Diabetic Myonecrosis: A Vascular Complication
By Daniyal Nadeem1, Saira Furqan2, Nanik Ram2Affiliations
doi: 10.29271/jcpsp.2022.Supp.S162ABSTRACT
Diabetic myonecrosis is an unpublicised problem that can occur in patients having either type 1 or type 2 diabetes mellitus. It usually affects patients who have long-standing, uncontrolled diabetes with associated microvascular complications. We report a case of a 78-year female with diabetic myonecroses admitted to a tertiary care hospital. Although it is a self-limiting disease, we lay down the management approach of how to exclude the extensive list of differential diagnoses and limit the life-threatening complications as urgent evaluation is critical. Clinicians taking care of patients with uncontrolled diabetes should be mindful and aware of the complication of diabetic myonecrosis in a patient presenting with pain in any limb and having negative venous doppler ultrasound for deep vein thrombosis. Magnetic resonance imaging (MRI) is the most specific and sensitive modality for diagnosis. Muscle biopsy can be used for anomalous cases. Although diabetic muscle infarction is a rare pathology, it presents a high risk for diabetes-related morbidity and mortality.
Key Words: Myonecrosis, Diabetes mellitus, Doppler ultrasound, Magnetic resonance imaging.
INTRODUCTION
Diabetic myonecrosis, also known as diabetic muscle infarction (DMI), is a rare complication of long-standing, poorly controlled diabetes mellitus. It presents as an acute, non-traumatic, tender swelling, and pain of any of the limbs, but is more common in the lower extremities. The presentation can easily be confused with deep vein thrombosis (DVT); hence, a doppler examination is needed to rule out DVT. It is usually self-limited and resolves on conservative treatment and physiotherapy.1
In a small number of cases, it can be aggressive leading to limb destruction and if left untreated can lead to mortality. Diabetic myonecrosis was first reported by Angerall and Stener in 1965.2 Since then fewer than 200 cases have been reported worldwide in literature.3 DMI is often associated with other complications of diabetes such as nephropathy, retinopathy and/or neuropathy.4 Women are more frequently affected than men and most of the affected patients also have one or more microvascular complications. The differential diagnoses include conditions such as cellulitis, polymyositis, necrotizing fasciitis, neoplasms, and DVT. The diagnosis can be confidently established on clinical presentation and magnetic resonance imaging (MRI) findings.5 Herein, we present a case of diabetic myonecrosis in a 78-year female.
CASE REPORT
A 78-year female presented to the emergency room with chief complaints of drowsiness and pain in the right leg for 7 days. She had been using sedative analgesics (co-proxamol, gabapentin) for pain since last 7 days. The pain had been gradually increasing in intensity and became excruciating. She was unable to walk and could not bear any weight on the right leg. She had a history of type 2 diabetes mellitus for more than 10 years, hypertension and non-B, non-C chronic liver disease (CLD). She had no history of trauma, insect-bite, surgery, or injection in the area.
On physical examination, she was sleepy but oriented in time, person, and place. She had a blood pressure of 120/50 mmHg, pulse rate, 80 beats per minute, and respiratory rate, 20 breaths per minute. Her temperature was 36.4°C and was maintaining oxygen saturation of 97% on room air. The right calf was swollen and tender on palpation. The skin was erythematous and warm to touch. Motor and sensory examinations of the lower limb were unremarkable. Lower limb pulses were palpable. Precordial, respiratory, and abdominal examinations were unremarkable. Fundoscopy revealed bilateral non-proliferative retinopathy.
Laboratory investigations were suggestive of metabolic acidosis and acute kidney injury (Table I). MRI of the right leg showed asymmetric diffuse enlargement of muscles of the anterior compartment of the right leg. There were heterogeneous T1 and T2 hyperintense signals in these regions as well. There was no evidence of abscess, calcification, or air. No evidence of intra or intermuscular collections. Bones were unremarkable (Figures 1-3). Doppler ultrasound of right leg was negative for DVT. A diagnosis of diabetic myonecrosis was made.
