Diagnostic Value of Preoperative Haemoglobin, Albumin, Lymphocyte and Platelet (HALP) Score in Predicting Tumour Budding in Colorectal Cancer
By Ugur Topal1, Serkan Guler2, Zafer Teke1, Erdal Karakose3, Idris Kurtulus2, Hasan Bektas2Affiliations
doi: 10.29271/jcpsp.2022.06.751ABSTRACT
Objective: To investigate the value of preoperative haemoglobin, albumin, lymphocyte, and platelet (HALP) score in predicting tumour budding in colorectal carcinoma.
Study Design: Observational study.
Place and Duration of Study: University of Health Sciences, Başakşehir Çam and Sakura City Hospital İstanbul/Turkey, between May 2020 and May 2021.
Methodology: The colorectal cancer patients who underwent surgery were divided into two groups according to the presence or absence of tumour budding. A total of 110 patients were included in the study, and there were 31 patients in group 1 and 79 patients in group 2. The predictive value of the HALP score in predicting tumour budding at the determined cut-off point was evaluated.
Results: The mean HALP score was similar in both groups (p=0.459). The rate of lymphovascular invasion was higher in group 2 (p=0.002), and T3 and T4 tumours were more common in group 2 (p<0.001). The number of metastatic lymph nodes was higher in group 2 (p=0.049). When the patients in group 2 were divided into subgroups according to the degree of tumour budding, the HALP score differed between intermediate and high budding groups (p=0.032). A HALP value of >31.6 predicted the presence of tumour budding with a sensitivity of 70.89% and a specificity of 48.39%.
Conclusion: The presence of tumour budding is associated with aggressive phenotypic features in colorectal carcinoma. The preoperative prediction of tumour budding can serve as a guide in the development of individualised therapy plans. The HALP score was associated with the presence of intermediate or high degree of tumour budding.
Key Words: Colorectal cancer, Tumor, Pathology, Hemoglobin, Albumin.
INTRODUCTION
Colorectal cancer (CRC) is the third most common type of cancer and the second leading cause of cancer-related death worldwide according to the GLOBOCAN 2018 database of the International Agency for Research on Cancer.1 The biomarkers that guide the diagnosis, treatment, and subtyping of diseases can be identified more easily. Thanks to developing technologies and novel techniques, the discovery of cancer biomarkers has become a major focus of cancer research in recent years. Given the rising incidence of CRC and the ongoing lack of absolute indicators for early diagnosis and treatment, new biomarkers are urgently needed for CRC.
Recent studies have shown that prognosis can differ among patients with at same cancer stage, leading researchers to look for new prognostic factors, such as tumour budding, which is believed to be a histological reflection of the epithelial-mesenchymal transition. It has been reported that budding cancer cells become resistant to apoptotic stimuli, chemotherapeutics, and immunogenic cell death as a result of a series of molecular events, and represent the first step of cancer metastasis in which migration to the extracellular matrix and invasion of the lymphatic and vascular structures begin.2,3 Many studies have reported tumour budding to be an independent prognostic factor associated with lymph node metastasis, local recurrence, and survival. European Society of Medical Oncology and International Tumour Budding Consensus Conference (ITBCC) guidelines included tumour budding as a criterion in the identification of high-risk patient groups.4-7
The HALP score is a comprehensive index that has been shown to have a prognostic role in gastrointestinal cancers,8,9 reflecting the components of patients' nutritional and immune status. The HALP score is a parameter that can be easily measured and easily calculated through clinical data of haemoglobin level, albumin level, lymphocyte count, and platelet count. Currently, no study has been conducted on the diagnostic value of HALP score in predicting tumour budding in patients with CRC.
The prediction of tumour budding based on immunonutritional indices is a new area of research in the literature removing the need to wait for pathology results. Thus, it has been found that patients who were erroneously believed to have a good prognosis based on clinicopathological factors until recently that could be studied, and who were followed up without additional therapy, would have had an additional survival advantage through actual prognosis-oriented therapy.
This study investigated the relationship between tumour budding and clinicopathological parameters in CRC patients who were diagnosed with adenocarcinoma and established the predictive value of the HALP score in predicting tumour budding.
