Diagnostic Value of Neutrophil/lymphocyte Ratio and Platelet/lymphocyte Ratio in Premature Rupture of Membranes Complicated by Sepsis
By Shujian Zhang1, Wei Zhang2, Xue Luan3, Zhengyong Jin1Affiliations
doi: 10.29271/jcpsp.2022.05.602ABSTRACT
Objective: To investigate the predictive value of neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR) in premature rupture of membranes complicated by sepsis.
Study Design: A descriptive study.
Place and Duration of Study: Department of Paediatrics, Affiliated Hospital of Yanbian University, China, from January 2020 to June 2021.
Methodology: Ninety-nine children with premature rupture of membranes and sepsis were included as group A and 99 children without sepsis were included as group B. Logistic regression analysis was used to analyse the risk factors for premature rupture of membranes complicated by sepsis. The diagnostic value of PLR and NLR in sepsis complicated by premature rupture of membranes was assessed by subject operating characteristic curve (ROC) curves.
Results: Univariate analysis showed significant differences between groups A and B in terms of mode of delivery, lymphocyte count, platelets, PLR and NLR (p <0.05). Logistic regression analysis showed that mode of delivery, platelets, PLR and NLR were independent risk factors for premature rupture of membranes complicated by sepsis (p <0.05). The ROC area for PLR was 0.781 (95% CI: 0.718-0.844, p <0.001), which was greater than that for NLR when premature rupture of membranes complicated by sepsis was predicted. When the PLR was >93.072, the sensitivity of predicting premature rupture of membranes complicated by sepsis was 67.7% and the specificity was 79.8%.
Conclusion: PLR and NLR have a high predictive value for premature rupture of membranes complicated by sepsis. Among them, the predictive value of PLR was greater than NLR.
Key Words: Premature rupture of membranes, Sepsis, PLR, NLR, Subject operating characteristic curve (ROC).
INTRODUCTION
Premature rupture of membranes (PROM) refers to the rupture of the membranes that enclose the amniotic fluid and the fetus before delivery, with the amniotic fluid flowing out through the vagina.1 The incidence of PROM ranges from 20% to 36% of all pregnancies.2,3 Sepsis is a common complication of PROM and has a significant impact on the growth and development of the 'fetus-newborn'.4 The study of PROM and sepsis has received considerable attention from clinicians.
The culture of microorganisms in the blood is considered the gold standard for the diagnosis of sepsis.5 In developing countries, inadequate testing levels and long testing times greatly affect the early diagnosis and rational drug treatment of children.6,7 The key to reducing deaths due to sepsis is early detection and accurate diagnosis. Therefore, early diagnosis before the detection of blood culture results has become the key to clinical management. In recent years, the detection of inflammatory markers in blood has become a hot topic of research for paediatric clinicians.8 Compared with blood cultures, the results of inflammatory indicators in blood are easy to apply clinically and easily available.
PLR and NLR are inflammatory indicators with diagnostic values derived from peripheral blood cells in recent years. Studies have shown that PLR and NLR can be used as early diagnostic indicators and prognostic predictors for a variety of acute and chronic inflammatory diseases.9,10 NLR combines neutrophil count and lymphocyte count and can predict the severity of sepsis.11 PLR, on the other hand, combines platelet count and lymphocyte count and is an important marker for assessing disease activity.12
The diagnostic value of PLR and NLR in PROM complicated by sepsis is unclear, therefore, the aim of this study was to investigate the diagnostic value of PLR and NLR in PROM complicated by sepsis.
METHODOLOGY
The descriptive study was approved by the Medical Ethics Committee of the College of Medicine of Yanbian University. Ninety-nine children admitted from January 2020 to June 2021 with PROM complicated by sepsis were selected as group A; 99 children with PROM without sepsis were selected as group B. Inclusion criteria: The diagnosis of PROM was based on examination and clinical manifestations and included the pregnant woman complained of sudden vaginal discharge or uncontrolled "leakage", and amniotic fluid containing fetal fat was seen to flow from the uterine orifice on speculum examination; the pH of vaginal fluid was >6.5; dried vaginal fluid was seen to be dentate or phyllodes-like on film insulin-like growth factor binding protein-1 and placental alpha microglobulin-1 if necessary; ultrasonography. Diagnostic criteria for sepsis: positive cultures of microorganisms in the blood with clinical signs of systemic infection such as high or low body temperature, rapid or suspended breathing, tachycardia or bradycardia, unstable body temperature, irregular respiratory rhythm, erratic heartbeat rhythm, and feeding intolerance. No antibiotic treatment prior to blood collection. Exclusion criteria were: infants younger than gestational age or older than gestational age; maternal use of medications related to fetal haematopoiesis during pregnancy; prenatal use of glucocorticoids; infants receiving postnatal cardiopulmonary resuscitation; twin or multiple pregnancies; maternal alcohol or tobacco use; maternal gestational diabetes mellitus or other conditions.