Table I: Laboratory investigations.
|
Investigation(s) |
Value(s) |
Reference range(s) |
|
Hemoglobin (Hb) |
9.5 g/dl |
11.1-14.5g/dl |
|
Mean corpuscular volume(MCV) |
89.9 fL |
76.0-96.0 fL |
|
White cell count (WBC) |
10.8 × 109/L |
4.0-10.0x 109/L |
|
Blood urea nitrogen (BUN) |
66mg/dL |
6-20mg/dL |
|
Serum creatinine (Cr) |
2.9mg/dL |
0.6–1.2 mg/dL |
|
potassium (k) |
6.8 mmol/L |
3.5-5.1 mol/L |
|
sodium (Na) |
114 mmol/ |
136-145 mmol/l |
|
Chloride(CL) |
84 |
98-107 mEq/L |
|
BIC |
14.7 |
20-31 mmol/L |
|
PH |
7.46 |
7.31-7.42 |
|
CO2 |
21.0mmHg |
32-45mmHg |
|
PO2 |
92.30mmHg |
70-108mmHg |
|
Creatinine phosphokinase (CPK) |
10987 U/L |
34–145 U/L |
|
HbA1c |
6.8% |
4.3-6.1 |
|
Random blood glucose |
327 mg/dL |
|
|
TSH |
1.839 ulU/ml |
0.5-8.9 |
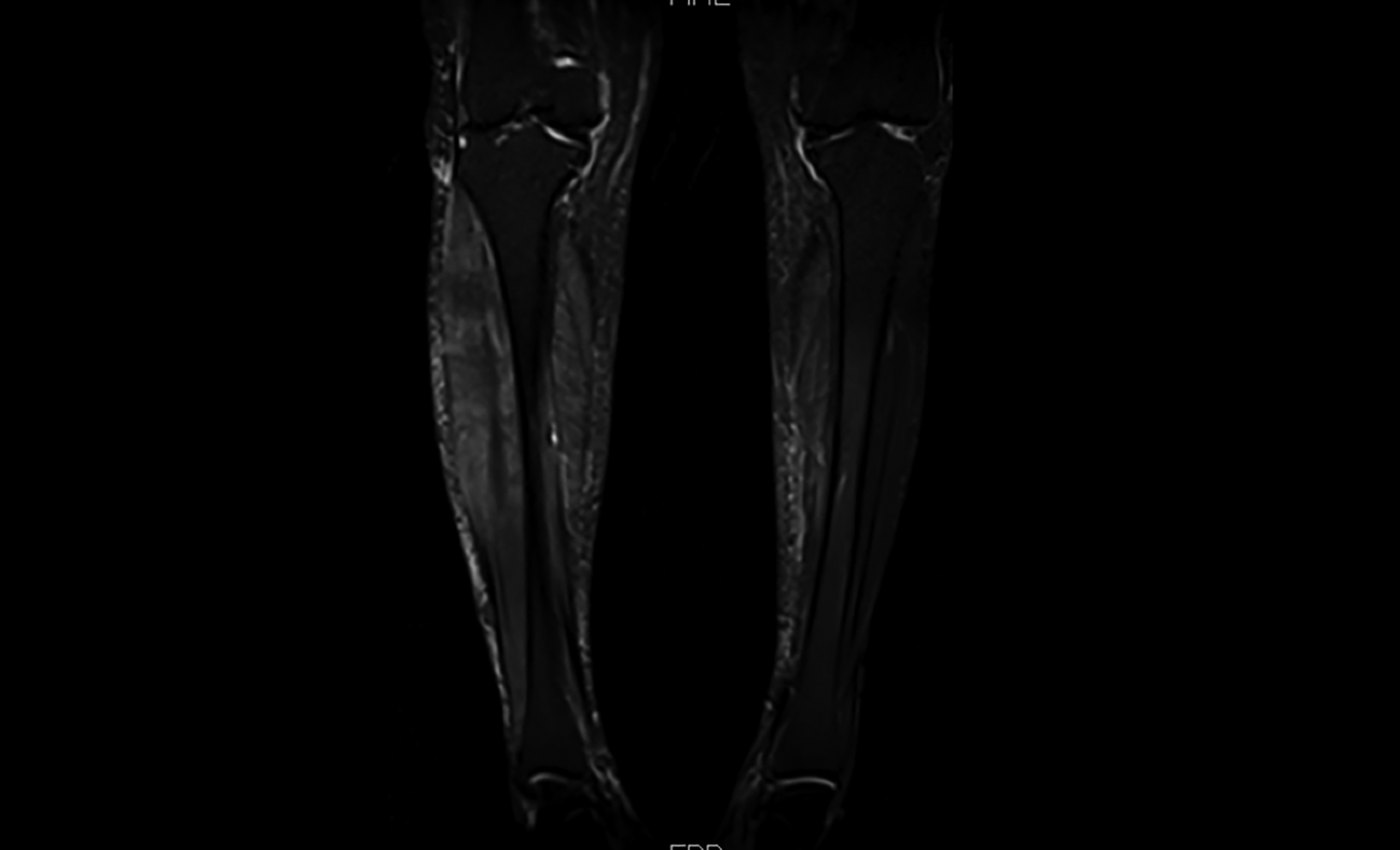 Figure 1: MRI transverse view hyperintense signals.
Figure 1: MRI transverse view hyperintense signals.
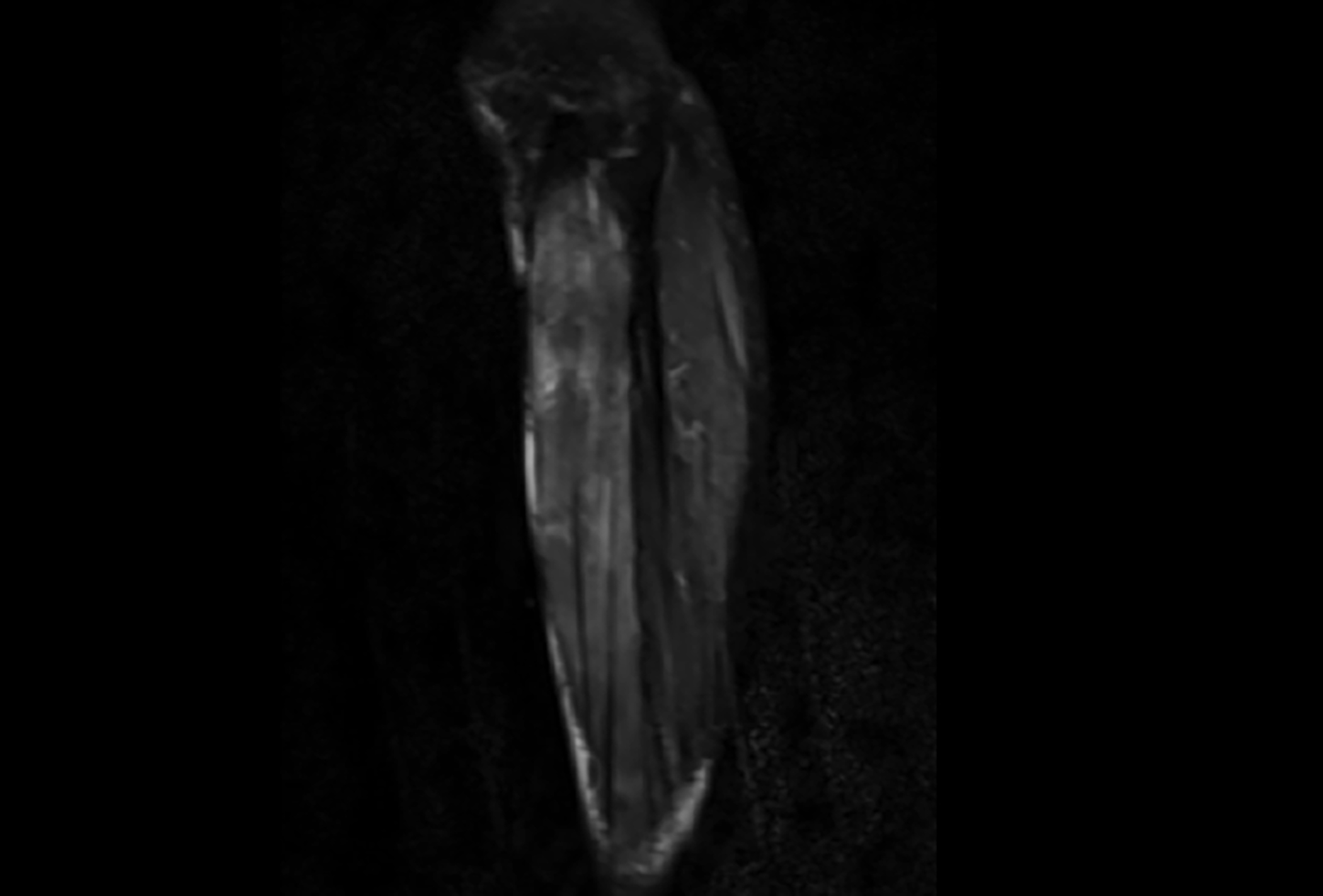 Figure 2: MRI Transverse view with hyperintense signals.
Figure 2: MRI Transverse view with hyperintense signals.
The patient developed respiratory distress and was shifted to the intensive care unit (ICU) where bilevel positive airway pressure (BIPAP) was administered. The central venous pressure (CVP) line was passed, and she was given IV fluids and analgesia. Blood and