Daptomycin MICs by Broth Microdilution Method Against Staphylococcus aureus in Clinical Specimens: A Cross-Sectional Study from Pakistan
By Mohammad Zeeshan1, Imran Ahmed1, Seema Irfan1, Moiz Ahmed Khan2, Afia Zafar1Affiliations
doi: 10.29271/jcpsp.2024.01.27ABSTRACT
Objective: To explore the distribution of daptomycin (DAP) minimum inhibitory concentrations (MICs) in Staphylococcus aureus isolated from complicated skin, soft tissue, and bloodstream infections collected from the Pakistani population using broth microdilution (BMD).
Study Design: Descriptive, cross-sectional study.
Place and Duration of the Study: Department of Pathology and Laboratory Medicine, The Aga Khan University Hospital, from May to October 2021.
Methodology: Through consecutive sampling techniques, 169 Staphylococcus aureus (S. aureus) isolated from clinical specimens including pus, tissue, and blood were collected. Patients’ data including age, gender, geographical location, specimen site, and methicillin susceptibility were collected from the laboratory data. BMD was used to determine MICs of clinical isolates and S. aureus ATCC 29213. DAP MIC ≤1.0 µg/ml was considered susceptible according to the Clinical and Laboratory Standards Institute M100.
Results: Among all the clinical isolates, 144 (85%) and 25 (15%) were from skin and soft tissue and blood, respectively. All isolates were susceptible to DAP with MIC50, MIC90, and MIC range of 0.25 µg/ml, 0.5 µg/ml, and 0.06 – 0.5 µg/ml, respectively.
Conclusion: These study findings demonstrated low in-vitro MICs for DAP against S. aureus in tested isolates from a diverse variety of patient specimens from across Pakistan.
Key Words: Daptomycin, Staphylococcus aureus, Broth microdilution, Minimum inhibitory concentrations.
INTRODUCTION
Staphylococcus aureus (S. aureus) is the topmost bacterial pathogen that causes all-cause all-age mortality due to sepsis.1 Moreover, it is also among the six leading pathogens for deaths associated with resistance.2 S. aureus survives and adapts in different environmental niches due to its intricate regulatory network which controls the production of virulence factors in both the temporal environment and host.3 Moreover, the unique mechanism of resistance against multiple important therapeutic agents further complicates the situation.4 It is the only gram-positive pathogen placed in the high-priority category by the World Health Organization (WHO) in its list of pathogens of global concern, which emphasises research and development for novel and effective antibiotic options.5
The therapeutic effectiveness and safety of vancomycin in the treatment of complex methicillin-resistant S. aureus (MRSA)-associated infections started to raise concerns due to the “minimum inhibitory concentrations (MICs) creep” phenomenon, nephrotoxicity, and challenging therapeutic drug monitoring (TDM). Hence, alternative agents were sought that could maintain the same bactericidal capabilities, having more stable MICs among susceptible strains over a long period and at the same time mitigating the effects of therapy-associated morbidity.6
Among the spectrum of anti-staphylococcal antibiotics as an alternative to vancomycin, the in-vitro evaluation of daptomycin (DAP) molecule against various gram-positive bacteria started in 1986 and was approved for skin and soft tissue infections (SSTIs) by the U.S. Food and Drug Administration in 2003. Moreover, the guideline recommended DAP as an alternate in patients with native and prosthetic valve endocarditis who are intolerant of vancomycin or with vancomycin-resistant staphylococcal infections and as an empirical therapy for staphylococcal and streptococcal SSTIs.7,8
Although daptomycin non-susceptible (DAP-R) S. aureus is not common, it has been associated with lower DAP doses and high-inoculum infections (e.g., infective endocarditis and abscesses). Moreover, vancomycin-associated development of vancomycin-intermediate S. aureus (VISA) phenotype had also been linked with increased resistance to DAP during therapy.9,10
The objective of this study was to explore the distribution of DAP MICs in S. aureus isolated from complicated SSTIs and bloodstream infections collected from the Pakistani population using broth microdilution (BMD).
