Development and Validation of In-house HEp-2 Cell Slides for Detection of Antinuclear Antibodies (ANA) by Indirect Immunofluorescence
By Muhammad Zain Arshad1, Dawood Ahmad1, Muhammad Hussain1, Hamid Nawaz Tipu1, Muhammad Omair Riaz1, Ayesha Tanveer2Affiliations
doi: 10.29271/jcpsp.2023.03.292ABSTRACT
Objective: To develop and validate in-house HEp-2 cell slides for the detection of ANA by indirect immunofluorescence.
Study Design: Cross sectional validation study.
Place and Duration of the Study: Department of Immunology, Armed Forces Institute of Pathology Rawalpindi, Punjab, Pakistan, from April to September 2022.
Methodology: This study involved development of in-house HEp-2 cell slides after procuring cell lines, sub-culturing and fixing them on different slides using variety of fixatives under different protocols. After standardisation of procedure, validation of procedure was done by testing sera of 305 patients for ANA detection at 1:40 dilution on in-house HEp-2 cell slides and subsequently on commercial HEp-2 cell slides (gold standard). Indirect immunofluorescence was observed by the two observers working independently and kept blinded from the results interpreted by each other. Data were collected on a pre-designed proforma and then analysed. Sensitivity, specificity, and positive predictive value (PPV) and negative predictive values (NPV) of in-house HEp-2 cell slides were calculated.
Result: Sera of 305 patients were tested on in-house and commercial HEp-2 cell slides. Sensitivity and specificity of in-house HEp-2 cell slides for ANA detection were 96.92% and 99.58%, respectively. PPV and NPV of in-house HEp-2 cell slides came out to be 98.43% and 99.17% respectively.
Conclusion: In-house HEp-2 cell slides are as effective as commercial HEp-2 cell slides for the detection of ANA and can be used as cost-effective alternative.
Key Words: Antinuclear antibodies (ANA), Human epithelial type-2 (HEp-2), Immunofluorescence.
INTRODUCTION
Antinuclear antibodies (ANA) are directed against nuclear antigens of the organism.1,2 ANAs are found in different autoimmune disorders (rheumatoid arthritis, systemic lupus erythematosus, Sjogren's syndrome, and mixed connective tissue diseases etc.) and even in healthy individuals. They can even appear well before the disease.2
Different methods are available to detect ANA in the serum, such as indirect immunofluorescence (IIF) and enzyme-linked immunosorbent assays (ELISA).3,4
Based on American College of Rheumatology recommendations, indirect immunofluorescence assays (IIFAs) using HEp-2 cell lines as substrate are the gold standard for ANA detection for the suspected autoimmune diseases.4
ANA test results are widely used for patient's screening, diagnosis, monitoring, and treatment. Fluorescence pattern detected on immunofluorescent microscope (homogenous, nucleolar, speckled, nuclear membrane, and centromere etc.) is very specific as per nature of the self-antigen and its location in the cell or organelle, therefore particular attention should be given to determine ANA pattern and titer.4 ANA testing on HEp-2 cells has its pitfalls because standardisation is difficult due to inter-manufacturer variability and subjective nature of indirect immunofluorescence. Additionally, anti Sjogren's syndrome related antigen A antibodies (SSA), Ro-52 and Jo-1 may not be easily detected on HEp-2 cells despite being present in high titers.4
The major step of ANA detection is the interpretation of observed patterns on HEp-2 cells for reaching correct diagnosis.5 A wide range of nuclear, cytoplasmic, and mitotic patterns can be detected, which are generated by more than 100 different autoantibodies.6 Important mitotic and cytoplasmic patterns are also observed on HEp-2 cells, leading to an alternative name of anti cellular antibodies presented in EASI/IUIS recommendations.7 Indirect immunofluorescence is undoubtedly the gold standard for ANA detection, titration and pattern determination in systemic autoimmune rheumatic diseases (SARD).7
Various substrates were used in the past, such as tissue sections, chicken erythrocytes, desquamated cells, HeLa cells, and rodent liver or composite multi-block substrates. HEp-2 cells were introduced in 1975 with higher sensitivity for antinuclear antibodies testing. These are cultured human cells of laryngeal squamous cell carcinoma, and are now used as a substrate.8 Due to the difference in sensitivity between substrates, it is important to specify the type of substrate being used for ANA testing.8 The advantages of HEp-2 cells over rodent substrate include increased sensitivity allowing identification of multiple patterns, increased specificity, better visualisation of nuclear complexity, clear visibility of cells due to monolayer, easy identification of antigens due to high cellular division, and absence of obscuring intercellular matrix along with homogenous antigen distribution.