urine cultures were sent. After 2 days in ICU, she was shifted out of ICU and was managed conservatively with analgesia and physiotherapy. Her blood and urine cultures were negative. Her glucose levels were controlled by insulin and blood pressure was optimized. Creatine phosphokinase (CPK) showed a declining trend (from 10987 IU/L on day 0 to 6887 to 5369 to 3473 IU/L on subsequent days) (Figure 4). Her pain subsided and hence she was discharged after seven days of hospitalisation. On the follow-up visit 15 days later her glycemic control was good. Moreover, creatinine (Cr) and blood urea nitrogen (BUN) were in normal ranges (Cr, 0.7 mg/dl and BUN, 20 mg/dl) and a year later, CPK was 78 IU/L.
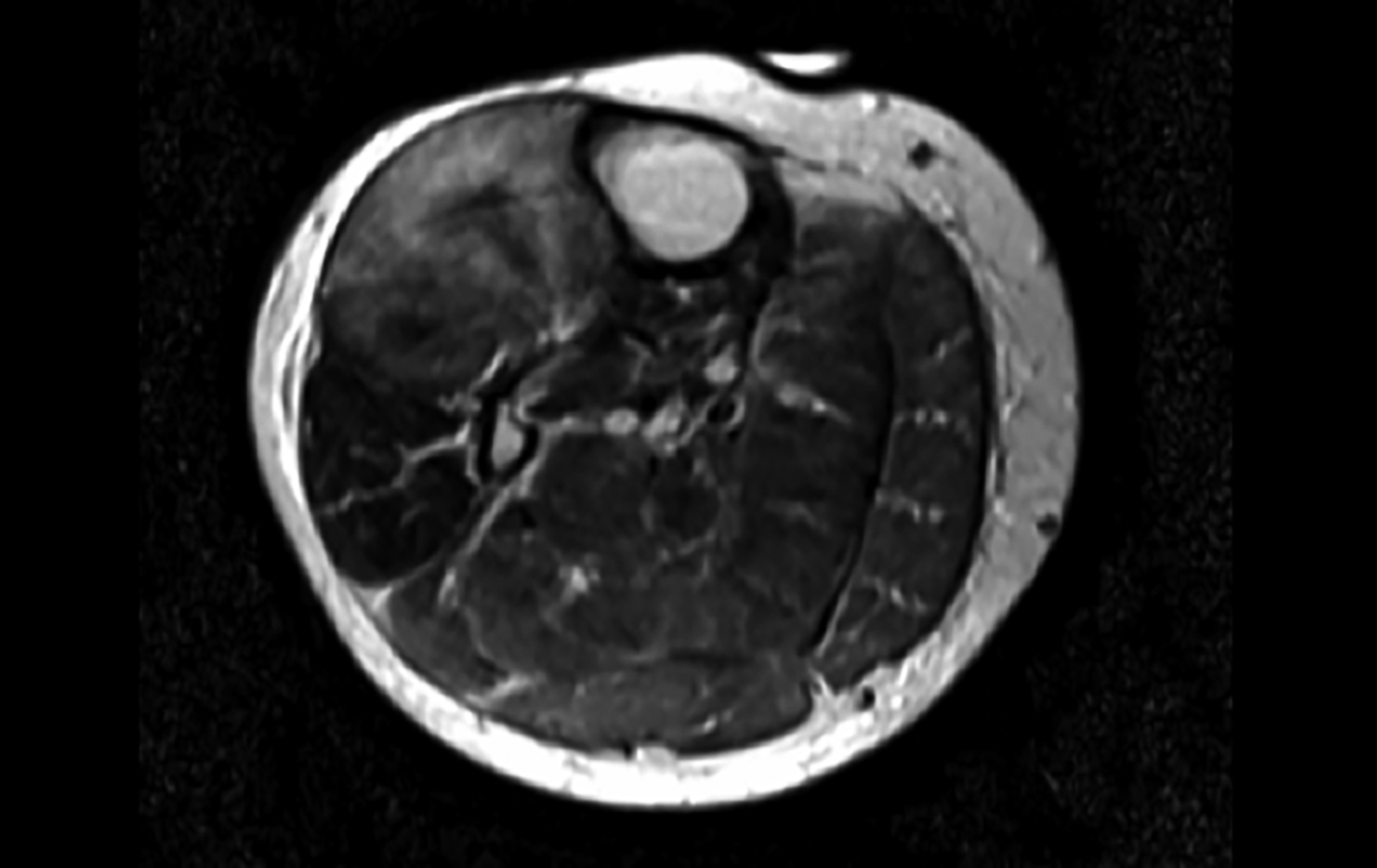 Figure 3: MRI Coronal view showing hyperintense signals.
Figure 3: MRI Coronal view showing hyperintense signals.
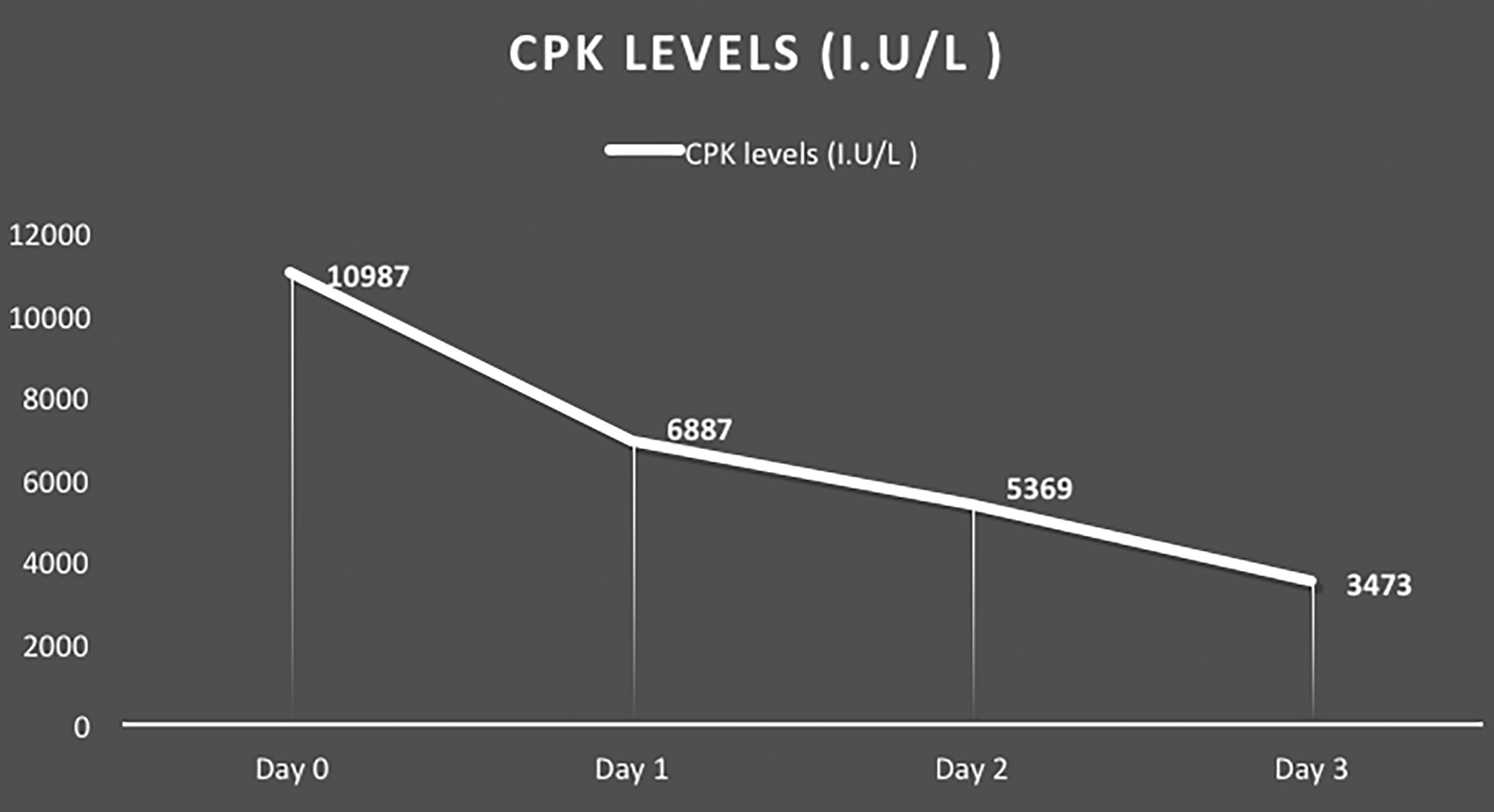 Figure 4: Creatine phosphokinase (CPK) levels of the patient.
Figure 4: Creatine phosphokinase (CPK) levels of the patient.
DISCUSSION
Diabetic myonecrosis can occur in both Type 1 and Type 2 Diabetes Mellitus. The duration of diabetes mellitus and poor glycemic control are the two main risk factors for developing diabetic myonecrosis. In a systematic review conducted on 116 patients, the majority were females (61.5%) with a mean