METHODOLOGY
This was a descriptive, cross-sectional study conducted over a 6-month period from May to October 2021 at the Department of Pathology and Laboratory Medicine, The Aga Khan University Hospital, Karachi, Pakistan. All clinical specimens of pus, tissue, and blood for culture submitted to the laboratory during the period of the study were included. Exclusion criteria included all clinical specimens other than pus, tissue, and blood and specimens submitted before the study’s sample collection period. These samples originated from patients either admitted to the hospital or were submitted at laboratory collection units located across the country, transported in controlled conditions, and processed in the central laboratory of Karachi according to the standard recommendations.11
The sample size was calculated from the findings of a previous single-centre study from Pakistan determining the efficacy of DAP against clinical isolates of MRSA,12 using the WHO sample size software. A minimum sample size of 158 S. aureus isolates was estimated to be needed to detect an expected DAP efficacy of 97.78%, keeping a 95% confidence interval and a 2.3% margin of error.
S. aureus was identified with the Staphaurex latex agglutination test (ThermoFisher SCIENTIFIC, USA), an agglutination test that detects the presence of bound coagulase and Protein A, and conventional tube coagulase test. Patients’ demographic information, specimens’ source, collection date, and methicillin susceptibility status, were recorded on a proforma.
Disc diffusion method with cefoxitin (30 µg) disc was used for methicillin susceptibility testing. Zone sizes ≤21 mm were labelled as resistant and ≥22 mm as susceptible.13 Identified strains were saved in Glycerol Phosphate Buffer (GPB) at -20oC.
Daptomycin drug powder was obtained from Sigma-Aldrich (Merk KGaA, Darmstadt, Germany) and MICs were determined by broth microdilution (BMD) method. The antibiotic stock solution was prepared by choosing a suitable range of DAP MICs against S. aureus as per the Clinical and Laboratory Standards Institute (CLSI). According to CLSI M100-S31, DAP MIC of ≤1 µg/ml was susceptible. Four-fold doubling dilutions of DAP above and below 1 µg/ml were chosen within the range of 0.06 – 16 µg/ml for susceptibility testing. The potency ‘P’ of DAP powder given by the manufacturer (Sigma-Aldrich) was 900 µg/mg. DAP stock solution was prepared.
The bacterial isolates were revived on Sheep Blood Agar (SBA) and incubated at 37oC for 24 hours in ambient air. A turbidity index of 0.5 McFarland was prepared from the isolated colonies in phosphate-buffered saline (PBS). Inoculum purity plates were set on SBA at the time of inoculation and were evaluated before reading the MICs. BMD was performed in 96-well U-bottom plates (Corning 96 Well Poly-D-Lysine Treated Microplate; Thermo Fisher) according to CLSI M7-A10. Doubling dilutions of the DAP were made in cation-adjusted Mueller Hinton broth (CA-MHB; Sigma-Aldrich, Merk KGaA, Darmstadt, Germany). Bacterial suspension was added to the wells to get a final inoculum of 5x105 CFU/mL in each well.
An equal concentration of the media broth and the bacterial suspension was also dispensed in a growth control well without adding the antibiotic to ensure quality control of bacterial growth as well as for a visual comparison of bacterial inhibition in the antibiotic dispensed wells. Similarly, sterility control of CA-MHB and antibiotic suspension were also set. The quality of the MIC results was assessed by processing S. aureus ATCC 29213 with the isolates and was deemed accurate only if the MIC for the ATCC strain was within the acceptable range provided by CLSI M100. MIC50, MIC90, and MIC range were calculated for the isolates and analysed.