METHODOLOGY
Patients who underwent surgery for CRC between May 2020 and May 2021 were included in this single-centre observational study conducted at the University of Health Sciences, Başakşehir Çam and Sakura City Hospital, Istanbul, Turkey, between May 2020 and May 2021. Clinical data were collected from the hospital patient files, while histopathological data were collected from the pathology reports in the digital patients archive. The patients who underwent palliative surgery, those under the age of 18, those who were pregnant, those with chronic inflammatory diseases (tuberculosis, sarcoidosis, etc.), autoimmune diseases and haematological diseases, those using corticosteroids, and those with inaccessible records were excluded from the study.
In accordance with the recommendations of the College of American Pathologists Protocol for Reporting of Colorectal Carcinoma (2018), tumour budding was determined as defined at the ITBCC 2016 meeting, being single cells or clusters of up to four cells at the invasive margin of the tumour.4 All tumour-containing slides were examined for the presence of tumour budding, along the invasive margin of the tumour. In cases with tumour budding, the tumour buds were counted within the area of 0.785 mm2 identified as having the highest density of tumour buds (hotspot), at the invasive margin of the tumour and 20x magnification.4 For field standardisation, the number of tumour buds identified in the 20x magnification objective was divided by the normalisation factor corresponding to the 20x field size of the microscope. Subsequently, cases identified with 0–4 tumour buds were scored as low budding (Bd1), those with 5-9 tumour buds as intermediate budding (Bd2), and those with ≥10 buds as high budding (Bd3). The patients were divided into two groups according to the absence or presence of tumour buds. Group 1 patients were budding absent and group 2 patients were budding present, based on their tumour budding findings. A total of 110 patients were included in the study. There were 31 patients in group 1 and 79 patients in group 2.
The groups were examined for demographic data, tumour marker levels, neoadjuvant treatment, type of operation, tumour localisation, histopathological diagnosis, tumour size, degree of differentiation, tumour depth of invasion, lymphovascular invasion, perineural invasion, total lymphocyte count, pathological stage, and lymph node metastasis.
The HALP score was calculated preoperatively using the equation: haemoglobin (g/dL) × albumin (g/L) × lymphocytes (109/L)/ platelets (109/L).
The pathological disease stage was determined in accordance with the 8th Edition of the TNM Classification.10
A receiver operating characteristic (ROC) analysis was conducted and a ROC curve was created to establish a cut-off point for the HALP score. The patients were divided into two groups according to the presence or absence of budding, and a ROC analysis was performed for the two groups. The diagnostic value of the HALP score at the established cut-off point was examined.
The factual investigation of the information was performed utilizing IBM SPSS Measurements for Windows, form 23.0 (IBM Corp., Armonk, NY, USA). The mean and standard deviation values were given for the parameters conforming to the normal distribution, and the median and range values were given for the parameters not conforming to the normal distribution. The normality of the data was analysed with a Shapiro-Wilk test; categorical variables were compared using Chi-square and Fisher’s tests; an Independent Samples (Student's) t-test was used for the normally distributed groups, and a Mann-Whitney U test for the non-normally distributed groups. With the aim to determine the source of difference between the groups Bonferroni method being among Post Hoc tests has been applied. The sensitivity and specificity of the HALP score were calculated based on the tumour budding, and a cut-off point was established through an examination of the area under the ROC curve. The statistical significance level was set at <0.05 for all tests.
RESULTS
A ROC analysis was conducted and a ROC curve was created to establish a cut-off point for the HALP score. The ROC analysis yielded an area under the ROC curve of 54.6%. The cut-off point gives an answer rate of 54.6% correctly. At the cut-off point, a HALP score of >31.6 predicted the presence of tumour budding with a sensitivity of 70.89% and a specificity of 48.39%. The results are presented in Figure 1 and Table I.
An analysis of the relationship between the HALP score and the degree of tumour budding in patients with tumour budding revealed a statistically significant difference between the intermediate and high budding groups (19.7 vs. 30.6, p = 0.032). The results are presented in Table I.