Gestational age, gender of the newborn, birth weight, delivery pattern, lymphocyte count, platelet count, neutrophil count, NLR and PLR were collected from the children in Group A and Group B. Blood was collected immediately on admission for microbiological culture and routine blood tests. The statistical software SPSS 26.0 was used to statistically process the above information. Normality tests, Q-Q plots, Shapiro-wilk, Kolmogorov smirnov method tests were used to assess the normality of variables. Those that did not obey the normal distribution were expressed as median and quartiles, and the Mann-Whitney test was used for comparison between the two groups. Count data were expressed as sample size and percentages, and the chi-square test was used for comparison between groups. Logistic regression models were applied to analyse risk factors for PROM complicated by sepsis. The area under the subject operating characteristic (ROC) curve was used to determine the sensitivity and specificity of PLR and NLR in predicting PROM complicated by sepsis. Statistical significance was defined as p <0.05.
RESULTS
The results of the Table I showed that the differences between groups A and B were statistically significant in terms of mode of delivery, lymphocyte count, platelets, PLR and NLR; however, there were no statistically significant differences in terms of gender, gestational age, birth weight and neutrophil count (p = 0.473, 0.181, 0.713, and 0.129 respectively, Table I).
Table I: General information about the children in both groups.
|
Index |
Group A (n=99) |
Group B (n=99) |
p-value |
|
Gender [n(%)] |
|
|
0.473 |
|
Male Female |
59 (59.6) |
54 (54.5) |
|
|
40 (40.4) |
45 (45.5) |
|
|
|
Gestational week |
38 (35-39) |
38 (37-39) |
0.181 |
|
Delivery route [NSD, n (%)] |
52 (52.5) |
37 (37.4) |
0.032 |
|
Birth weight (kg) |
3.15 (2.40-3.50) |
3.05 (2.58-3.49) |
0.713 |
|
Lymphocyte (109/L) |
2.75 (2.04-3.67) |
3.12 (2.55-4.05) |
0.007 |
|
Neutrophil (109/L) |
8.79 (5.12-12.46) |
7.60 (6.70-9.23) |
0.129 |
|
PLT (109/L) |
277 (245-331) |
237 (211-262) |
<0.001 |
|
NLR |
2.98 (2.64-4.14) |
2.47 (1.95-3.01) |
<0.001 |
|
PLR |
110.09 (79.64-147.86) |
71.04 (54.73-90.37) |
<0.001 |
|
The chi-square test was used for gender and delivery route; The Mann-Whitney test was used for Gestational week, Birth weight, Lymphocyte, Neutrophil, PLT, NLR, and PLR. |