Frequencies, percentages, median, and IQR were computed for the patients belonging to different cities in Pakistan, and gender, age groups, and methicillin susceptibility status were tabulated according to susceptibility results of DAP MICs. Microsoft Excel software (Microsoft Excel 2013 {15.0.5553.1000} 32-bit) was used to enter and analyse the data.
RESULTS
A total of 169 clinical isolates were included in the study. The median age of the patient population was 34 years (IQR: 15.5 – 50 years). Among all, 104 (61.5%) were males and 65 (38.5%) were females. The distribution of specimens is shown in Figure 1.
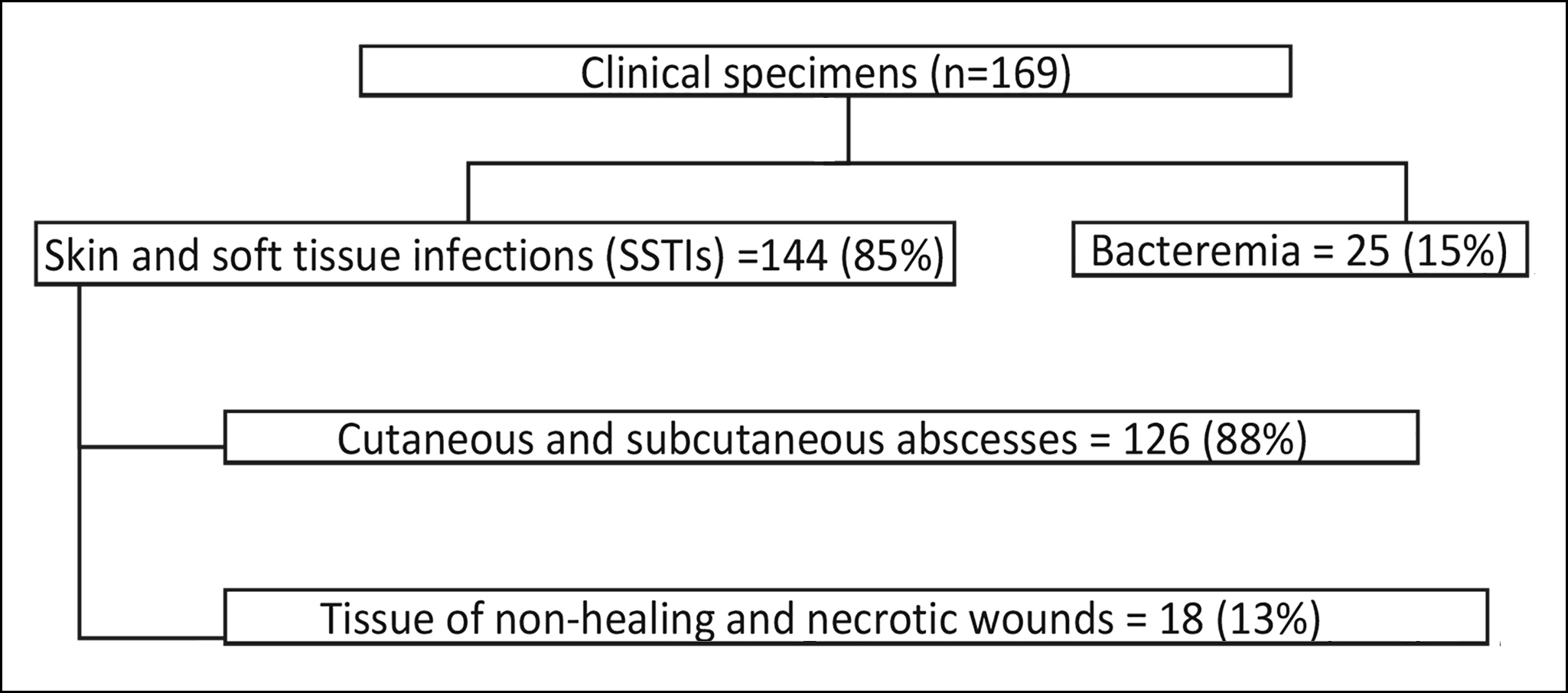 Figure 1: The distribution of specimens.