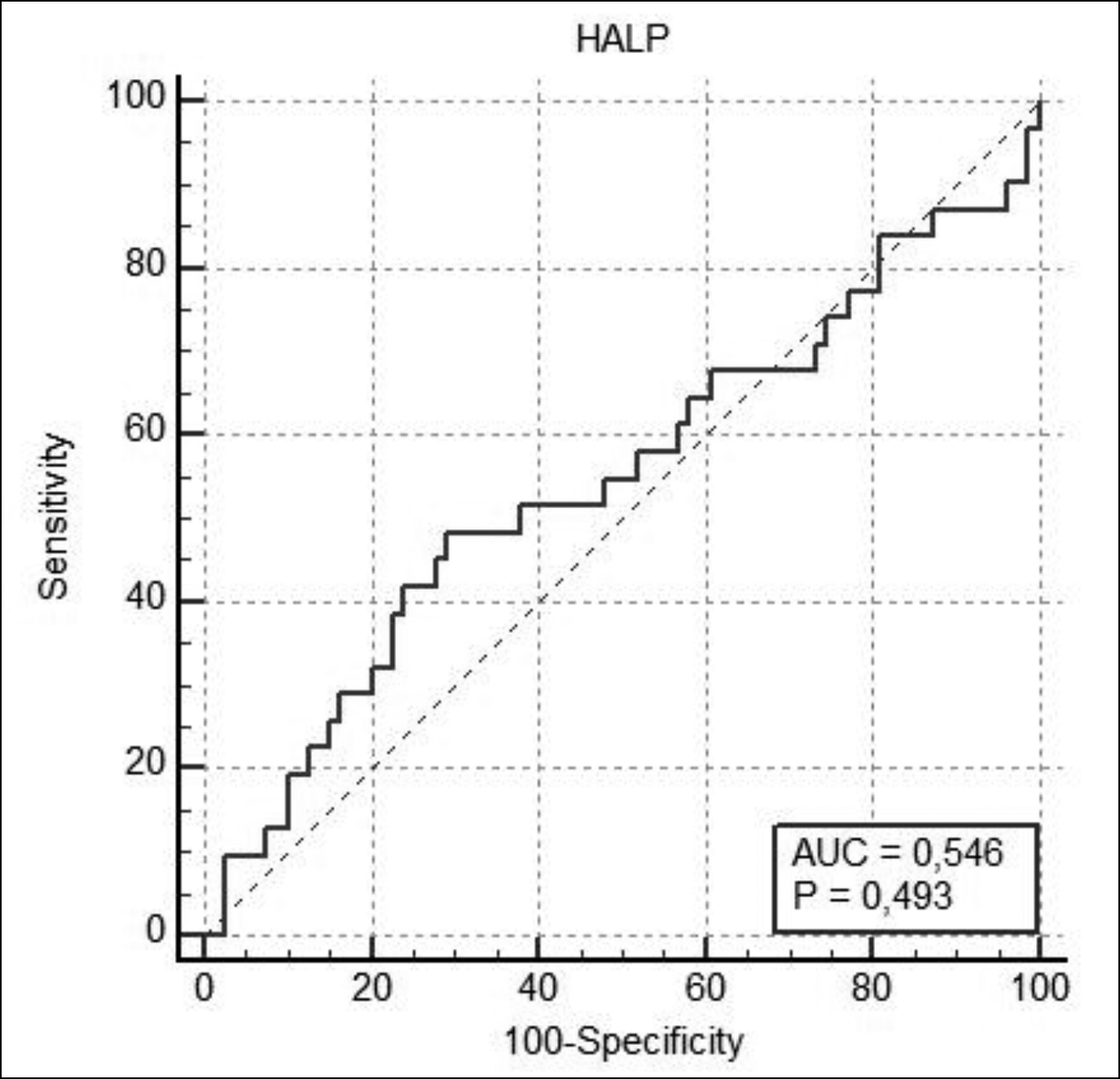 Figure 1: Receiver operating characteristic curve analysis of the HALP score for tumour budding.
Figure 1: Receiver operating characteristic curve analysis of the HALP score for tumour budding.