As part of ANA reporting, three parameters are considered; fluorescence pattern, substrate, and titer. Most laboratories, consider a titer over 1:80 to be significant for the diagnosis of autoimmune diseases.8,9 Occasionally, patients with autoimmune diseases will test negative on mouse kidney or rat liver but positive on human substrates like HEp-2 cell lines.10,11 Automated pattern recognition systems have recently been introduced that is comparable with manual interpretation.11
Although, IIF is an excellent screening test for experienced hands, the technical limitations of processing and interpreting IIF slides including the tedious slide handling, manual interpretation, need for highly trained technicians and use of dark room can make adaptation of IIF difficult to fit in the workflow of modern automated laboratories.12
Most of the indirect immunofluorescence assay for ANA are done on commercial products which are very expensive and the practical data for development and validation of in-house HEp-2 cell slide is scarce. The objective of conducting this study was to find out optimal conditions for the development of in-house HEp-2 cell slides using different available fixatives and then validation of method by keeping commercial HEp-2 cell slides as reference.
METHODOLOGY
This cross-sectional validation study was carried out at the Department of Immunology, Armed Forces Institute of Pathology Rawalpindi, Punjab, Pakistan, from April to September 2022 after formal approval by institutional review board (IRB/22/958) and obtaining written informed consent from patients.
Inclusion criteria were patients of any age and gender presenting at (Armed Forces Institute of Pathology) for ANA testing, Sera from patients with unequivocal results e.g either positive or negative along with recognisable pattern. Patients with ambiguous test results were excluded.
Sample size (n) was calculated using sensitivity and specificity calculator by keeping the following given assumptions; expected sensitivity 87.7%,13 expected specificity 67.8%,13 prevalence (p) 13.8%,14 precision (acceptable) 0.10, and confidence level (CI) as 95%. Calculated sample size was 305.
The study involved development of in-house HEp-2 cell slides after procuring cell lines and subculturing 20ul of HEp-2 cell suspension having viable count of 4 hundred thousand per ml on wells of multi-well plastic slides. Slides were then incubated in 5% CO2 incubator at 35-36oC for 90 minutes after which each well was enriched with 10μl of 10% HMEM (hepes minimum essential medium). Slides were incubated in CO2 incubator for further 12 hours. After incubation, microscopy was performed and each well was again enriched with 10μl of 10% HMEM. Slides were again incubated in CO2 incubator till 24 hours passed after initial sub-culturing on slides. Each slide was rinsed thrice with PBS and then fixed by keeping it in ice-cold pure methanol for 15 minutes. After removing from methanol, each slide was rinsed thrice with PBS and kept in PBS till its use or stored at -60oC for upto 10 days after wrapping in aluminium foil. After standardisation of procedure, validation of procedure was done by testing sera of 305 patients for ANA detection at 1:40 dilution on in-house HEp-2 cell slides and subsequently on commercial HEp-2 cell slides-EUROIMMUN Medizinische Labordiagnostika AG (Gold Standard) (Figure 1). Indirect immuno-fluorescence was observed by two observers working independently and kept blinded from results interpreted by each other (Figure 2).
Data were collected on predesigned proforma and analysed using SPSS version 23.0. Sensitivity, specificity, positive predictive value, and negative predictive values of in-house HEp-2 cell slides were calculated using 2x2 contigency table (Table I).