Figure 1: The distribution of specimens.
The geographical distribution of specimens across the country is given in Table I. Most of the isolates were from Karachi, Sindh, probably due to the large footprint of laboratory collection units providing specimen collection and transportation to the central laboratory from outpatient clinics and hospitals across the city of Karachi.
Both methicillin-susceptible and resistant isolates of S. aureus were included in the study; the predominant isolates were MRSA (123/169, 73%).
DAP MIC for the ATCC 29213 was within the range of 0.12 – 1.0 µg/ml. The isolates processed for BMD showed pure growth on purity plates. All the S. aureus isolates were found to be sensitive to DAP with MIC50 of 0.25 µg/ml, and MIC90 of 0.5 µg/ml (Figure 2).
The distribution of methicillin susceptibility among the isolates and its association with their DAP MICs is demonstrated in Figure 3; however, it did not reach statistical significance.
Table I: Distribution of specimens received from various regions of Pakistan.
|
Geographical distribution |
Total sample % (n) |
|
Sindh |
65 (109) |
|
Punjab |
18 (32) |
|
Baluchistan |
8 (13) |
|
KPK |
5 (8) |
|
Azad Kashmir |
1(1) |
|
Total specimens |
100 (169) |
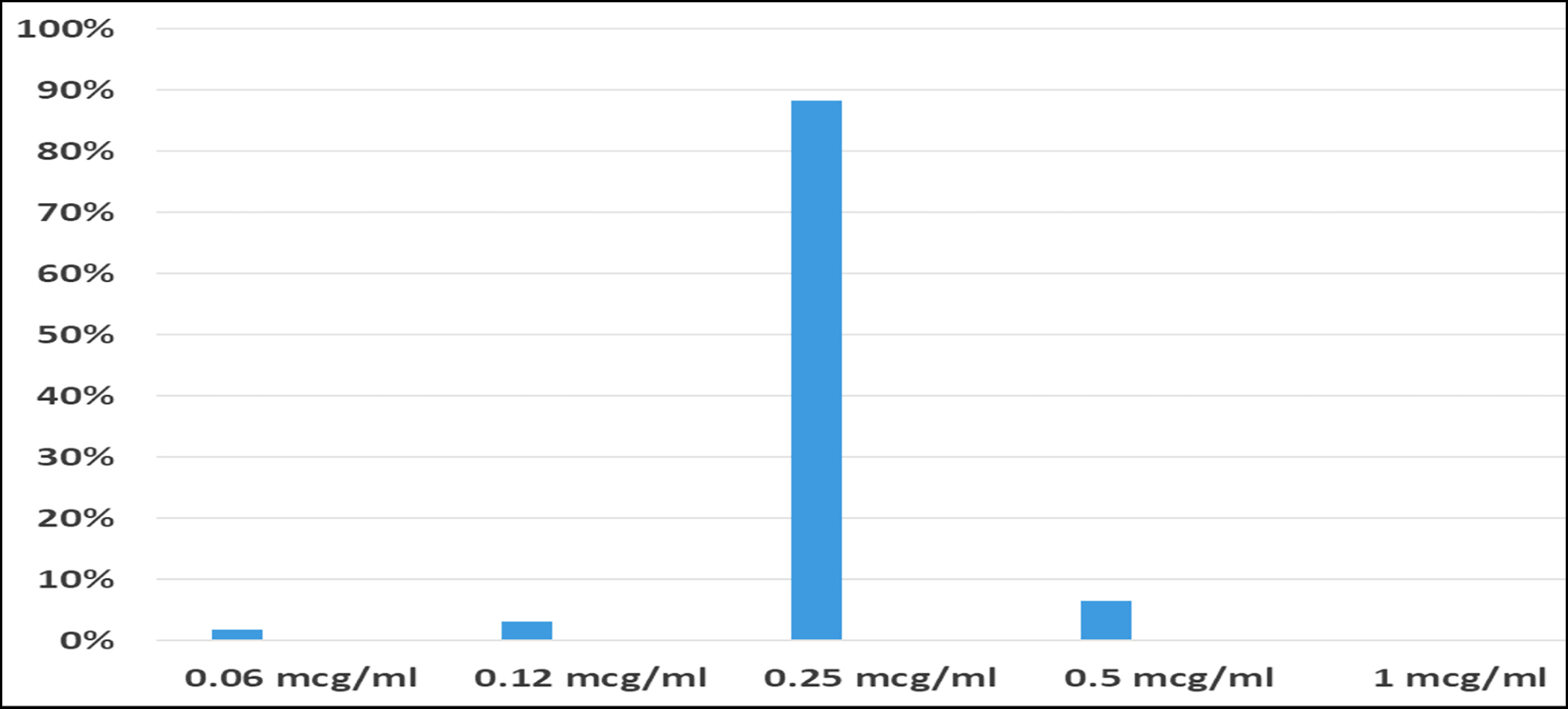 Figure 2: Daptomycin minimum inhibitory concentrations (MICs) of S. aureus.
Figure 2: Daptomycin minimum inhibitory concentrations (MICs) of S. aureus.
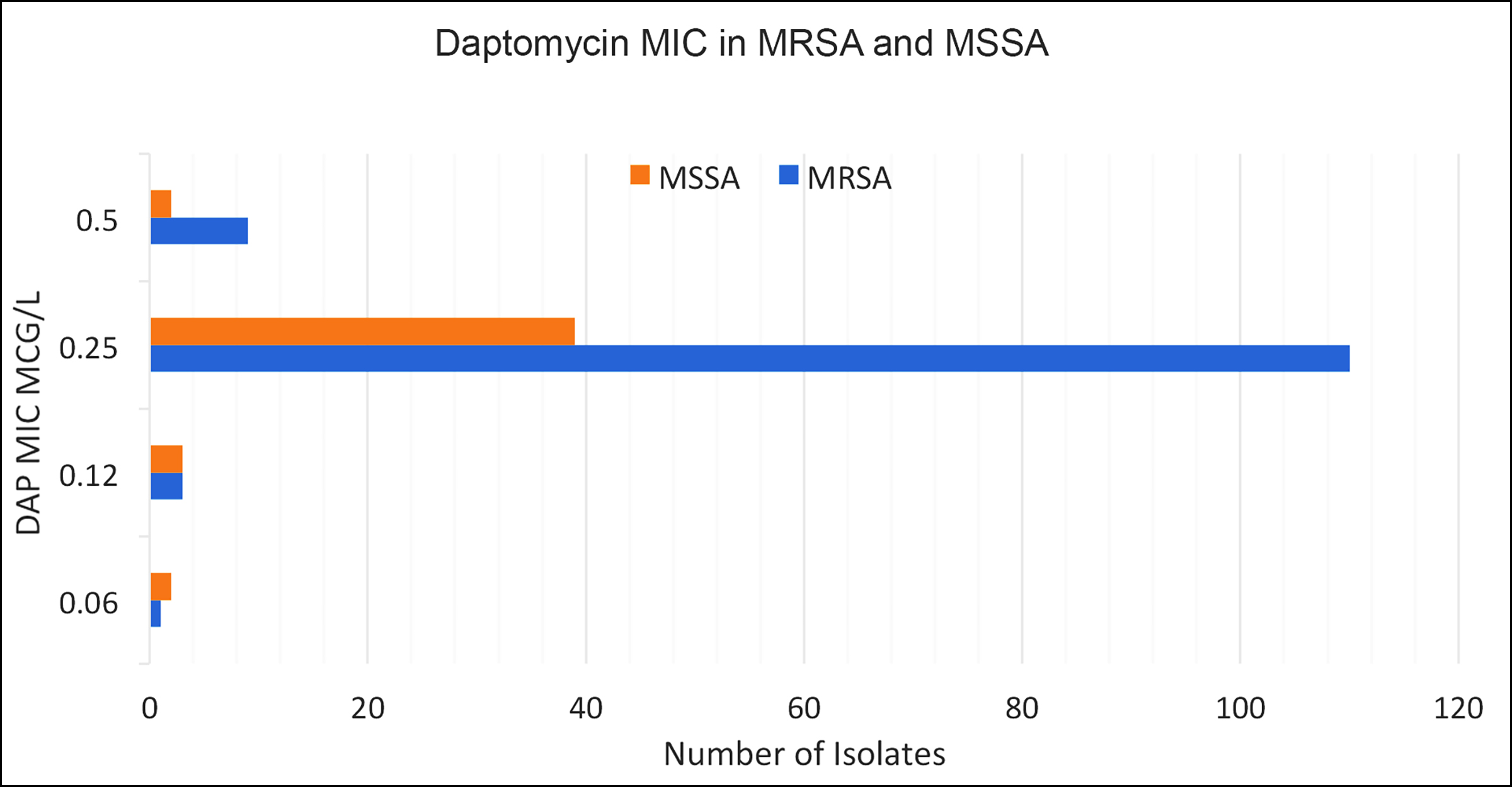 Figure 3: Methicillin-susceptible and resistant isolates and DAP MICs.
Figure 3: Methicillin-susceptible and resistant isolates and DAP MICs.
DISCUSSION