|||
Table II: Multivariate logistic regression analysis of PROM complicated by sepsis.
|
Risk factor |
B |
S.E. |
p-value |
|
Delivery route |
1.190 |
0.4 |
0.003 |
|
Lymphocyte |
0.486 |
0.339 |
0.151 |
|
PLT |
0.016 |
0.005 |
0.002 |
|
NLR |
0.454 |
0.197 |
0.021 |
|
PLR |
0.036 |
0.012 |
0.003 |
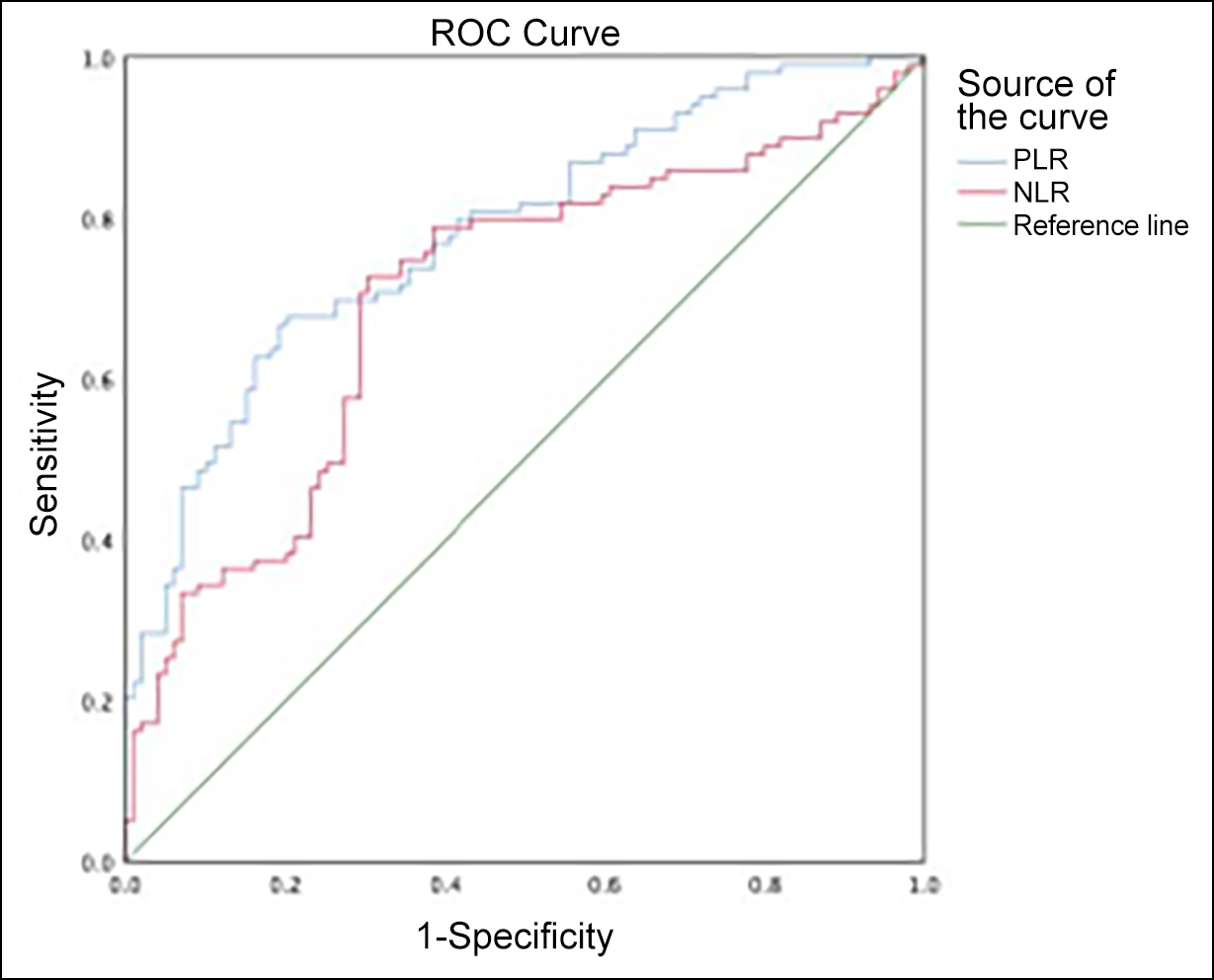 Figure 1: ROC curves for NLR and PLR predict PROM complicated by sepsis.
Figure 1: ROC curves for NLR and PLR predict PROM complicated by sepsis.
We conducted a logistic regression analysis of parturition pattern, lymphocyte count, platelets, PLR and NLR, which showed that parturition pattern, platelets, PLR and NLR were independent risk factors for premature neonatal sepsis (p =0.03, 0.151, 0.002, 0.003, and 0.021 respectively, Table II).
The ROC area for predicting PLR for PROM complicated by sepsis was 0.781 (95% CI: 0.718-0.844, p<0.001). The optimal threshold for PLR affecting PROM complicated by sepsis was 93.072, which had a sensitivity of 67.7% and specificity of 79.8% for predicting PROM complicated by sepsis.
The ROC area for predicting NLR for PROM complicated by sepsis was 0.701 (95% CI: 0.627-0.775, p<0.001). The optimal threshold for NLR affecting PROM complicated by sepsis was 2.743, which had a sensitivity of 72.7% and specificity of 69.7% for predicting PROM complicated by sepsis (Figure 1). PLR and NLR predicted PROM complicated by sepsis, with PLR predicting premature neonatal sepsis with a higher ROC area of 0.781 (95% CI: 0.718-0.844, p<0.001) than NLR.
DISCUSSION
PROM with sepsis is a serious neonatal disease that greatly affects fetal-neonatal growth and healthy development. PROM is a risk factor for neonatal sepsis and is associated with high mortality and the development of infectious diseases in children in the neonatal period.2,13 Its clinical signs are atypical and non-specific early on and can easily be misdiagnosed as other infectious diseases. A positive microbiological culture in the blood is the gold standard for the diagnosis of PROM with sepsis, but its detection time is long, usually taking 1-3 days, and its sensitivity is low. Studies have reported that only 19.2% of detections are positive.14 Therefore, the early diagnosis of PROM with sepsis has been given high priority by paediatricians.