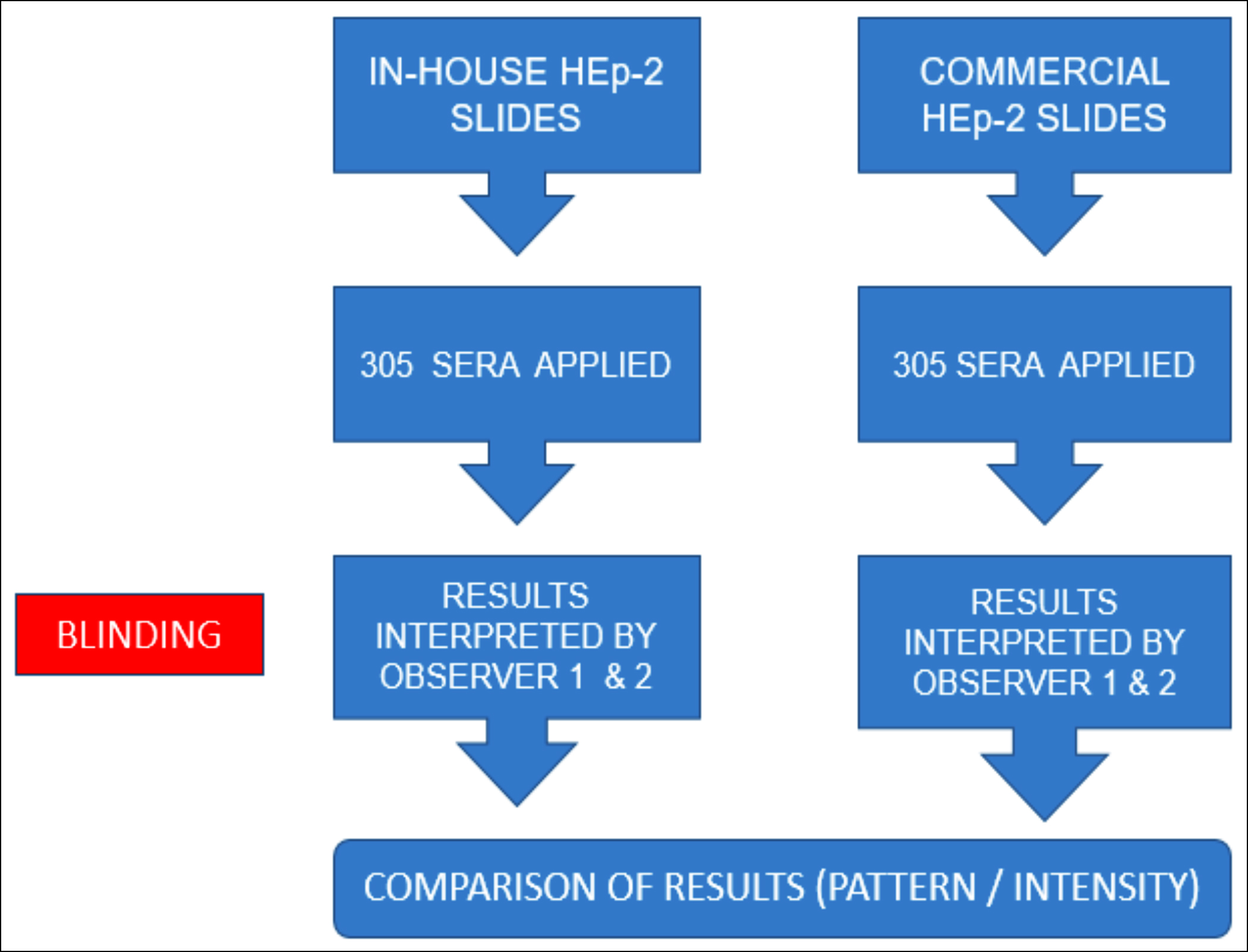 Figure 1: Steps of validation.
Figure 1: Steps of validation.
Table I: Comparison of immunofluorescence on commercial and in-house HEp-2 cell slides.
|
|
Commercial HEp-2 Cell Slide Results |
Total |
||
|
Positive |
Negative |
|||
|
In-House HEp-2 Cell Slide Results |
Positive |
63 (TP)* |
1 (FP)** |
64 |
|
Negative |
2 (FN)*** |
239 (TN)**** |
241 |
|
|
Total |
65 |
240 |
305 |
|
|
* True Positives (TP) = 20.65 %, ** False Positives (FP) = 0.32%, *** False Negatives (FN) = 0.65%, **** True Negatives (TN) = 78.36%. |
||||
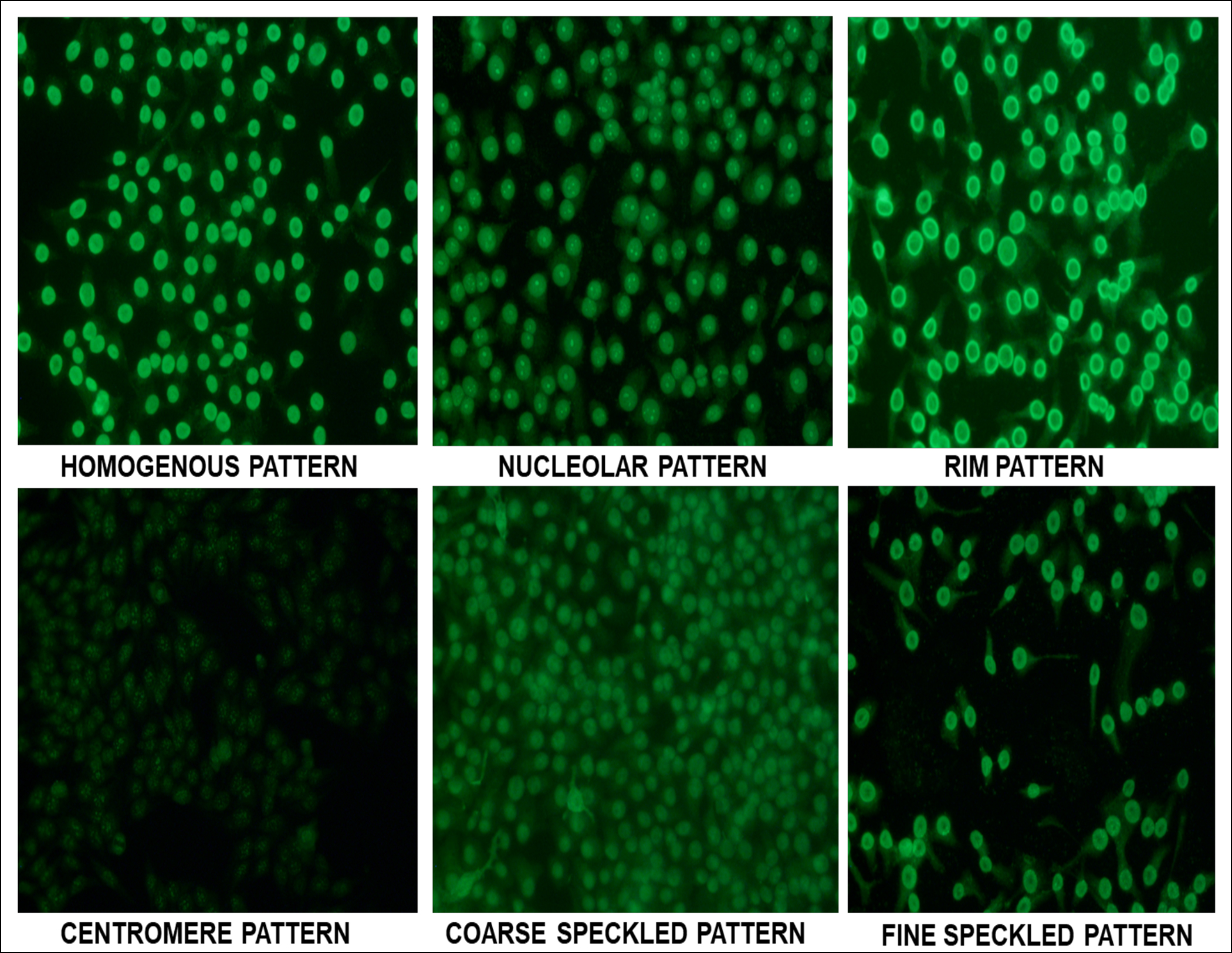 Figure 2: Common ANA patterns on in-house HEp-2 cell slides.
Figure 2: Common ANA patterns on in-house HEp-2 cell slides.
RESULTS
Out of those tested (n = 305), 122(40%) were males and 183(60%) were females. Sensitivity and specificity of in-house HEp-2 cell slides for ANA detection were 96.92% and 99.58%, respectively, whereas PPV and NPV of in-house HEp-2 cell slides came out to be 98.43% and 99.17%, respectively (Table I). Inter-observer agreement was 99.7% and percentage agreement was 97%.
DISCUSSION
IIF using HEp-2 cell slides is the gold standard method for ANA detection. Khuntikij et al. in Thailand-based study showed that the optimal time for harvesting cells was 42 hours.15 The spreading of cells on slide was 90-100% confluent and 2-5 dividing were observed under phase contrast microscope at magnification of 400X. For ANA pattern determination, both 1% paraformaldehyde and methanol-acetone fixed cells represented correct ANA positivity for homogeneous, speckled, peripheral, nucleolar, and centromere patterns. However, cells fixed with 1% paraformaldehyde comparatively showed more background staining than cells fixed with methanol-acetone mixture. Their results suggested that the methanol-acetone mixture was the better fixative for ANA screening by IIF.15 In this study, the best time for harvesting HEp-2 cells for further fixation and staining was 24 hours at which there was maximum confluency with minimum cell death. This time difference may be because Khuntikij et al. seeded wells with HEp-2 cell suspension of 3 hundred thousand per ml,15 whereas according to the present observation, it was better to seed well with cell suspension of 4 hundred thousand per ml. In the present study, the best fixative for HEp-2 cell was found to be methanol which showed better nuclear details and less cytoplasmic staining whereas cytoplasmic staining was more pronounced in formaldehyde fixation.