Pakistan has a high antimicrobial resistance burden; unregulated antibiotic use and inadequate surveillance to monitor the emergence of antimicrobial resistance are important contri-buting factors to the situation. The bacterial cell wall is the target site for both vancomycin and DAP. Prior use of vanco-mycin in vancomycin-intermediate S. aureus (VISA) has been linked to the emergence of DAP non-susceptible strains. The unprecedented vancomycin use in healthcare institutes of Pakistan, unknown vancomycin MICs of clinical isolates, and the presence of VISA in the Pakistani population can be the suitable situations for the development of DAP resistance in S. aureus.14,15 The institutional data (unpublished) of vancomycin MIC had also shown the rising trends of MIC 2 µg/ml from 0.3 to 2.8%. Although DAP is not available in Pakistan and not approved by the drug regulatory authority of Pakistan (DRAP), the studies had shown increasing DAP resistance in S. aureus, Enterococcus faecalis and Enterococcus faecium without prior DAP exposure.16 This study provided the baseline data for DAP susceptibility trends against methicillin-resistant and suscep-tible S. aureus isolated in clinical specimens collected from the different geographical regions of Pakistan.
This study found that all SSTIs and bacteremia-associated S. aureus isolates were DAP susceptible, and the MIC of the majority of isolates was ≤0.25 µg/ml. Another unicentric study conducted in Rawalpindi, Pakistan, examined the in-vitro efficacy of DAP among MRSA isolates using an E-test. Of the 270 isolates, 97.78% were susceptible to DAP while 6 of the isolates showed higher MICs of >1 µg/ml. These 6 isolates that had MIC >1 µg/ml were not rechecked with BMD method; hence, there is a lack of reasonable comparison of the DAP MICs between different methods for determining whether these isolates were truly resistant to DAP.12
