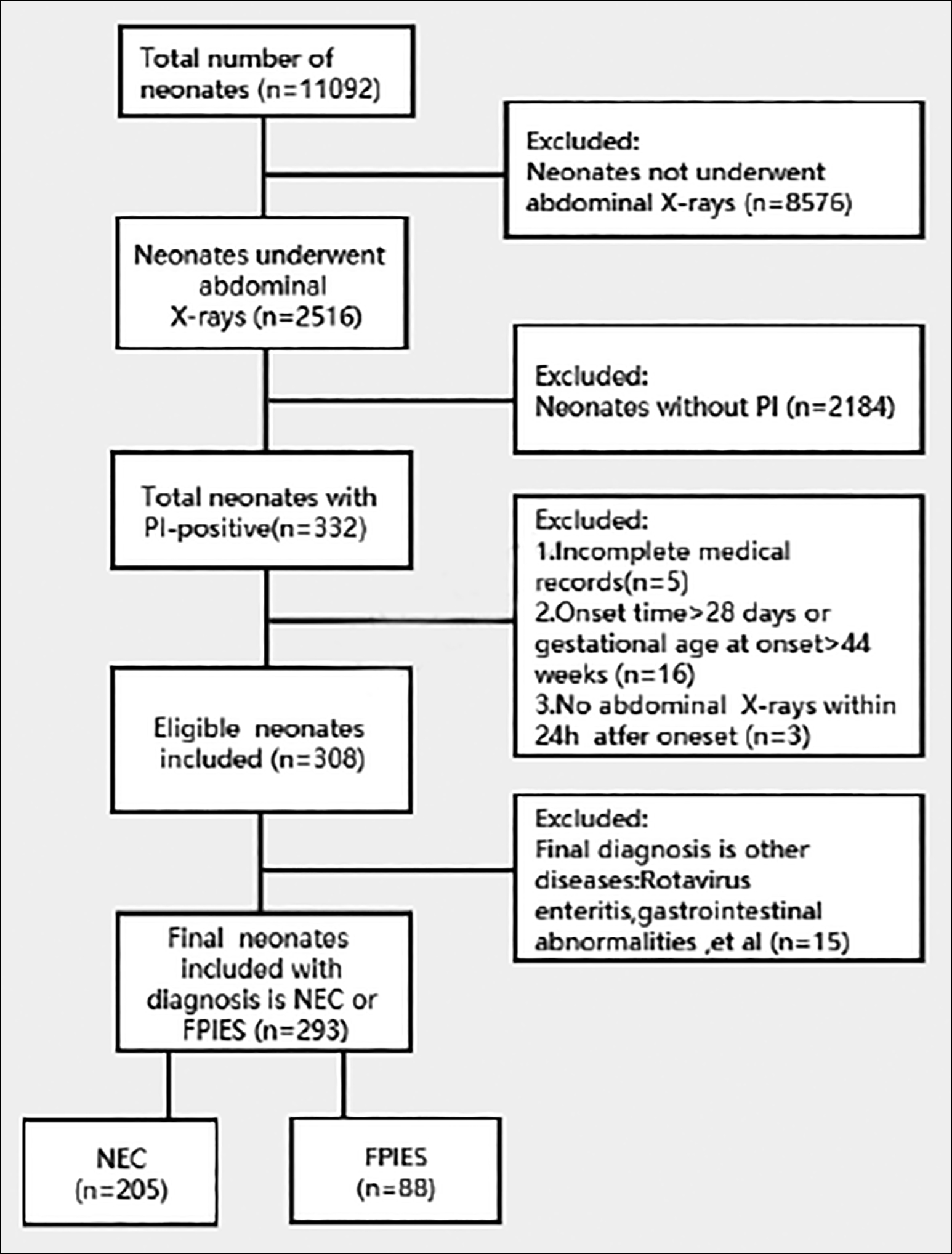 Figure 1: Patient selection flow chart.
Figure 1: Patient selection flow chart.
The study protocol was approved by the Ethics Committee of CHCMU (Registration No: 139/2020). Data were retrieved from Electronic Medical Record (EMR). Data obtained included patients’ demographic data, onset time of symptoms, complications, manifestations, laboratory findings, and the prognosis. All data were reviewed and analysed anonymously by the authors. The process of inclusion and exclusion is shown in the figure of the patient selection flow chart (Figure 1).