Table I: Proposed cut-off points for the HALP score for the prediction of tumour budding and relationship between the degree of tumour budding and HALP score.
|
|
HALP score |
|
|||
|
AUC |
0.546 |
|
|||
|
95% Cl (%) |
0.448–0.641 |
|
|||
|
Cut-off |
>31.6 |
|
|||
|
Specificity |
48.39 |
|
|||
|
95% Cl (%) |
30.2–66.9 |
|
|||
|
Sensitivity (%) |
70.89 |
|
|||
|
95% Cl (%) |
59.6–80.6 |
|
|||
|
PPV |
39.5 |
|
|||
|
NPV |
77.8 |
|
|||
|
+LR |
1.66 |
|
|||
|
-LR |
0.73 |
|
|||
|
p |
0.493 |
|
|||
|
|
Low (a) |
Intermediate (b) |
High (c) |
p |
|
|
HALP score |
24.1 (5.5–150) |
19.7 (6.5–43.7) |
30.6 (17.4–70.1) |
0.039* |
|
|
Post Hoc Bonferroni c-b; p = 0,032. |
|
||||
The male gender was dominant in both groups (71% vs. 60.8%, p = 0.317). The mean age was similar in both groups (67 years vs. 62 years, p = 0.298). Preoperative laboratory parameters and tumour marker levels were similar in both groups (p>0.05). The mean HALP score was similar in both groups (28.5 vs. 24.2, p = 0.459). The results are presented in Table II.
The majority of the operations were performed under elective conditions (90.3% vs. 84.85, p = 0.448) and the most common tumour localisation was the rectum (38.7% vs. 32.9%, p = 0.631). Operation-related variables are presented in Table III.
The most common pathological grade was moderate differentiation (74.2% vs. 88.6%, p = 0.111). The rate of lymphovascular invasion was higher in group 2 (38.7% vs. 70.9%, p = 0.002). The T3 and T4 tumours were more common in group 2 (p <0.001). The number of lymph nodes harvested was similar in both groups (19 vs. 23, p = 0.291), although the number of metastatic lymph nodes was higher in group 2 (0 vs. 1, p = 0.049). The results are presented in Table III.
DISCUSSION
This study was conducted to investigate the relationship between tumour budding and clinic pathological parameters in CRC patients and to establish the predictive value of the HALP score for tumour budding. It was found that tumour budding was associated with such poor histo pathological factors as the presence of lymphovascular invasion, a high T stage, and an increased number of metastatic lymph nodes. The HALP score was not statistically significantly associated with the presence of tumour budding. However, the subgroup analysis of patients with tumour budding revealed a higher HALP score to be associated with the presence of intermediate or high degree of tumour budding.
The inflammatory response and nutritional status have been shown to be associated with the prognosis of cancer patients.11 The HALP score integrates four haematological parameters, and previous studies have demonstrated its prognostic significance in CRC. Inflammation-based ratios are representative biomarkers of host inflammatory response that can predict cancer prognosis. The reliability of the HALP score has been demonstrated in various studies in the literature. Jiang et al. studied the prognostic value of the HALP score in locally advanced CRC patients and found that patients with lower HALP scores exhibited an increased risk of death (HR = 1.46, 95% CI 1.11–1.92; p = 0.007) and cancer-related death (HR = 1.78, 95% CI 1.31–2.43; p < 0.001). These patients had also lower 5-year overall survival rate (60.7% vs. 74.0%; log-rank p = 0.001).8 Similarly, Yalav et al. found the HALP score to be closely associated with clinicopathological features and to be an independent prognostic factor for survival in CRC patients who underwent curative resection.12 Likewise, Dagmura et al. grouped CRC patients by age, as above and below 80 years, and established an association between HALP score and survival. The HALP score was significantly higher in the >80 years of age group than in the younger age group.13
The HALP score has been shown to be a safe parameter in solid tumours other than CRC, 14-17 and its predictive power has been proven in various benign conditions. Tian et al. investigated the association between HALP score and poor outcomes in patients with acute ischaemic stroke and found that an increased HALP score was associated with reduced risk of recurrent stroke and mortality within 90 days and 1 year after the onset of stroke.18 Park et al. reported that the HALP score at the time of diagnosis may reflect the cross-sectional activity of anti-neutrophil cytoplasmic antibody-associated vasculitis.19 It was hypothesised that the HALP score may be of value in the prediction of tumour budding, although the diagnostic value of the HALP score in predicting tumour budding was limited in this study.