Although studies had shown the high categorical agreement between the two methods for both S. aureus and Enterococcus; E-test showed at least 1 doubling dilution higher in most of the tested isolates as compared with the BMD method.17,18 Poor drug penetration and inappropriate calcium concentration in media were the important limitations highlighted in the literature in assessing the DAP E-test MICs.19
DAP provided an apt alternative to the potentially nephrotoxic vancomycin which required continuous TDM and exhibited rising MICs over the past decade in Pakistan. A study conducted in a tertiary care hospital in Karachi reported the isolation of VISA strains from patients with bloodstream infections.14 Another study from Peshawar reported the isolation of VISA strains from various clinical specimens.15 Moreover, vanco-mycin susceptibility testing and reporting on disc diffusion methods and limited vancomycin TDM in the clinical laboratories of Pakistan are blind spots for clinicians that can create serious problems.20
Polymicrobial infections produce synergistic interactions between microbes which can manifest in various forms, well-known as ‘polymicrobial synergism’. One such extensively studied interaction involves tolerance to various antimicrobial agents leading to the treatment failure and raised morbidity.21 In this study, 31 of the S. aureus isolates (18%) were from polymicrobial cultures. DAP MICs in these isolates were on the lower side (0.006 – 0.5 µg/ml) and were seemingly unaffected by polymicrobial synergistic effects. In addition, there had been cases of polymicrobial infections which have been successfully treated with DAP as a part of combination therapy.22 Therefore, the promising in-vitro effects of DAP suggested its potential role as a therapeutic agent in monotherapy as well as in combination therapy.