Calcitoninogen (PCT) and C-reactive protein (CRP) were once considered to be promising clinical markers for detection15 and are released in large quantities in the blood when the body's response to infection occurs, but CRP and PCT have low specificity and peak levels of PCT at 12 hours of infection and CRP at 24-48 hours, with little significance for the early diagnosis of children with PROM with sepsis. This is of little significance in the early diagnosis of PROM with sepsis. Peripheral blood analysis is a commonly used test in clinical paediatrics, which is easy to perform, short and readily available.16 When infectious disease occurs, colony-stimulating factors, chemokines and cytokines are heavily activated and lymphocyte activation is restricted and reduced in number. At this time, the number of megakaryocytes increases, allowing for massive platelet production.17 In addition, granulocyte lifespan increases, allowing for an elevated number of neutrophils.18 However, a single test is controversial in the diagnosis of sepsis complicated by PROM. It has been shown that a severe inflammatory response occurs with apoptosis leading to a large depletion of lymphocytes and a decrease in their number.6 In addition, Can et al. reported that platelet count cannot be used as a marker to detect sepsis.19 In contrast, the results of this study showed statistically significant differences in lymphocyte counts and platelets between group A and group B. Next, we performed a logistic regression analysis of lymphocyte counts and platelets, which showed that platelets were an independent risk factor for PROM complicated by sepsis, while lymphocyte counts were not.
Recently, a series of novel inflammatory markers have been derived from peripheral blood cells: e.g. PLR and NLR, which have attracted attention in the diagnosis of clinical inflammatory diseases.20 PLR and NLR are more stable when combined with two single indicators than single neutrophil counts, lymphocyte counts and platelet counts. Studies have shown that NLR and PLR can better predict the severity and prognosis of pneumonia, cardiovascular and metabolic diseases, etc.21 The results of this study showed that the difference between groups A and B was statistically significant in terms of PLR and NLR. The results of the ROC curve showed that the ROC area for predicting PLR for PROM complicated by sepsis was 0.781 (95% CI: 0.718-0.844, p <0.001). The optimal threshold for PLR affecting PROM complicated by sepsis was 93.072, which had a sensitivity of 67.7% and specificity of 79.8% for predicting PROM complicated by sepsis. The ROC area for predicting PROM complicated by sepsis NLR was 0.701 (95% CI: 0.627-0.775, p<0.05). The optimal threshold for NLR affecting PROM with sepsis was 2.743, which had a sensitivity of 72.7% and specificity of 69.7% for predicting PROM with sepsis. the area under the PLR curve for predicting PROM with sepsis was greater than the NLR, which had a greater predictive value.
CONCLUSION
PLR and NLR are independent risk factors for PROM complicated by sepsis. The predictive value of PLR and NLR for PROM complicated by sepsis is high, and the predictive value of PLR is higher than that of NLR.
ETHICAL APPROVAL:
This retrospective descriptive study, involving human participants, was in accordance with the ethical standards of the Institutional and National Research Committee; and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Ethics Committee of Yanbian University School of Medicine approved this study.
PATIENTS’ CONSENT:
Informed consent were obtained from the patients to publish the data concerning this case.
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS' CONTRIBUTION:
SZ: Design, literature search, data acquisition, data analysis, and manuscript preparation and editing.
WZ: Revising it critically for important intellectual content.
XL: Drafting the work and manuscript preparation and editing.
ZJ: Agreement to be accountable for all aspects of the work.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin No. 188: Prelabor rupture of membranes. Obstet Gynecol 2018; 131(1):e1-e14. doi: 10.1097/AOG.0000000 000002455.
- Duan SY, Kong XY, Xu FD, Lv HY, Ju R, Li ZK, et al. [Impact of premature rupture of membranes on neonatal complications in preterm infants with gestational age <37 weeks]. Nan Fang Yi Ke Da Xue Xue Bao 2016; 36(7): 887-91.
- He XG, Xu FD, Li JF, Wu WS, Liu SJ. [Effect of different antibiotic use strategies on infection in neonates with premature rupture of membranes and high-risk factors for neonatal infection]. Zhongguo Dang Dai Er Ke Za Zhi 2020; 22(4):310-315. doi: 10.7499/j.issn.1008-8830.1910170.
- Lee SM, Park JW, Kim BJ, Park CW, Park JS, Jun JK, et al. Acute histologic chorioamnionitis is a risk factor for adverse neonatal outcome in late preterm birth after preterm premature rupture of membranes. PLoS One 2013; 8(12): e79941. doi: 10.1371/journal.pone.0079941.