Tebo et al. in US based study concluded that at a screening titer of 1:80, ANA prevalence among U.S. based population greater than 12 years of age was 13.8%.14 Satoh et al. reported ANA prevalence gradually increases with age (p = 0.01) and was more among females as compared to males (17.8% vs. 9.6%, p <0.001), where the female-to-male ratio was maximum at 40–49 years of age. In ANA-positive individuals, nuclear staining was detected in 84.6%.16 In the present study, 21% females and 22% males presenting to tertiary care were ANA positive with overall prevalence of 21.3%. Avery et al. in an international study evaluated ANA, anti-ENA, and anti-dsDNA antibody prevalence among patient cohorts from primary care to tertiary care, as 6.2%, 10.8% and 16.0% respectively.17 Aktamov et al. conducted a study to determine the prevalence of anti-nuclear autoantibodies (ANA) among adult German population and evaluated the relation between ANAs and metabolic and cardiovascular disorders. In the subject study, 33% out of the 1196 participants were ANA positive (titer greater than 1:80). Weak, moderate, and strong positive ANA proportions were 29%, 3.3%, and 1.3%, respectively. ANA positivity was more prevalent among women as compared to men across all titers (p = 0.03).18
Monce et al. reported that fluorescence was more pronounced and easier to detect on the acetone fixed slides as compared to alcohol/acetone fixed slides.19 According to their test results, fixation of HEp-2 cells with a pure acetone yielded 97.5% sensitivity when anti-SS-A (Ro) antibody positive samples were tested, however, only 81.3% sensitivity was observed on HEp-2 cells fixed with alcohol/acetone solution mixture when detecting anti-SS-A (Ro) antibodies.19 Khuntikij et al. showed results similar to the present study, whereas findings by Tsiakalou et al. and Monce et al. were in contrast to the present findings.20 Brito et al. in one of the Brazil based study quoted sensitivity and specificity of HEp-2 cells at 1:80 dilution as 87.7% and 67.8% respectively and prevalence in healthy individuals as 13.2%.13 In the present study, 21.3% of patients presenting to tertiary care facility were tested ANA positive. This increase in ANA positivity may be due to demographic factors and high risk testing in the case. In the present study, in-house HEp-2 cells slides had a sensitivity and specificity of 96.92% and 99.58%, respectively.
Hahm et al. revealed that aldehyde fixatives conserved the cell structures best and acetone fixatives revealed remarkable changes. These findings corroborated the present research.21 Limitations of the present study include negligible previous data availability and minor inter-observer variation because of subjective nature of interpretation of indirect immunofluorescence.
CONCLUSION
In-House HEp-2 cell slides are as effective as commercial HEp-2 cell slides for the detection of ANA and can be used as cost-effective alternative.
ETHICAL APPROVAL:
This study was approved by the institutional ethical review board (IERB) and an ethical approval letter was issued to the authors.
PATIENTS’ CONSENT:
Informed written consents were obtained from the patients to publish the data.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
MZA: Designed the study and analysed it.
DA: Conception of idea and revised it critically.
MH: Contributed to data collection.
HNT, MOR: Compiled the data and supervised the study.
AT: Contributed to data analysis.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Cascio D, Taormina V, Raso G. An automatic HEp-2 specimen analysis system based on an active contours model and an SVM classification. Appl Sci 2019; 9(2):307. doi.org/ 10.3390/app9020307
- Ashraf S, Afzal N, Kashif M, Shahzad F, Sajjad W, Latif W, et al. Prevalence of autoantibodies in healthy adults in Pakistani population. Bangladesh J Med Sci 2016; 15(4): 579-82. doi.org/10.3329/bjms.v15i4.27834.
- Berwal A, Bairy I, Gupta A. Demystifying antinuclear antibodies and other serological tests in dermatology practice. Clin Dermatol Rev 2019; 3(1): 29-33. doi.10. 4103/CDR.CDR_49_18.