Furthermore, the findings of this study may guide physicians in using DAP as an alternative to vancomycin and should prompt the Drug Regulatory Authority of Pakistan to make the drug available in the country so it can be used when there are limitations.
The limitation of this study was that the authors did not check susceptibilities to DAP against vancomycin-resistant enterococcus (VRE) which accounts for a significant number of infections in hospitalised and long-term care patients.
CONCLUSION
The present study findings demonstrated low in-vitro MICs for DAP against S. aureus in tested isolates from the diverse clinical specimens from across Pakistan suggesting a promising and safe alternative to vancomycin that can be used as mono-therapy as well as in combination with fewer side effects.
ETHICAL APPROVAL:
The Ethical Review Committee at The Aga Khan University approved the protocol of this study and the approval number is Ref #. 2020-5753-15344.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
MZ: Conceived the study design, analysed and interpreted the data, drafted and reviewed the manuscript.
IA: Conceived the study design, bench experiment, drafted and reviewed the manuscript.
SI: Supervised, reviewed and edited the manuscript.
MK: Conducted the bench experiment, data analysis, and review of the manuscript.
AZ: Supervised, reviewed, wrote and edited the manuscript.
All authors read and approved the final version of the manu-script to be published.
REFERENCES
- Ikuta KS, Swetschinski LR, Aguilar GR, Sharara F, Mestrovic T, Gray AP, et al. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022; 400(10369):2221-48. doi: 10.1016/S0140-6736(22)02 185-7.
- Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022; 399(10325):629-55. doi: 10.1016/S0140-6736(21)027 24-0.
- Jenul C, Horswill AR. Regulation of Staphylococcus aureus virulence. Microbiol Spectr 2019; 7(2):10.1128/micro biolspec.GPP3-0031-2018. doi: 10.1128/microbiolspec. GPP3-0031-2018.
- Foster TJ. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol Rev 2017; 41(3):430-49. doi: 10.1093/femsre/fux007.
- Manzanares-Leal G, Ramírez-Durán N, Moreno-Pérez P, Sánchez Y, Calderón M, Sandoval-Trujillo H. New bacterial species resistant to antibiotics, current situation. One Health Triad, Unique Scientific Publishers, Pakistan: Faisal-abad 2023; 2:102-8.
- Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methi-cillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, The Infectious Diseases Society of America, The Pediatric Infectious Diseases Society, and The Society of Infectious Diseases Pharma-cists. Am J Health Syst Pharm 2020; 77(11): 835-64. doi: 10.1093/ajhp/zxaa036.
- Gould FK, Denning DW, Elliott TS, Foweraker J, Perry JD, Prendergast BD, et al. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: A report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 2012; 67(2): 269-89. doi: 10.1093/jac/dkr450.
- Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by The Infectious Diseases Society of America for the treatment of methi-cillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18-55. doi: 10.1093/cid/ciq146.
- Heidary M, Khosravi AD, Khoshnood S, Nasiri MJ, Soleimani S, Goudarzi M. Daptomycin. J Antimicrob Chemother 2018; 73(1):1-11. doi: 10.1093/jac/dkx349.
- Salemi R, Zega A, Aguglia E, Lo Verde F, Pigola G, Stefani S, et al. Balancing the Virulence and Antimicrobial Resistance in VISA DAP-R CA-MRSA Superbug. Antibiotics 2022; 11(9): 1159.
- Garcia LS. Clinical microbiology procedures handbook. American Society for Microbiology Press; 2010.
- Shah AA, Abbasi SA, Ali Y, Maqbool A. In vitro efficacy of Daptomycin against clinical isolates of Methicillin-resistant Staphylococcus aureus (MRSA). J Pak Med Assoc 2021; 71(1(B):338-40. doi: 10.47391/JPMA.145.
- CLSI, Performance Standard for Antimicrobial Susceptibility Testing. 33rd ed. CLSI Supplement M100. Clinical and Laboratory Standards Institute; 2018.
- Hakim S, Arshed S, Iqbal M, Javaid S. Vancomycin sensi-tivity of Staphylococcus aureus isolates from hospital pati-ents in Karachi, Pakistan. Libyan J Med 2007; 2(4): 176-9. doi: 10.4176/070624.
- Ullah A, Qasim M, Rahman H, Khan J, Haroon M, Muhammad N, et al. High frequency of methicillin-resistant Staphylo-coccus aureus in Peshawar Region of Pakistan. Springerplus 2016; 5:600. doi: 10.1186/s40064-016- 2277-3.
- Tran TT, Munita JM, Arias CA. Mechanisms of drug resis-tance: Daptomycin resistance. Ann N Y Acad Sci 2015; 1354:32-53. doi: 10.1111/nyas.12948.
- Bushra KP, Sudhaharan S, Teja VD. Comparison of Vancomycin MICs by Broth Microdilution Method, E-Test and Vitek 2C among MRSA Isolates in Tertiary Care Centre, Hyderabad, India. Natl Lab Med 2022; 11(3): M001-M004.
- Riedel S, Neoh KM, Eisinger SW, Dam LM, Tekle T, Carroll KC. Comparison of commercial antimicrobial susceptibility test methods for testing of Staphylococcus aureus and Enterococci against vancomycin, daptomycin, and linezo-lid. J Clin Microbiol 2014; 52(6):2216-22. doi: 10. 1128/ JCM.00957-14.
- Koeth L, DiFranco J. Comparison of daptomycin E-test MICs on Mueller Hinton, IsoSensitest and brain heart infusion agars from Europe against 20 Staphylococcus aureus isolates. Eur J Clin Microbiol Infect Dis 2010; 29(10): 1261-4. doi: 10.1007/s10096-010-0996-x.
- Arshad F, Saleem S, Jahan S, Tahir R. Assessment of vanco-mycin MIC creep phenomenon methicillin-resistant Staphylococcus aureus isolate in a tertiary care hospital of Lahore. Pak J Med Sci 2020; 36(7):1505-10. doi: 10.12669/ pjms.36.7.3273.
- Little W, Black C, Smith AC. Clinical implications of poly-microbial synergism effects on antimicrobial suscepti-bility. Pathogens 2021; 10(2):144. doi: 10.3390/pathogens 10020144.
- Pasticci MB, Di Filippo P, Pasqualini L, Mencacci A, Pallotto C, Malincarne L, et al. Tolerability and efficacy of long-term treatment with daptomycin, ceftazidime and colistin in a patient with a polymicrobial, multidrug-resistant prosthetic joint reinfection: A case report. J Med Case Rep 2014: 8: 186. doi: 10.1186/1752-1947-8-186.