Table II: Demographic data and preoperative laboratory findings.
|
|
Group 1 |
Group 2 |
p-value |
|
31 (%) |
79 (%) |
||
|
Gender |
|||
|
Male |
22 (71) |
48 (60.8) |
0.317 |
|
Female |
9 (29) |
31 (39.2) |
|
|
Ageb |
67 (34) (43–77) |
62 (71) (17–88) |
0.298 |
|
Neoadjuvant treatment |
9 (29) |
15 (19) |
0.251 |
|
Neutrophil countb (109/L) |
3.82 (11.5) (2–13.5) |
4.63 (11) (1.8–12.7) |
0.271 |
|
Haemoglobin levela (g/dL) |
11.6 ± 2.3 |
11.6 ± 1.9 |
0.946 |
|
Albumin levela (g/L) |
40.8 ± 5.3 |
40.4 ± 5.1 |
0.763 |
|
Lymphocyte countb (109/L) |
1.31 (3.8) (0.6–4.4) |
1.49 (3.0) (0.5–3.5) |
0.182 |
|
Platelet countb (109/L) |
246 (422) (104–526) |
276 (276) (45–697) |
0.092 |
|
HALP score |
28.5 (7.82) (4–82.2) |
24.2 (14.45) (5.5–150) |
0.459 |
|
CEA levelb (ng/mL) |
2.64 (453.1) (0.9–454) |
4.3 (753.2) (0.8–754) |
0.120 |
|
CA19-9 levelb (ng/mL) |
13.45 (1188.5) (2.5–1191) |
18.9 (14641) (2–14643) |
0.286 |
|
Chi-square and Fisher’s Exact tests; a: Independent Samples (Student’s) t-test (ort±ss); b: Mann-Whitney U-test ((Range) (Min-Max)); CEA: Carcinoembryonic antigen; CA 19-9: Carbohydrate antigen 19-9. |
|||
Table III: Surgical variables and pathological results.
|
|
Group 1 |
Group 2 |
p-value |
|
31 (%) |
79 (%) |
||
|
Emergency & Elective |
|
|
|
|
Emergency |
3 (9.7) |
12 (15.2) |
0.448 |
|
Elective |
28 (90.3) |
67 (84.8) |
|
|
Localized |
|||
|
Rectosigmoid |
2 (6.5) |
11 (13.9) |
0.631 |
|
Rectum |
12 (38.7) |
26 (32.9) |
|
|
Right colon |
4 (12.9) |
17 (21.5) |
|
|
Sigmoid |
8 (25.8) |
14 (17.7) |
|
|
Left colon |
5 (16.1) |
10 (12.7) |
|
|
Transverse colon |
- |
1 (1.3) |
|
|
Type of operation |
|||
|
Open |
20 (64.5) |
61 (77.2) |
0.370 |
|
Laparoscopic |
6 (19.4) |
11 (13.9) |
|
|
Robotic |
5 (16.1) |
7 (8.9) |
|
|
Postoperative hospital stayb |
7 (31) (4–35) |
6 (37) (3–40) |
0.349 |
|
Pathological grade |
|||
|
Poorly differentiated |
2 (6.5) |
4 (5.1) |
0.111 |
|
Intermediately differentiated |
23 (74.2) |
70 (88.6) |
|
|
Well-differentiated |
6 (19.4) |
5 (6.3) |
|
|
Lymphovascular invasion |
12 (38.7) |
56 (70.9) |
0.002** |
|
Perineural invasion |
11 (35.5) |
39 (49.4) |
0.188 |
|
T stage |
|
|
|
|
0 |
2 (6.5) |
1 (1.3) |
<0.001** |
|
1 |
3 (9.7) |
- |
|
|
2 |
7 (22.6) |
3 (3.8) |
|
|
3 |
11 (35.5) |
50 (63.3) |
|
|
4a |
4 (12.9) |
21 (26.6) |
|
|
4b |
4 (12.9) |
4 (5.1) |
|
|
N stage |
|||
|
0 |
20 (64.5) |
29 (36.7) |
0.291 |
|
1 |
1 (3.2) |
3 (3.8) |
|
|
1a |
4 (12.9) |
13 (16.5) |
|
|
1b |
2 (6.5) |
14 (17.7) |
|
|
1c |
- |
3 (3.8) |
|
|
2 |
- |
2 (2,5) |
|
|
2a |
2 (6.5) |
6 (7.6) |
|
|
2b |
2 (6.5) |
9 (11.4) |
|
|
M stage |
|||
|
0 |
29 (93.5) |
66 (83.5) |
0.169 |
|
1 |
2 (6.5) |
13 (16.5) |
|
|
Tumour sizeb (cm) |
3.25 (6.9) (0.1–7) |
33 (7.3) (1.3–8.6) |
0.154 |
|
Harvested lymph nodesb |
19 (77) (1–78) |
23 (122) (6–128) |
0.291 |
|
Metastatic lymph nodesb |
0 (9) (0–9) |
1 (44) (0–44) |
0.049* |
|
Chi-square and Fisher’s Exact tests. b: Mann-Whitney U-test ((Range) (Min-Max)). |
|||
While a subgroup analysis of patients with tumour budding revealed that a high HALP score was associated with the presence of intermediate or high degree of tumour budding, the authors could not demonstrate the prognostic value of the HALP score due to the short-term follow-up of their patients. The authors attributed this to the similar preoperative characteristics of patients. The patient groups in this study had a homogeneous distribution.