The cases’ final diagnoses were retrospectively reviewed by three attending physicians in consensus. The diagnostic criteria and definitions were abdominal radiographic finding of PI were reached based on these imaging features: translucent vesicle-like shadow under the mucosa of the intestinal wall, and ring-like, semi-ring-like, and strip-like shadows under the serous membrane.7,8 Two radiologists with 6 and 8 years of clinical experience retrospectively reviewed all images by consensus. The radiologists were unaware of surgical, physical, or other imaging findings of the patients. NEC was diagnosed according to the modified Bell's staging9 criteria, which is presence of clinical signs such as abdominal distension and emesis or gross blood in the stool (with an absence of fissure); having radiographic or ultrasound findings of pneumatosis intestinalis or portal vein gas. FPIES was diagnosed based on the International Consensus Guidelines for the Diagnosis and Management of FPIES criteria.10 The symptoms were relieved after the patient was treated by switching to a highly hydrolysed formula or changing the mother's diet if the baby is being breastfed; a history of direct or indirect exposure to any suspected substances should be there. Infants recovered well mainly undergoing oral intake restrictions, without antibiotics or antibiotics treatment for less than 3 days. Oral food challenge results should be referred when necessary.
Statistical analysis was performed using SPSS 22.0 software (IBM, Armonk, New York). Normally distributed continuous data were described as the mean ± standard deviation (M ± SD) and tested by the Student t-test. Skewed data were described as the median and interquartile range (IQR) and analysed by the Mann-Whitney U-test. Categorical data were shown in percentages and analysed by the Chi-square test or Fisher's Exact test. A p-value <0.05 was considered statistically significant.
Table I: Demographic data of NEC and FPIES.
|
Variables |
NEC (n=205) |
FPIES (n=88) |
p-value |
|
Male — n (%) |
112 (54.63) |
40 (45.45) |
0.149 |
|
Eutocia — n (%) |
87 (42.44) |
34 (38.64) |
0.545 |
|
GA (M ±SD, w) |
34.53±3.57 |
35.88±2.92 |
0.001 |
|
GA <35W — n (%) |
99(48.29) |
23 (26.14) |
<0.001 |
|
BW (M ±SD, kg) |
2.57±0.83 |
2.85±0.69 |
0.004 |
|
Onset time in days, median (IQR) |
9 (4-14) |
12 (9-16.75) |
0.001a |
|
NEC: Necrotizing enterocolitis; FPIES: Food protein-induced enterocolitis syndrome; M ±SD: Mean ± standard deviation; Onset time: Onset time of happening symptoms of the digestive tract; W: Weeks; GA: Gestational age; Kg: Kilograms; BW: Birth weight; a: Mann-Whitney U-test. |
|||
RESULTS
During the study period, a total of 11092 neonates were admitted to CHCMU. Of these, radiographic PI-positive was found in 332 cases of 2516 (13.20%). After reviewing the cases, 293 cases were finally included and divided into group NEC (n=205, 69.97%) and group FPIES (n=88, 30.03%) according to the diagnostic criteria of NEC and FPIES.
NEC was significantly related to (p<0.05) lower birth weight, younger gestational age (especially <35 weeks), and earlier onset time (Table I). NEC had significantly higher rates (p<0.05) of mothers’ antenatal steroid therapy, sepsis, and anemia when compared with FPIES (Table II).
Meanwhile, the occurrences of emesis, abdominal distension, abdominal tenderness and decreased bowel sounds were higher in NEC. Abdominal tenderness and absent bowel sounds were only observed in NEC, but the rates of diarrhea and hematochezia were lower in NEC compared to FPIES.