duration of diabetes mellitus of 14.3 years.6 The thigh muscles are the most commonly affected group followed by calf muscles. The presentation is frequently unilateral with occasional bilateral involvement. Complications of vasculopathy, especially microvascular, such as nephropathy (71%), retinopathy (59%), and neuropathy (55%) were co-morbid conditions.4
The pathophysiology of myonecrosis related to diabetes is unknown. Several theories have been proposed including arteriosclerosis, microangiopathy, ischemia-reperfusion injury, and alterations in the coagulation-fibrinolysis system. Chester and Banker advocated an atheroembolic occurrence, but the results of their study were more consistent with arteriosclerosis obliterans, which has now become the widely accepted plausible hypothesis.7 Some other hypotheses regarding the aetiology of diabetic myonecrosis include abnormal coagulation pathways such as enhanced factor VII activity and increased plasminogen activator inhibitor. Another hypothesis stems from the presence of phospholipid antibodies but all these hypotheses need further studies and data to substantiate.8
Clinical manifestations of diabetic myonecrosis include acute or subacute pain, swelling, and tenderness, typically in the thigh or calf. There is usually involvement of more than one muscle compartment. However, in this patient, only the anterior compartment of leg was involved with the sparing of all other compartments. Differential diagnosis of diabetic myonecrosis includes polymyositis, necrotizing fasciitis, DVT, soft tissue infections, and hematomas leading to compartment syndrome. According to a new study, an increase in CPK was witnessed in patients of with diabetes type 2. The study found that most cases were due to primary myopathy such as metabolic myopathy leading to a rise in CPK levels. They proposed that a further neurologic workup is warranted in patients with diabetes with high CPK levels.9 To diagnose this syndrome, MRI is the preferred modality as it is non-invasive, sensitive and provides complete anatomical delineation. Muscle biopsy is always the gold standard but is only used in anomalous cases, as it is invasive and requires expertise.4 Regarding the more invasive and active management such as surgery, Kapur and Mckendry described in their study that the recovery period is almost double in patients undergoing surgery (13 weeks) than in the conservative treatment group (5.5 weeks).10 The recurrence of diabetic myonecrosis in the same muscle or contralateral limb is greater than 50% and there can be at least one episode per year after the first one. The prognosis of patients after a myonecrosis event is not good and according to a recent review, most of the patients die in the next 5 years.10