The association between tumour budding and pathological T stage has been investigated in several studies, most of which found tumour budding to be associated with more advanced pT stages.5,20-22 In the present study, tumour budding was found to be associated with a high pT level, which is a poor histological parameter, in accordance with the findings of previous studies.
Lymph node metastasis remains one of the most valuable prognostic factors in CRC. Earlier studies have shown that the depth of submucosal invasion, the tumour grade at the deepest invasive front and the presence of lymphovascular invasion are predictive markers for lymph node metastasis in CRC. It has been reported that tumour budding, which represents a differentiated histology at the invasive margin, is useful for the prediction of lymph node metastasis or haematogenous metastasis.23,24 Many studies on tumour budding in CRC revealed a significant association between tumour budding and lymphovascular invasion, and it has been suggested that tumour buds may be the part of the tumour that acquires the ability to invade lymphatic vessels.5,23,25 In the present study, tumour budding was found to be associated with an increased number of metastatic lymph nodes and increased lymphovascular invasion, supporting the findings of previous studies.
When tumours begin to bud, single tumour cells or small cell nests detach from the main tumour, and the budding of these cells is the first step in cancer metastasis, given their capacity to migrate through the extracellular matrix, to invade lymphatic and vascular structures, and to metastasize to regional lymph nodes and distant organs.2 In the present study, tumour budding was found to be associated with an increased number of metastatic lymph nodes and poor histological parameters, in line with the findings of previous studies.
It was believed that these results support the theoretical view explaining the onset of the metastatic cascade with tumour budding. The association between tumour budding and poor histopathological factors was not only related to the presence of budding, but also the degree of budding. The fact that the patient groups are generally heterogeneous in the published studies in the literature, and that the different studies have come up with the different findings to support this argument.
Tumour budding has initially been described in cancer patients who have not received neoadjuvant therapy and has been used to determine the need for neoadjuvant therapy. Currently, the determination of tumour budding score in CRC patients who received neoadjuvant treatment is a controversial issue in the literature, and it has not been clearly stated in the literature that this score cannot be used in CRC patients who received neoadjuvant therapy. CRC patients receiving neoadjuvant treatment may have bud-like false appearances due to regression in the tumour, and some pathologists do not prefer to report this score. However, since this issue is not yet clear enough in the literature, it was preferred to use this scoring system in CRC patients who received neoadjuvant therapy in this study.