Table II: Perinatal factors and complications of NEC and FPIES.
|
Variables |
NEC (n = 205) |
FPIES (n = 88) |
p-value |
||||
|
n |
% |
n |
% |
||||
|
Perinatal factors |
Gestational diabetes mellitus |
32 |
15.61 |
18 |
20.45 |
0.312 |
|
|
Gestational cholestasis |
8 |
3.90 |
6 |
6.82 |
0.369 b |
||
|
Maternal dexamethasone treatment |
63 |
30.73 |
10 |
11.36 |
<0.001 |
||
|
Meconium-stained amniotic fluid |
9 |
4.39 |
4 |
4.55 |
>0.999b |
||
|
Premature rupture of membrane |
63 |
30.73 |
18 |
20.45 |
0.071 |
||
|
Intrauterine distress |
16 |
7.80 |
4 |
4.55 |
0.311 |
||
|
Maternal allergy history |
16 |
7.80 |
8 |
9.09 |
0.713 |
||
|
Multiple births |
6 |
2.93 |
4 |
4.55 |
0.494b |
||
|
Type of feeding |
Formula-fed |
121 |
59.02 |
32 |
36.36 |
0.002 |
|
|
Mixed-fed |
39 |
19.02 |
27 |
30.68 |
|||
|
Breastfed |
45 |
21.95 |
29 |
32.95 |
|||
|
Complications/comorbidities |
Metabolic acidosis |
8 |
3.90 |
7 |
7.95 |
0.158 b |
|
|
Hypoglycemia |
11 |
5.37 |
2 |
2.27 |
0.196 b |
||
|
Sepsis |
73 |
35.61 |
7 |
7.95 |
<0.001 |
||
|
ABO hemolytic disease |
33 |
16.10 |
8 |
9.09 |
0.113 |
||
|
Pathological jaundice of the newborn |
55 |
26.83 |
27 |
30.68 |
0.501 |
||
|
Patent ductus arteriosus |
30 |
14.63 |
10 |
11.36 |
0.455 |
||
|
Anemia |
55 |
26.83 |
13 |
14.77 |
0.025 |
||
|
Hypoproteinemia |
12 |
5.85 |
8 |
9.09 |
0.314 |
||
|
High lactic acid |
33 |
16.10 |
20 |
22.73 |
0.177 |
||
|
Respiratory failure |
7 |
3.41 |
6 |
6.82 |
0.220 b |
||
|
Potassium anomaly |
16 |
7.80 |
3 |
3.41 |
0.161 |
||
|
Abnormal clotting |
12 |
5.85 |
5 |
5.68 |
0.954 |
||
|
NEC: Necrotizing enterocolitis; FPIES: Food protein-induced enterocolitis syndrome; b: Fisher's exact test. |
|||||||
Table III: Symptoms, signs, and examinations of NEC and FPIES.
|
Variables |
NEC (n = 205) |
FPIES (n = 88) |
p-value |
|||
|
n |
% |
n |
% |
|||
|
Symptoms and Signs |
Fever |
32 |
15.61 |
10 |
11.36 |
0.342 |
|
Emesis |
91 |
44.39 |
25 |
28.41 |
0.010 |
|
|
Diarrhea |
43 |
20.98 |
52 |
59.09 |
<0.001 |
|
|
Hematochezia |
110 |
53.66 |
71 |
80.68 |
<0.001 |
|
|
Abdominal distension |
145 |
70.73 |
27 |
30.68 |
<0.001 |
|
|
Abdominal tender |
16 |
7.80 |
0 |
0 |
0.004b |
|
|
Bowel sounds for 1 Minute 1-3 |
132 |
64.39 |
12 |
13.64 |
<0.001 |
|
|
Bowel sounds for 1 Minute=0 |
8 |
3.90 |
0 |
0 |
0.110b |
|
|
Capillary refill time >3 seconds |
11 |
5.37 |
1 |
1.14 |
0.116 b |
|
|
Examinations |
Abnormal leukocyte |
61 |
29.76 |
18 |
20.45 |
0.100 |
|
Platelet count <100(x 109/L) |
20 |
9.76 |
1 |
1.14 |
0.009 |
|
|
Abnormal CRP |
44 |
21.46 |
7 |
7.95 |
0.005 |
|
|
NEC: Necrotizing enterocolitis; FPIES: Food protein-induced enterocolitis syndrome; b: Fisher's exact test. |
||||||
Table IV: Outcomes of NEC and FPIES.
|
Variables |
NEC (n = 205) |
FPIES (n = 88) |
p-value |
|
|
Length (days) of hospital stay, (M±SD) |
21.11±16.28 |
9.80±5.22 |
<0.001 |
|
|
Clinical outcomes |
Medical treatment/cure (n, %) |
195 (95.12%) |
88 (100.00) |
0.036b |
|
Surgical treatment/death (n, %) |
10 (4.88) |
0 (0.00) |
||
|
NEC: Necrotizing enterocolitis; FPIES: Food protein-induced enterocolitis syndrome; M±SD: Mean ±standard deviation; b: Fisher's exact test. |
||||
The rates of continuous elevated C-reactive protein (CRP) as well as thrombocytopenia were higher in NEC than in FPIES (Table III).