- Tipu HN, Bashir MM. Determination of specificity and pattern of antinuclear antibodies (ANA) in systemic rheumatic disease patients positive for ANA testing. J Coll Physicians Surg Pak 2018; 28(1):40-3. doi: 10.29271/ jcpsp.2018.01.40.
- Gupta V, Bhavsar A. Feature importance for human epithelial (HEp-2) cell image classification. J Imaging 2018; 4(3): 46. doi.org/10.3390/jimaging4030046.
- Perner P, Perner H, Müller B. Mining knowledge for HEp-2 cell image classification. Artif Intell Med 2002; 26(1-2): 161-73. doi: 10.1016/s0933-3657(02)00057-x.
- Damoiseaux J, Andrade LEC, Carballo OG, Conrad K, Francescantonio PLC, Fritzler MJ, et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: The inter-national consensus on ANA patterns (ICAP) perspective. Ann Rheum Dis 2019; 78(7):879-89. doi: 10.1136/annrheumdis-2018-214436.
- Kumar Y, Bhatia A, Minz RW. Antinuclear antibodies and their detection methods in diagnosis of connective tissue diseases: Diagn Pathol 2009; 4:1. doi: 10.1186/1746- 1596-4-1.
- Ghosh P, Dwivedi S, Naik S, Agarwal V, Verma A, Aggarwal A, et al. Antinuclear antibodies by indirect immuno-fluorescence: Optimum screening dilution for diagnosis of systemic lupus erythematosus. Indian J Med Res 2007; 126(1):34-8.
- Cook L. New methods for detection of anti-nuclear antibodies. Clinical Immunol Immunopathol 1998; 88(3): 211-20. doi: 10.1006/clin.1998.4560.
- Evans J. Antinuclear antibody testing in systemic autoimmune disease. Clin Chest Med 1998; 19(4):613-25. doi: 10.1016/s0272-5231(05)70106-4.
- Buchner C, Bryant C, Eslami A, Lakos G. Anti-nuclear antibody screening using HEp-2 cells. J Vis Exp 2014; 88: e51211. doi: 10.3791/51211.
- Brito FdA, Santos SME, Ferreira GA, Pedrosa W, Gradisse J, Costa LC, et al. Detection of anti-nuclear antibodies by indirect immunofluorescence on HEp-2 cells: Setting the appropriate screening dilution for the diagnosis of autoimmune rheumatic diseases. Revis Bras Reumatol (English Edition) 2014; 54(1):13-20.
- Tebo AE. Recent approaches to optimize laboratory assessment of antinuclear antibodies. Clin Vaccine Immunol 2017; 24(12):e00270-17. doi: 10.1128/CVI. 00270-17.
- Khuntikij P SS, Graidist P, Thongsuksai P. Preparation of HEp-2 cells for antinuclear antibody detection using immunofluorescent technique. J Med Tech Assoc Thailand April 2010; 38(1).
- Satoh M, Chan EKL, Ho LA, Rose KM, Parks CG, Cohn RD, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum 2012; 64(7):2319-27. doi: 10.1002/art.34380.
- Avery TY, van de Cruys M, Austen J, Stals F, Damoiseaux JGmc. anti-nuclear antibodies in daily clinical practice: prevalence in primary, secondary, and tertiary care. J Immunol Res 2014; 2014:401739. doi: 10.1155/2014/ 401739.
- Akmatov MK, Röber N, Ahrens W, Flesch-Janys D, Fricke J, Greiser H, et al. Anti-nuclear autoantibodies in the general German population: Prevalence and lack of association with selected cardiovascular and metabolic disorders—findings of a multicenter population-based study. Arthritis Res Ther 2017; 19(1):127. doi: 10.1186/s13075-017-1338-5.
- Moncé NM, Cappel VL, Saqueton CB. A comparison of two fixatives on IFA HEp-2 slides for the detection of antinuclear antibodies. J Immunoassay 1994; 15(1):55-68. doi: 10. 1080/15321819408009571.
- Tsiakalou V, Tsangaridou E, Polioudaki H, Nifli A-P, Koulentaki M, Akoumianaki T, et al. Optimized detection of circulating anti-nuclear envelope autoantibodies by immuno-fluorescence. BMC Immunol 2006; 20. doi: 10. 1186/1471- 2172-7-20.
- Hahm D, Anderer U. Establishment of HEp-2 cell preparation for automated analysis of ANA fluorescence pattern. Cytometry A 2006; 69(3):178-8. doi: 10.1002/cyto.a. 20223.