Determining the optimal cut-off points for biomarkers requires further research, as the only way of achieving more accurate results related to their predictive power. Prognostic tools are needed for the development of individualised cancer therapy programs. The assessment of tumour budding may help improve tumour staging systems and treatment approaches, and may serve as an additional pathological parameter in the determination of tumour behavior. The preoperative prediction of tumour budding can guide individualised therapy modalities.
The most important limitation of this study is its retrospective design. Other limitations are the limited number of patients and the lack of long-term oncological follow-up results, as the patient with the earliest record underwent surgery one and a half years ago. The authors believe, however, that this study contributes to the literature as the first study to investigate the association between HALP score and tumour budding.
CONCLUSION
The results of this study revealed the presence of tumour budding to be associated with aggressive phenotypic features in CRC, and its ability to be used as a practical and reliable parameter for the determination of greater potential for malignancy. The authors recommend that tumour budding should be studied at least as present or absent in routine histopathological examination of CRC.
ETHICAL APPROVAL:
The study was approved by the local Ethics Committee of the institute (Institutional Review Board (IRB) No. 2021.03.19).
PATIENTS’ CONSENT:
Consent for publication was obtained from the patients whose data are included in this manuscript.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
UT: Conception, design, interpretation, literature search, and writing the manuscript.
SG: Conception, design, data collection and processing, interpretation, and literature search.
ZT: Supervision, writing manuscript, and critical review.
EK: Conception, design, supervision, and critical review.
IK: Supervision, literature search, and critical review.
HB: Conception, design, supervision, literature search, writing manuscript, and critical review.
All authors approved the final revision of this manuscript to be published.
REFERENCES
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6):394-424. doi: 10.3322/caac.21492.
- De Smedt L, Palmans S, Sagaert X. Tumour budding in colorectal cancer: What do we know and what can we do? Virchows Archiv 2016; 468(4): 397-408. doi: 10.1007/s00428-015-1886-5.
- Ozer SP, Barut SG, Ozer B, Catal O, Sit M. The relationship between tumor budding and survival in colorectal carcinomas. Rev Assoc Med Bras (1992) 2019; 65(12):1442-7. doi: 10.1590/1806-9282.65.12.1442.
- Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, et al. Recommendations for reporting tumor budding in colorectal cancer based on the international tumor budding consensus conference (ITBCC) 2016. Mod Pathol 2017; 30(9):1299-311. doi: 10.1038/modpathol.2017.46.
- Koelzer VH, Zlobec I, Lugli A. Tumor budding in colorectal cancer--ready for diagnostic practice? Hum Pathol 2016; 47(1):4-19. doi: 10.1016/j.humpath.2015. 08.007.
- Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 2012; 23(10): 2479-516. doi: 10.1093/annonc/mds236.
- Lugli A, Karamitopoulou E, Zlobec I. Tumor budding: A promising parameter in colorectal cancer. Br J Cancer 2012; 106(11): 1713-7. doi: 10.1038/bjc.2012.127.
- Jiang H, Li H, Li A, Tang E, Xu D, Chen Y, et al. Preoperative combined hemoglobin, albumin, lymphocyte and platelet levels predict survival in patients with locally advanced colorectal cancer. Oncotarget 2016; 7(44): 72076-83. doi: 10.18632/oncotarget.12271.
- Chen XL, Xue L, Wang W, Chen HN, Zhang WH, Liu K, et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: A retrospective cohort study. Oncotarget 2015; 6(38): 41370-82. doi: 10. 18632/oncotarget.5629.
- Weiser MR. AJCC 8th Edition: Colorectal cancer. Ann Surg Oncol 2018; 25(6):1454-5. doi: 10.1245/s10434- 018-6462-1.
- McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009; 12(3):223-6. doi: 10.1097/MCO. 0b013e32832a7902.
- Yalav O, Topal U, Unal AG, Eray IC. Prognostic significance of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients undergoing curative resection for colorectal cancer. Ann Ital Chir 2021; 92 :283-92.