Diabetic myonecrosis is a rare complication of long-standing poorly controlled diabetes mellitus. Clinicians taking care of patients with uncontrolled diabetes should be mindful and aware of the complication in patients presenting with pain in any limb and negative venous doppler ultrasound for DVT. MRI is the most specific and sensitive modality for diagnosis. Muscle biopsy can be used for anomalous cases. Because the differential diagnosis is extensive and includes several limb and life-threatening conditions, urgent evaluation is critical.
PATIENT’S CONSENT:
Informed consent was obtained from the patient to publish the data.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
DN: Wrote this manuscript.
SF: Edited and proofread the manuscript.
NR: Wrote and managed the case as a primary physician.
REFERENCES
- Khanna HK, Stevens AC. Diabetic myonecrosis: A rare complication of diabetes mellitus mimicking deep vein thrombosis. American J Case Report 2017; 18:38-41. doi: 10.12659/ajcr.900903.
- Angervall L, Stener B. Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia 1965; 1(1):39-42. doi.org/10.1007/BF01338714.
- Joshi T, D’Almeida E, Luu J. Diabetes myonecrosis – A rare complication. Diabetes Research and Clinical Practice 2015; 109(3):e18-e20. doi.org/10.1016/j.diabres.2015.06.004.
- Trujillo-Santos AJ. Diabetic muscle infarction: an underdiagnosed complication of long-standing diabetes. Diabetes Care 2003; 26(1):211-5. Doi. 10.2337/diacare. 26.1.211.
- Mazoch MJ, Bajaj G, Nicholas R, Pandey T, Jambhekar K, Ram R, et al. Diabetic myonecrosis: Likely an under-recognized entity. Orthopedics 2014; 37(10):e936-9. Doi. 10.3928/01477447-20140924-91.
- Habib GS, Nashashibi M, Saliba W, Haj S. Diabetic muscular infarction: emphasis on pathogenesis. Clinical Rheumatology 2003; 22(6):450-1. Doi.10.1007/s10067- 003-0789-z.
- Chester CS, Banker BQ. Focal infarction of muscle in diabetics. Diabetes Care 1986; 9(6):623. Doi. 10.2337/ diacare.9.6.623.
- Bjornskov EK, Carry MR, Katz FH, Lefkowitz J, Ringel SP. Diabetic muscle infarction: A new perspective on pathogenesis and management. Neuromuscular Disorders 1995; 5(1):39-45. Doi. 10.1016/0960-8966(94)e0027-6.
- Frank M, Finsterer J. Creatine kinase elevation, lactacidemia, and metabolic myopathy in adult patients with diabetes mellitus. Endocrine Practices 2012; 18(3): 387-93. Doi. 10.4158/EP11316.OR.
- Kapur S, McKendry RJ. Treatment and outcomes of diabetic muscle infarction. J Clinical Rheumatol 2005; 11(1):8-12. Doi. 10.1097/01.rhu.0000152142.33358.f1.