The average hospitalisation time in group NEC (21.11±16.28 days) was significantly (p<0.05) longer than group FPIES (9.80±5.22 days). Only group NEC had cases who required surgical intervention or died finally (Table IV).
DISCUSSION
Radiographic PI is considered an important diagnostic hallmark of NEC, but it can also present in some other diseases.11 In this study, all cases had radiographic PI-positive, of which 69.97% (n=205) were NEC and the other 30.03% (n=88) were FPIES. PI is associated with bowel inflammation with or without pathophysiologic necrosis in these two diseases.12
More than 85% of NEC occurs in preterm infants with gestational age less than 32 weeks or birth weight less than 1500 g.1,13,14 Prematurity is the most important risk factor for NEC because of immature intestinal barriers and local host defenses, such as secretory immunoglobulin A and mucosal enzymes.1 In this study, NEC was significantly associated with low birth weight, and short gestational age of less than 35 weeks. Our results are consistent with the findings of other studies.1,2 NEC usually happens earlier than FPIES, and the median onset time of symptoms is 7-12 days after birth. The median onset time of NEC in our study was 9 days also is consistent with the previous study.15
An antenatal steroid is widely used to prompt lung maturity of preterm infants. Some studies indicate that the administration of antenatal steroids has shown to be protective against NEC in preterm infants with gestational age of more than 25 weeks.16 However, our study indicated that antenatal steroid was a risk factor for NEC. The reason might be that antenatal steroid was accompanied by preterm infants, which was a primary risk factor for NEC. Another study found that glucocorticoids could interfere and inhibit intestinal epithelial repair and proliferation, thereby promoting the occurrence of NEC.17 However, the exact effects of antenatal steroids on intestinal development still need further exploration.
Recent randomised controlled trials and meta-analyses indicate a risk reduction of NEC using human milk formula.18 This study also revealed that formula-feeding can increase the risk of NEC, whereas breast milk has a protective effect on intestinal function. There are bioactive factors such as proteins, polyunsaturated fatty acids, oligosaccharides, and microbial content, present in breast milk that can influence the infant's gut immune maturation.18 This study also showed that group NEC had a higher rate (59.02%) of formula feeding and a lower rate (21.95%) of breastfeeding than group FPIES (p=0.002). According to the current classification, food allergy by definition is an immune-mediated adverse food reaction.19 There is no exact epidemiological data on neonatal food allergy. Cow's milk protein allergy is the most common FPIES in newborns.20 There was no difference in breastfeeding rate and formula-feeding rate in our FPIES, which might be due to the small sample size of our study.
Fox reported that NEC was associated with conditions that cause reduced mesenteric blood flow, foremost among these were congenital heart disease, particularly patent ductus arteriosus (PDA) and sepsis.21 In this study, the incidence of sepsis in group NEC (35.61%) was significantly higher than in group FPIES (7.95%), but the incidence of PDA between the two groups had no difference (p=0.455). The reasons for these results might be: 1. NEC is coagulative necrosis with severe inflammation affecting the terminal ileum and colon, so sepsis is a common underlying disease in NEC.22 The cardiac structures of term or near term infants are close to the mature, our cases in the research are mainly term or near term infants, so the number of cases with PDA was small in the two groups. NEC complicated by anemia was more common than FPIES in this study. Our conclusion is in agreement with the study of Patel et al.,23 who had demonstrated that it was severe anemia rather than the packed cell transfusion contribution significantly to the development of NEC.