- Dagmura H, Daldal E, Okan I. The efficacy of hemoglobin, albumin, lymphocytes, and platelets as a prognostic marker for survival in octogenarians and nonagenarians undergoing colorectal cancer surgery. Cancer Biother Radiopharm 2021; doi : 10.1089/cbr.2020.4725.
- Arıkan TB, Sozuer E, Topal U, Lale A, Yilmaz AZ. The value and prognostic significance of preoperative hemoglobin and albumin levels, and the lymphocyte and platelet count (HALP) scores in predicting pancreatic fistula in patients undergoing pancreaticoduodenectomy due to periampullary region tumors. Acta Medica Mediterr 2021; 37:1341. doi 10.19193/0393-6384_ 2021_2_206.
- Yalav O, Topal U, Unal AG, Sarıtaş AG. Clinical value of hemoglobin and albumin levels and lymphocyte and platelet count (HALP) combination in predicting postoperative complications, lymph node positivity and prognosis in gastric cancer patients who underwent curative surgical resection. Cyprus J Med Sci 2020; 5: 145-52.doi. 10.5152/cjms.2020.1747.
- Xu SS, Li S, Xu HX, Li H, Wu CT, Wang WQ, et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol 2020; 26(8):828-38. doi: 10.3748/wjg. v26.i8.828.
- Guo Y, Shi D, Zhang J, Mao S, Wang L, Zhang W, et al. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is a novel significant prognostic factor for patients with metastatic prostate cancer undergoing cytoreductive radical prostatectomy. J Cancer 2019; 10(1):81-91. doi: 10.7150/jca.27210. eCollection 2019.
- Tian M, Li Y, Wang X, Tian X, Pei LL, Wang, et al. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is associated with poor outcome of acute ischemic stroke. Front Neurol 2021; 11: 610318. doi: 10.3389/ fneur.2020.610318.
- Park PG, Yoo BW, Song JJ, Park YB, Lee SW. Will the HALP score help to assess the activity and predict the prognosis of antineutrophil cytoplasmic antibody-associated vasculitis? Clin Exp Rheumatol 2020; 124(2): 236-7. PMID: 32141432
- Graham RP, Vierkant RA, Tillmans LS, Wang AH, Laird PW, Weisenberger DJ, et al. Tomur budding in colorectal carcinoma: Confirmation of prognostic significance and histologic cutoff in a population-based cohort. Am J Surg Pathol 2015; 39(10):1340-6. doi: 10.1097/PAS.0000 000000000504.
- Zlobec I, Hädrich M, Dawson H, Koelzer VH, Borner M, Mallaev M, et al. Intratumoural budding (ITB) in preoperative biopsies predicts the presence of lymph node and distant metastases in colon and rectal cancer patients. Br J Cancer 2014; 110(4): 1008-13. doi: 10.1038/bjc. 2013.797. Epub 2013 Dec 24
- Satoh K, Nimura S, Aoki M, Hamasaki M, Koga K, Iwasaki H, et al. Tumor budding in colorectal carcinoma assessed by cytokeratin immunostaining and budding areas: possible involvement of c-Met. Cancer Sci 2014; 105(11): 1487-95. doi: 10.1111/cas.12530.
- Kaneko I, Tanaka S, Oka S, Kawamura T, Hiyama T, Ito M, et al. Lymphatic vessel density at the site of deepest penetration as a predictor of lymph node metastasis in submucosal colorectal cancer. Dis Colon Rectum 2007; 50(1): 13-21. doi: 10.1007/s10350-006-0745-5.
- Cappellesso R, Luchini C, Veronese N, Lo Mele M, Rosa-Rizzotto E, Guido E, et al. Tumor budding as a risk factor for nodal metastasis in pT1 colorectal cancers: A meta-analysis. Hum Pathol 2017; 65: 62-70. doi: 10.1016/j.humpath.2017.04.013.
- Karamitopoulou E, Zlobec I, Kolzer V, Kondi-Pafiti A, Patsouris ES, Gennatas K, et al. Proposal for a 10-high-powerfields scoring method for the assessment of tumor budding in colorectal cancer. Mod Pathol 2013; 26(2):295-301. doi: 10.1038/modpathol.2012.155.