- Iroh Tam PY, Bendel CM. Diagnostics for neonatal sepsis: Current approaches and future directions. Pediatr Res 2017; 82(4):574-83. doi: 10.1038/pr.2017.134.
- Li T, Dong G, Zhang M, Xu Z, Hu Y, Xie B, et al. Association of neutrophil-lymphocyte ratio and the presence of neonatal sepsis. J Immunol Res 2020; 2020:7650713. doi: 10.1155/2020/7650713.
- Celik IH, Hanna M, Canpolat FE, Pammi M. Diagnosis of neonatal sepsis: The past, present and future. Pediatr Res 2021; 91(2):337-50. doi: 10.1038/s41390-021-01696-z.
- Zhang S, Luan X, Zhang W, Jin Z. Platelet-to-lymphocyte and neutrophil-to-lymphocyte ratio as predictive biomarkers for early-onset neonatal sepsis. J Coll Physicians Surg Pak 2021; 30(7):821-4. doi: 10.29271/jcpsp.2021. 07.821.
- Stojkovic Lalosevic M, Pavlovic Markovic A, Stankovic S, Stojkovic M, Dimitrijevic I, Radoman Vujacic I, et al. Combined diagnostic efficacy of neutrophil-to-lymphocyte ratio (nlr), platelet-to-lymphocyte ratio (PLR), and mean platelet volume (MPV) as biomarkers of systemic inflammation in the diagnosis of colorectal cancer. Dis Markers 2019; 2019:6036979. doi: 10.1155/2019/6036979.
- Qin B, Ma N, Tang Q, Wei T, Yang M, Fu H, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol 2016; 26(3):372-6. doi: 10.3109/14397595.
- Djordjevic D, Rondovic G, Surbatovic M, Stanojevic I, Udovicic I, Andjelic T, et al. Neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume-to-platelet count ratio as biomarkers in critically ill and injured patients: Which ratio to choose to predict outcome and nature of bacteremia? Mediators Inflamm 2018; 2018:3758068. doi: 10.1155/ 2018/3758068.
- Mukhopadhyay S, Taylor JA, Von Kohorn I, Flaherman V, Burgos AE, Phillipi CA, et al. Variation in sepsis evaluation across a national network of nurseries. Pediatrics 2017; 139(3):e20162845. doi: 10.1542/peds.2016-2845.
- Liu J, Feng ZC, Wu J. The incidence rate of premature rupture of membranes and its influence on fetal-neonatal health: A report from mainland China. J Trop Pediatr 2010; 56(1):36-42. doi: 10.1093/tropej/fmp051.
- Jyothi P, Basavaraj MC, Basavaraj PV. Bacteriological profile of neonatal septicemia and antibiotic susceptibility pattern of the isolates. J Nat Sci Biol Med 2013; 4(2):306-9. doi: 10.4103/0976-9668.116981.
- Khan F. C-reactive Protein as a Screening Biomarker in Neonatal Sepsis. J Coll Physicians Surg Pak 2019; 29(10):951-3. doi: 10.29271/jcpsp.2019.10.951.
- Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Curr Opin Infect Dis 2012; 25(3):321-7. doi: 10.1097/QCO.0b013e3283528c9b.
- Hudzik B, Szkodzinski J, Gorol J, Niedziela J, Lekston A, Gasior M, et al. Platelet-to-lymphocyte ratio is a marker of poor prognosis in patients with diabetes mellitus and ST-elevation myocardial infarction. Biomark Med 2015; 9(3):199-207. doi: 10.2217/bmm.14.100.
- Yoon NB, Son C, Um SJ. Role of the neutrophil-lymphocyte count ratio in the differential diagnosis between pulmonary tuberculosis and bacterial community-acquired pneumonia. Ann Lab Med 2013; 33(2):105-10. doi: 10.3343/alm.2013. 33.2.105.
- Can E, Hamilcikan Ş, Can C. The value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio for detecting early-onset neonatal sepsis. J Pediatr Hematol Oncol 2018; 40(4):e229-e32. doi: 10.1097/MPH.0000000 000001059.
- Wu Y, Chen Y, Yang X, Chen L, Yang Y. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int Immunopharmacol 2016; 36:94-9. doi: 10.1016/j.intimp.2016.04.006.
- Ussell CD, Parajuli A, Gale HJ, Bulteel NS, Schuetz P, de Jager CPC, et al. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: A systematic review and meta-analysis. J Infect 2019; 78(5):339-48. doi: 10.1016/j.jinf.2019.02.006.