NEC and FPIES both have a set of similar signs and symptoms which vary depending on the severity of the disease and make the differential diagnosis difficult.12 The present conclusion is also in agreement with this conclusion, but we found that abdominal tenderness and absent bowel sounds were only observed in NEC, and FPIES presented with diarrhea and hematochezia more common. These different presentation may depend on the different etiology. NEC is characterised by progressive systemic and gastrointestinal symptoms and can lead to peritonitis easily, while FPIES involves specific areas of the digestive tract, so they presented with some different presentations. This finding may provide significant guidance and aid clinicians to assess the diagnosis.
Previous research reported thrombocytopenia and significantly elevated C-reactive protein (CRP) on the second day after onset had the predictive value for NEC.24,25 On the other hand, elevated CRP also can be seen under infectious or non-infectious inflammatory conditions. Similarly, in this study, it was found that the rate of abnormal CRP in NEC was much higher than in FPIES (p=0.005). Therefore, continuous normal CRP may be used to exclude NEC; however, further prospective multicentre studies with large sample sizes are needed for data validation. In this study, thrombocytopenia (platelet count<100x109/L) was more common in NEC than FPIES (p=0.009), this finding strongly suggests that thrombocytopenia might be one of the potential markers to distinguish FPIES from NEC. Therefore, for the identification of NEC and FPIES, it is emphasised that complete blood count and CRP should be followed up routinely to assess the disease progression.
Due to the different causes of NEC and FPIES, most of NEC requires long-term antibiotics, fasting, and even require surgical treatment, while FPIES only needs timely diet evasion to recover, so the main treatment options and prognosis are different.5,6 This study proved that the average hospitalisation time in NEC was much longer and the prognosis was also worse than in FPIES. This conclusion has confirmed the importance to identify NEC and FPIES again. It was a retrospective study about NEC compared with FPIES, though some indicators to identify meaningfully, but had no specificity, still lack of effective identification method, the author thinks that based on more multi-centres, prospective studies eventually form a valuable NEC or FPIES prediction model for clinical use.
CONCLUSION
NEC is the main cause of PI-positive in neonates, but FPIES can't be ignored for the main treatment protocols and outcome are different. When PI-positive, although infants diagnosed with NEC or FPIES lack specific signs and symptoms, there are still clinical characteristics that need to be focused on: risk factors (BW, GA, onset time, mother's antenatal steroid therapy, formula feeding, sepsis, and anemia), abdominal signs (abdominal tenderness and absent bowel sounds), the results of CRP and platelet, which may help clinicians to identify them.
ETHICAL APPROVAL:
Human studies were reviewed and approved by the Clinical Research Ethics Committee of Chongqing Medical University (Registration No. 2020/R/139).
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
YH: Study design, data analysis, validation, data interpretation, and writing.
ZH: Data analysis, validation, visualisation.
KL: Study design.
WH: Literature search, figures, study design, data collection, data analysis, data interpretation, writing, validation.
All authors approved the final version of the manuscript to be published.
DATA AVAILABILITY STATEMENT:
The dataset used and/or analysed during the current study are available from the corresponding author upon reasonable request. All data generated or analysed during this study are included in this published article. Proposals should be submitted to weihong@cqmu.edu.cn.
REFERENCES
- Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK. Incidence and timing of presentation of necrotizing enterocolitis in preterminfants. Pediatrics 2012; 129(2):e298-e304.doi:10.1542/peds.2011-2022.
- Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med 2015; 372(4):331-40.doi:10.1056/NEJMoa1403489.
- 3.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R,Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978; 187(1):1-7.doi:10.1097/00000658-197801000-00 001.
- Lenfestey MW, de la Cruz D, Neu J. Food protein-induced enterocolitis instead of necrotizing enterocolitis? A neonatal intensive care unit case series. J Pediatr 2018; 200:270-3.doi:10.1016/j.jpeds.2018.04.048.
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the nomenclature review committee of the world allergy organization, October 2003. J Allergy Clin Immunol 2004; 113(5):832-6. doi: 10.1016/j.jaci.2003. 12.591.
- Suda K, Yanai T, Toma M, Aiyoshi T, Sasaki T, Muraji T, et al. Aggressive gastrointestinal food allergy in neonates and its possible relationship to necrotising enterocolitis. Int J Surg Case Rep 2017; 36:175-8. doi:10.1016/j.ijscr.2017.05.038.
- Pear BL. Pneumatosis intestinalis: A review. Radiol 1998; 207(1):13-19. doi:10.1148/radiology.207.1.9530294.
- Kreiss C, Forohar F, Smithline AE, Brandt LJ. Pneumatosis intestinalis complicating C. difficile pseudomembranous colitis. Am J Gastroenterol 1999; 94(9):2560-1:2560-1. doi:10. 1111/j.1572-0241.1999.01397.x.
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: Treatment based on staging criteria. Pediatr Clin North Am 1986; 33(1):179-201. doi:10.1016/s0031-3955(16). 34975-6.
- Nowak-Wegrzyn A, Chehade M, Groetch ME, Spergel JM, Wood RA, Allen K, et al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: Executive summary-workgroup report of the adverse reactions to foods committee, american academy of allergy, asthma & immunology. J Allerg Clin Immunol 2017; 139:1111-26, e4.
- Gordon PV, Swanson JR, Attridge JT, Clark R. Emerging trends in acquired neonatal intestinal disease: Is it time to abandon Bell's criteria? J Perinatol 2007; 27:661-71.doi: 10.1038/sj.jp.7211782.
- Neu J, Modi N, Caplan M. Necrotizing enterocolitis comes in different forms: Historical perspectives and defining the disease. Semin Fetal Neonatal Med 2018; 23(6):370-3. doi:10.1016/j.siny.2018.07.004.
- Thompson AM, Bizzarro MJ. Necrotizing enterocolitis in newborns: Pathogenesis, prevention and management. Drugs 2008; 68(9):1227-38. doi:10.2165/00003495- 200868090-00004.
- Neu, J. Necrotizing enterocolitis: The future. Neonatol 2020; 117(2): 240-4. doi:10.1159/000506866.
- Al Tawil K, Sumaily H, Ahmed IA, Sallam A, Al Zaben A, Al Namshan M, et al. Risk factors, characteristics and outcomes of necrotising enterocolitis in late preterm and term infants. J Neonatal Perinatal Med 2013; 6(2):125-30. doi:10.3233/NPM-1365912.
- Rose AT, Patel RM. A critical analysis of risk factors for necrotizing enterocolitis. Semin Fetal Neonatal Med 2018; 23(6):374-9.doi:10.1016/j.siny.2018.07.005.
- Jung S, Fehr S, Harder-d'Heureuse J, Wiedenmann B, Dignass AU. Corticosteroids impair intestinal epithelial wound repair mechanisms in vitro. Scand J Gastroenterol 2001; 36(9):963-70. doi:10.1080/003655201750305495.
- Altobelli E, Angeletti PM, Verrotti A. The impact of human milk on necrotizing enterocolitis: A systematic review and meta-analysis. Nutrients 2020; 12(5):1322.doi:10.3390/ nu12051322.
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, et al. Revised nomenclature for allergy for global use: Report of the nomenclature review committee of the world allergy organization, October 2003. J Allergy Clin Immunol 2004; 113(5):832-6. doi: 10.1016/j.jaci.2003.12.591.
- Sicherer SH. Clinical aspects of gastrointestinal food allergy in childhood. Pediatrics 2003; 111(6 Pt 3):1609-16. PMID: 12777600.
- Fox TP, Godavitarne C. What really causes necrotising enterocolitis? ISRN Gastroenterol 2012; 2012:628317.doi:10. 5402/2012/628317.
- Afzal MF, Abbas E, Hamid MH, Nasir H, Laeeq A, Hanif A. Share risk factors for candidaemia in neonates with sepsis in a tertiary care hospital in Pakistan. J Pak Med Assoc 2020; 70(9):1568-71. doi: 10.5455/JPMA.35996.
- Patel RM, Knezevic A, Shenvi N, Josephson CD, Keene S, Roback JD, et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA 2016; 315:889-97. doi:10.1001/jama.2016.1204.
- Maheshwari A. Role of platelets in neonatal necrotizing enterocolitis. Pediatr Res 2020; 10.1038/s41390-020-1038-8. doi:10.1038/s41390-020-1038-8.
- Pourcyrous M, Korones SB, Yang W, Boulden TF, Bada, HS. C-reactive protein in the diagnosis, management, and prognosis of neonatal necrotizing enterocolitis. Pediatrics 2005; 116(5):1064-9. doi:10.1542/peds.2004-1806.